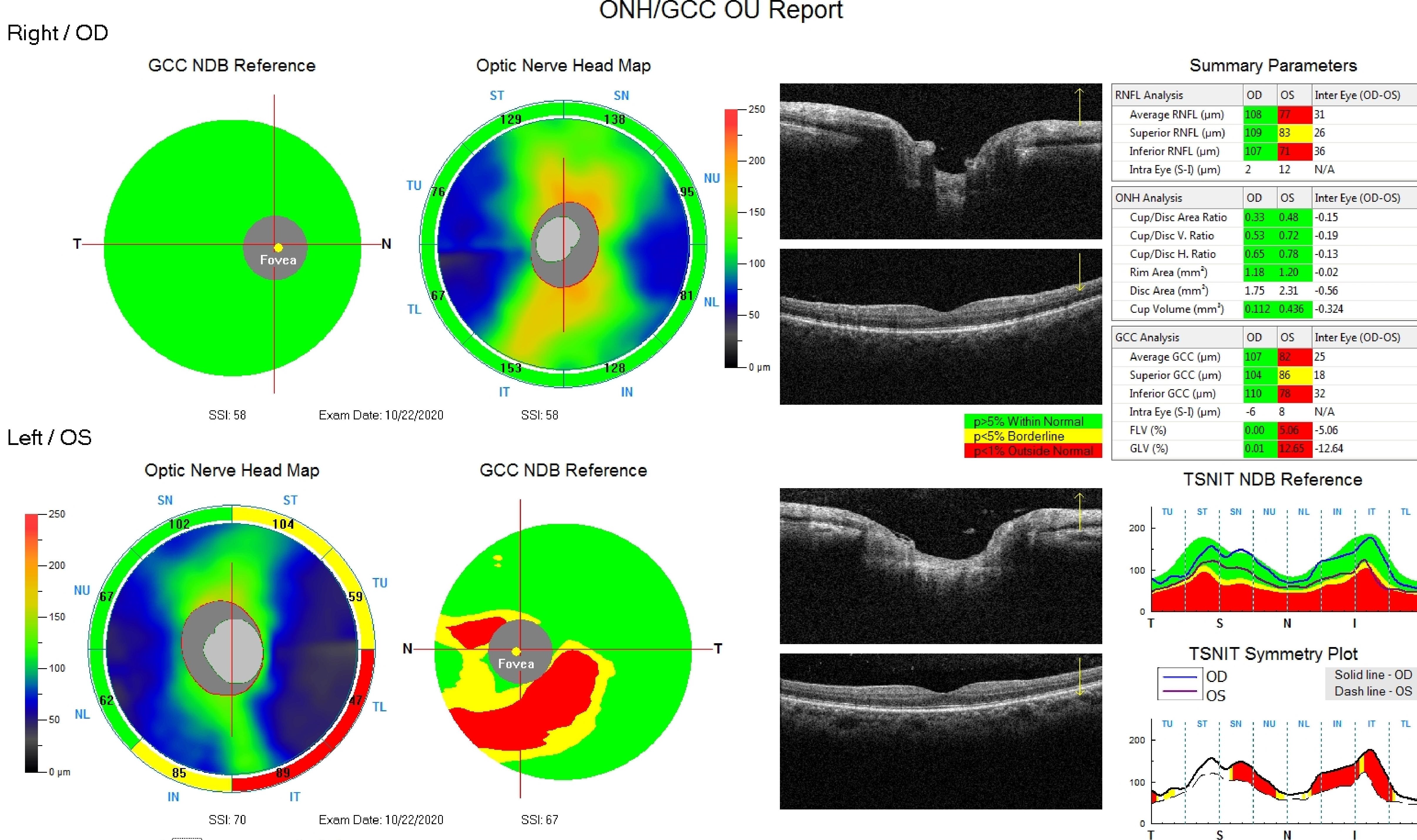
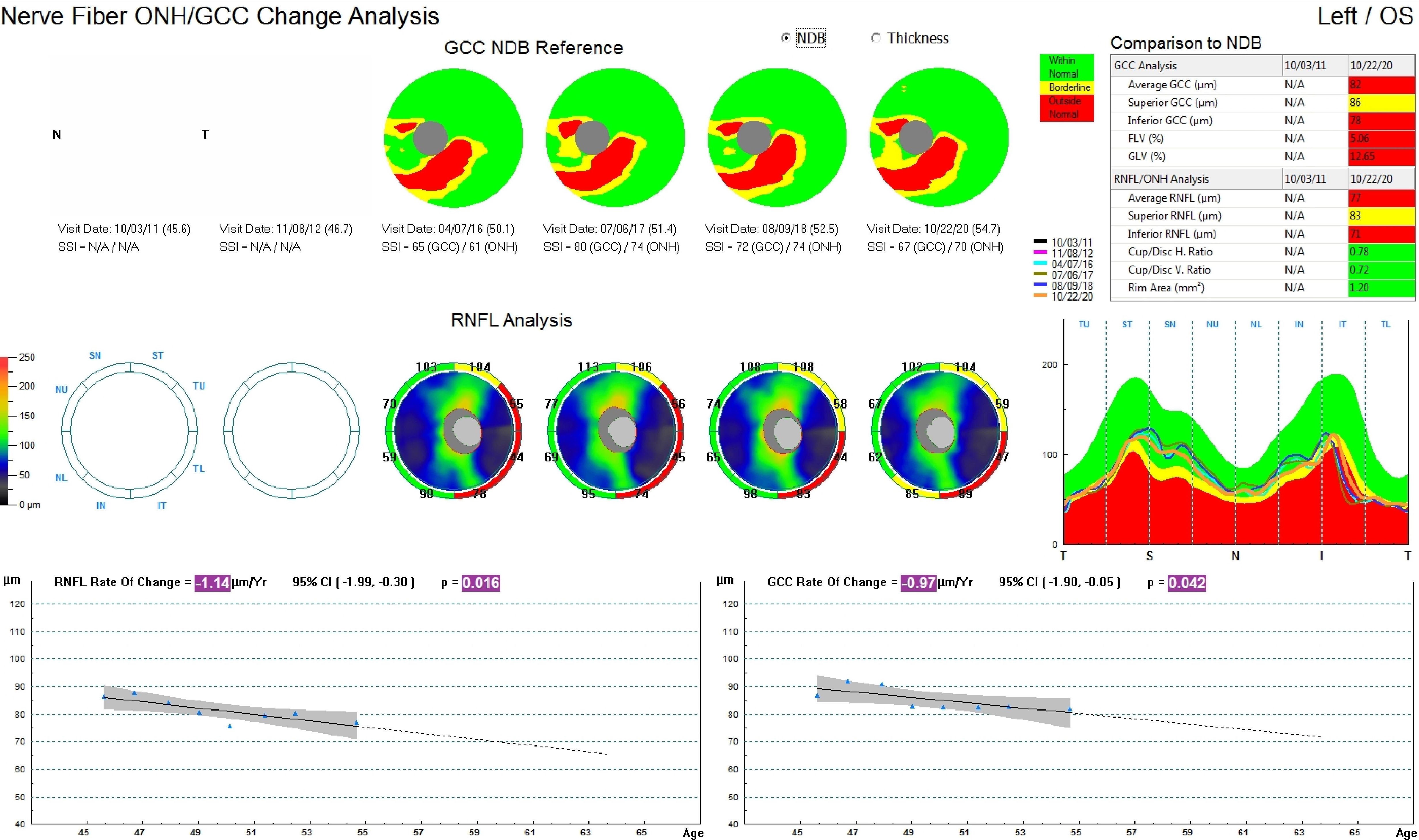
[1]
POSNER A, SCHLOSSMAN A. Syndrome of unilateral recurrent attacks of glaucoma with cyclitic symptoms. Archives of ophthalmology (Chicago, Ill. : 1929). 1948 Apr:39(4):517-35
[PubMed PMID: 18123283]
[2]
Megaw R, Agarwal PK. Posner-Schlossman syndrome. Survey of ophthalmology. 2017 May-Jun:62(3):277-285. doi: 10.1016/j.survophthal.2016.12.005. Epub 2016 Dec 22
[PubMed PMID: 28012873]
Level 3 (low-level) evidence
[3]
Shazly TA, Aljajeh M, Latina MA. Posner-Schlossman glaucomatocyclitic crisis. Seminars in ophthalmology. 2011 Jul-Sep:26(4-5):282-4. doi: 10.3109/08820538.2011.605821. Epub
[PubMed PMID: 21958175]
[4]
Huq M, Sanan N, Daniels P, Hostoffer R. Posner-Schlossman Syndrome in Common Variable Immunodeficiency. Case reports in ophthalmological medicine. 2020:2020():8843586. doi: 10.1155/2020/8843586. Epub 2020 Oct 15
[PubMed PMID: 33123397]
Level 3 (low-level) evidence
[5]
Kass MA, Becker B, Kolker AE. Glaucomatocyclitic crisis and primary open-angle glaucoma. American journal of ophthalmology. 1973 Apr:75(4):668-73
[PubMed PMID: 4696670]
[6]
Green RJ. Posner-Schlossman syndrome (glaucomatocyclitic crisis). Clinical & experimental optometry. 2007 Jan:90(1):53-6
[PubMed PMID: 17177667]
[7]
Kim TH, Kim JL, Kee C. Optic disc atrophy in patient with Posner-Schlossman syndrome. Korean journal of ophthalmology : KJO. 2012 Dec:26(6):473-7. doi: 10.3341/kjo.2012.26.6.473. Epub 2012 Nov 12
[PubMed PMID: 23204806]
[8]
Takusagawa HL, Liu Y, Wiggs JL. Infectious theories of Posner-Schlossman syndrome. International ophthalmology clinics. 2011 Fall:51(4):105-15. doi: 10.1097/IIO.0b013e31822d6ab4. Epub
[PubMed PMID: 21897144]
[9]
THEODORE FH. Observations on glaucomatocyclitic crises (Posner-Schlossman syndrome). The British journal of ophthalmology. 1952 Apr:36(4):207-10
[PubMed PMID: 14916093]
[10]
Sokolić P. Developmental factor in the etiopathogenesis of glaucomatocyclitic crisis. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 1970:161(5):446-50
[PubMed PMID: 4993003]
[11]
Raitta C, Vannas A. Glaucomatocyclitic crisis. Archives of ophthalmology (Chicago, Ill. : 1960). 1977 Apr:95(4):608-12
[PubMed PMID: 557968]
[12]
Chee SP, Bacsal K, Jap A, Se-Thoe SY, Cheng CL, Tan BH. Clinical features of cytomegalovirus anterior uveitis in immunocompetent patients. American journal of ophthalmology. 2008 May:145(5):834-40. doi: 10.1016/j.ajo.2007.12.015. Epub 2008 Feb 6
[PubMed PMID: 18255045]
[13]
Babu K, Konana VK, Ganesh SK, Patnaik G, Chan NSW, Chee SP, Sobolewska B, Zierhut M. Viral anterior uveitis. Indian journal of ophthalmology. 2020 Sep:68(9):1764-1773. doi: 10.4103/ijo.IJO_928_20. Epub
[PubMed PMID: 32823392]
[14]
Choi CY, Kim MS, Kim JM, Park SH, Park KH, Hong C. Association between Helicobacter pylori infection and Posner-Schlossman syndrome. Eye (London, England). 2010 Jan:24(1):64-9. doi: 10.1038/eye.2009.34. Epub 2009 Feb 20
[PubMed PMID: 19229276]
[15]
Bloch-Michel E, Dussaix E, Cerqueti P, Patarin D. Possible role of cytomegalovirus infection in the etiology of the Posner-Schlossmann syndrome. International ophthalmology. 1987 Dec:11(2):95-6
[PubMed PMID: 2833455]
[16]
Kongyai N, Sirirungsi W, Pathanapitoon K, Tananuvat N, Kunavisarut P, Leechanachai P, de Groot-Mijnes JD, Rothova A. Viral causes of unexplained anterior uveitis in Thailand. Eye (London, England). 2012 Apr:26(4):529-34. doi: 10.1038/eye.2011.363. Epub 2012 Jan 13
[PubMed PMID: 22241022]
[17]
Hwang YS, Shen CR, Chang SH, Lai CC, Liu CL, Chen KJ, Lin KK, Chen TL, Hsiao CH. The validity of clinical feature profiles for cytomegaloviral anterior segment infection. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2011 Jan:249(1):103-10. doi: 10.1007/s00417-010-1510-y. Epub 2010 Sep 21
[PubMed PMID: 20857136]
[18]
Koizumi N, Inatomi T, Suzuki T, Shiraishi A, Ohashi Y, Kandori M, Miyazaki D, Inoue Y, Soma T, Nishida K, Takase H, Sugita S, Mochizuki M, Kinoshita S, Japan Corneal Endotheliitis Study Group. Clinical features and management of cytomegalovirus corneal endotheliitis: analysis of 106 cases from the Japan corneal endotheliitis study. The British journal of ophthalmology. 2015 Jan:99(1):54-8. doi: 10.1136/bjophthalmol-2013-304625. Epub 2014 Jul 29
[PubMed PMID: 25075122]
Level 3 (low-level) evidence
[19]
Sobolewska B, Deuter C, Doycheva D, Zierhut M. Long-term oral therapy with valganciclovir in patients with Posner-Schlossman syndrome. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2014 Jan:252(1):117-24. doi: 10.1007/s00417-013-2535-9. Epub 2013 Nov 28
[PubMed PMID: 24287937]
[20]
Leleu I, Jhanji V, Touhami S, Westcott M, Angi M, Titah C, Rousseau A, Hamard P, Brasnu E, Manicom T, Blumen-Ohana E, Rozenberg F, Vauloup-Fellous C, Deback C, Labetoulle M, Sahel JA, Bodaghi B, Merabet L, Kobal A, Brignole-Baudouin F, Errera MH. Clinical Features and Diagnosis of Anterior Segment Inflammation Related to Cytomegalovirus in Immunocompetent African, Asian, and Caucasian Patients. Ocular immunology and inflammation. 2021 Jan 2:29(1):160-168. doi: 10.1080/09273948.2019.1662059. Epub 2019 Oct 23
[PubMed PMID: 31642720]
[21]
Shen SC, Ho WJ, Wu SC, Yu KH, Lin HC, Lin YS, Tsay PK, Chu PH. Peripheral vascular endothelial dysfunction in glaucomatocyclitic crisis: a preliminary study. Investigative ophthalmology & visual science. 2010 Jan:51(1):272-6. doi: 10.1167/iovs.09-3849. Epub 2009 Aug 13
[PubMed PMID: 19684007]
[22]
Hedayatfar A, Chee SP. Posner-Schlossman syndrome associated with cytomegalovirus infection: a case series from a non-endemic area. International ophthalmology. 2014 Oct:34(5):1123-9. doi: 10.1007/s10792-014-9928-6. Epub 2014 Mar 13
[PubMed PMID: 24622824]
Level 2 (mid-level) evidence
[23]
Burnstein Y, Shelton K, Higginbotham EJ. Glaucomatocyclitic crisis in a child. American journal of ophthalmology. 1998 Jul:126(1):136-7
[PubMed PMID: 9683163]
[24]
Harrington JR. Posner-Schlossman syndrome: a case report. Journal of the American Optometric Association. 1999 Nov:70(11):715-23
[PubMed PMID: 10618850]
Level 3 (low-level) evidence
[25]
Zhai RY, Xu H, Kong XM, Wang ZJ. [Effect of 2% ganciclovir eye drops on cytomegalovirus positive Posner-Schlossman syndrome]. [Zhonghua yan ke za zhi] Chinese journal of ophthalmology. 2018 Nov 11:54(11):833-838. doi: 10.3760/cma.j.issn.0412-4081.2018.11.007. Epub
[PubMed PMID: 30440154]
[26]
Päivönsalo-Hietanen T, Tuominen J, Vaahtoranta-Lehtonen H, Saari KM. Incidence and prevalence of different uveitis entities in Finland. Acta ophthalmologica Scandinavica. 1997 Feb:75(1):76-81
[PubMed PMID: 9088407]
[27]
Li J, Ang M, Cheung CM, Vania M, Chan AS, Waduthantri S, Yang H, Chee SP. Aqueous cytokine changes associated with Posner-Schlossman syndrome with and without human cytomegalovirus. PloS one. 2012:7(9):e44453. doi: 10.1371/journal.pone.0044453. Epub 2012 Sep 13
[PubMed PMID: 23028541]
[28]
Ohguro N, Sonoda KH, Takeuchi M, Matsumura M, Mochizuki M. The 2009 prospective multi-center epidemiologic survey of uveitis in Japan. Japanese journal of ophthalmology. 2012 Sep:56(5):432-5. doi: 10.1007/s10384-012-0158-z. Epub 2012 Jul 3
[PubMed PMID: 22752308]
Level 3 (low-level) evidence
[29]
Nakahara H, Kaburaki T, Takamoto M, Okinaga K, Matsuda J, Konno Y, Kawashima H, Numaga J, Fujino Y, Amano S. Statistical analyses of Endogenous Uveitis Patients (2007-2009) in central Tokyo area and Comparison with Previous Studies (1963-2006). Ocular immunology and inflammation. 2015 Aug:23(4):291-296. doi: 10.3109/09273948.2014.920036. Epub 2014 Aug 25
[PubMed PMID: 25154003]
[30]
Ohira S, Inoue T, Iwao K, Takahashi E, Tanihara H. Factors Influencing Aqueous Proinflammatory Cytokines and Growth Factors in Uveitic Glaucoma. PloS one. 2016:11(1):e0147080. doi: 10.1371/journal.pone.0147080. Epub 2016 Jan 15
[PubMed PMID: 26771310]
[31]
Jiang JH, Zhang SD, Dai ML, Yang JY, Xie YQ, Hu C, Mao GY, Lu F, Liang YB. Posner-Schlossman syndrome in Wenzhou, China: a retrospective review study. The British journal of ophthalmology. 2017 Dec:101(12):1638-1642. doi: 10.1136/bjophthalmol-2016-309863. Epub 2017 Apr 27
[PubMed PMID: 28450379]
Level 2 (mid-level) evidence
[32]
Chee SP, Jap A. Presumed fuchs heterochromic iridocyclitis and Posner-Schlossman syndrome: comparison of cytomegalovirus-positive and negative eyes. American journal of ophthalmology. 2008 Dec:146(6):883-9.e1. doi: 10.1016/j.ajo.2008.09.001. Epub
[PubMed PMID: 19027423]
[33]
Woo JH, Lim WK, Ho SL, Teoh SC. Characteristics of Cytomegalovirus Uveitis in Immunocompetent Patients. Ocular immunology and inflammation. 2015:23(5):378-83. doi: 10.3109/09273948.2014.950384. Epub 2014 Sep 10
[PubMed PMID: 25207970]
[34]
Huang X, Xu Y, Chen W, Zhu T, He L, Wang S, Peng S, Mei S, Wang Y, Zhao J. The genetic contribution of HLA-E*01:03 and HLA-E*01:03-G*01:01 to Posner-Schlossman syndrome in southern Chinese. Annals of translational medicine. 2019 Dec:7(23):749. doi: 10.21037/atm.2019.11.70. Epub
[PubMed PMID: 32042765]
[35]
Zhao J, Zhu T, Chen W, Fan BJ, He L, Yang B, Deng Z. Human Leukocyte Antigens-B and -C Loci Associated with Posner-Schlossman Syndrome in a Southern Chinese Population. PloS one. 2015:10(7):e0132179. doi: 10.1371/journal.pone.0132179. Epub 2015 Jul 10
[PubMed PMID: 26161794]
[36]
Hirose S, Ohno S, Matsuda H. HLA-Bw54 and glaucomatocyclitic crisis. Archives of ophthalmology (Chicago, Ill. : 1960). 1985 Dec:103(12):1837-9
[PubMed PMID: 4074175]
[37]
Koizumi N, Suzuki T, Uno T, Chihara H, Shiraishi A, Hara Y, Inatomi T, Sotozono C, Kawasaki S, Yamasaki K, Mochida C, Ohashi Y, Kinoshita S. Cytomegalovirus as an etiologic factor in corneal endotheliitis. Ophthalmology. 2008 Feb:115(2):292-297.e3
[PubMed PMID: 17669498]
[38]
Hess LK, Lee GA, Shah P. Bilateral simultaneous presentation of Posner-Schlossman syndrome. Clinical & experimental ophthalmology. 2017 Dec:45(9):925-927. doi: 10.1111/ceo.12981. Epub 2017 Jun 13
[PubMed PMID: 28486785]
[39]
Iwata K, Namba K, Abe H. [Early fundus changes caused by repeated small crises in the Posner-Schlossman syndrome: a model for glaucoma simplex]. Klinische Monatsblatter fur Augenheilkunde. 1982 Jan:180(1):20-6
[PubMed PMID: 7077989]
[40]
Liu PK, Tseng HY, Huang MY, Wu KY. Glaucomatocyclitic Crises May Occur in Patients with Narrow or Closed Angles. Journal of ophthalmology. 2017:2017():4074912. doi: 10.1155/2017/4074912. Epub 2017 Nov 19
[PubMed PMID: 29348928]
[41]
Hart CT, Weatherill JR. Gonioscopy and tonography in glaucomatocyclitic crises. The British journal of ophthalmology. 1968 Sep:52(9):682-7
[PubMed PMID: 5725167]
[42]
Choi JA, Park YR, La TY. Concurrence of iridocorneal endothelial syndrome in a patient with glaucomatocyclitic crisis. International journal of ophthalmology. 2014:7(2):384-6. doi: 10.3980/j.issn.2222-3959.2014.02.34. Epub 2014 Apr 18
[PubMed PMID: 24790889]
[43]
Köhler U. [Posner-Schlossman syndrome]. Ophthalmologica. Journal international d'ophtalmologie. International journal of ophthalmology. Zeitschrift fur Augenheilkunde. 1992:205(3):158-62
[PubMed PMID: 1475095]
[44]
Garala P, Bansal A. Acute Secondary Optic Neuropathy as a Complication of a Single Episode of Acutely Raised Intraocular Pressure: A Case Series. Journal of glaucoma. 2019 Jan:28(1):e10-e13. doi: 10.1097/IJG.0000000000001094. Epub
[PubMed PMID: 30234746]
Level 2 (mid-level) evidence
[45]
Darchuk V, Sampaolesi J, Mato L, Nicoli C, Sampaolesi R. Optic nerve head behavior in Posner-Schlossman syndrome. International ophthalmology. 2001:23(4-6):373-9
[PubMed PMID: 11944864]
[46]
Cao G, Tan C, Zhang Y, Kong X, Sun X, Ma Y, Chen J, Guan M. Digital droplet polymerase chain reaction analysis of common viruses in the aqueous humour of patients with Posner-Schlossman syndrome in Chinese population. Clinical & experimental ophthalmology. 2019 May:47(4):513-520. doi: 10.1111/ceo.13440. Epub 2018 Dec 3
[PubMed PMID: 30414235]
[47]
Rodier-Bonifas C, Cornut PL, Billaud G, Lina B, Burillon C, Denis P. [Cytomegalovirus research using polymerase chain reaction in Posner-Schlossman syndrome]. Journal francais d'ophtalmologie. 2011 Jan:34(1):24-9. doi: 10.1016/j.jfo.2010.10.008. Epub 2010 Nov 26
[PubMed PMID: 21112125]
[48]
Yokogawa H, Kobayashi A, Sugiyama K. Mapping owl's eye cells of patients with cytomegalovirus corneal endotheliitis using in vivo laser confocal microscopy. Japanese journal of ophthalmology. 2013 Jan:57(1):80-4. doi: 10.1007/s10384-012-0189-5. Epub 2012 Nov 3
[PubMed PMID: 23124832]
[49]
Shiraishi A, Hara Y, Takahashi M, Oka N, Yamaguchi M, Suzuki T, Uno T, Ohashi Y. Demonstration of "owl's eye" morphology by confocal microscopy in a patient with presumed cytomegalovirus corneal endotheliitis. American journal of ophthalmology. 2007 Apr:143(4):715-7
[PubMed PMID: 17386293]
[51]
Su CC, Hu FR, Wang TH, Huang JY, Yeh PT, Lin CP, Wang IJ. Clinical outcomes in cytomegalovirus-positive Posner-Schlossman syndrome patients treated with topical ganciclovir therapy. American journal of ophthalmology. 2014 Nov:158(5):1024-1031.e2. doi: 10.1016/j.ajo.2014.08.007. Epub 2014 Aug 12
[PubMed PMID: 25124264]
Level 2 (mid-level) evidence
[52]
Chee SP, Jap A. Cytomegalovirus anterior uveitis: outcome of treatment. The British journal of ophthalmology. 2010 Dec:94(12):1648-52. doi: 10.1136/bjo.2009.167767. Epub 2010 Jun 24
[PubMed PMID: 20576767]
[53]
Wong JX, Agrawal R, Wong EP, Teoh SC. Efficacy and safety of topical ganciclovir in the management of cytomegalovirus (CMV)-related anterior uveitis. Journal of ophthalmic inflammation and infection. 2016 Dec:6(1):10. doi: 10.1186/s12348-016-0078-z. Epub 2016 Mar 15
[PubMed PMID: 26976016]
[54]
Wong VW, Chan CK, Leung DY, Lai TY. Long-term results of oral valganciclovir for treatment of anterior segment inflammation secondary to cytomegalovirus infection. Clinical ophthalmology (Auckland, N.Z.). 2012:6():595-600. doi: 10.2147/OPTH.S30476. Epub 2012 Apr 17
[PubMed PMID: 22553419]
[55]
Hwang YS, Lin KK, Lee JS, Chang SH, Chen KJ, Lai CC, Huang JC, Kuo YH, Hsiao CH. Intravitreal loading injection of ganciclovir with or without adjunctive oral valganciclovir for cytomegalovirus anterior uveitis. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2010 Feb:248(2):263-9. doi: 10.1007/s00417-009-1195-2. Epub 2009 Sep 27
[PubMed PMID: 19784845]
[56]
Accorinti M, Gilardi M, Pirraglia MP, Amorelli GM, Nardella C, Abicca I, Pesci FR. Cytomegalovirus anterior uveitis: long-term follow-up of immunocompetent patients. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2014 Nov:252(11):1817-24. doi: 10.1007/s00417-014-2782-4. Epub 2014 Aug 20
[PubMed PMID: 25138606]
[57]
Maruyama K, Maruyama Y, Sugita S, Mori K, Yokoyama Y, Sanuki-Kunimatsu S, Nakagawa H, Kinoshita S, Mochizuki M, Nakazawa T. Characteristics of cases needing advanced treatment for intractable Posner-Schlossman syndrome. BMC ophthalmology. 2017 Apr 11:17(1):45. doi: 10.1186/s12886-017-0438-y. Epub 2017 Apr 11
[PubMed PMID: 28399831]
Level 3 (low-level) evidence
[58]
Broadway DC, Bates AK, Lightman SL, Grierson I, Hitchings RA. The importance of cellular changes in the conjunctiva of patients with uveitic glaucoma undergoing trabeculectomy. Eye (London, England). 1993:7 ( Pt 4)():495-501
[PubMed PMID: 7902819]
[60]
Zhong Y, Cheng Y, Liu X, Feng P. Trabeculectomy in the management of glaucomatocyclitic crisis with visual field defect. Ocular immunology and inflammation. 2010 Jun:18(3):233-6. doi: 10.3109/09273940903499864. Epub
[PubMed PMID: 20482405]
[61]
Pedersen IB, Ivarsen A, Hjortdal J. Graft rejection and failure following endothelial keratoplasty (DSAEK) and penetrating keratoplasty for secondary endothelial failure. Acta ophthalmologica. 2015 Mar:93(2):172-7. doi: 10.1111/aos.12518. Epub 2014 Aug 12
[PubMed PMID: 25130326]
[64]
Kasetsuwan N, Tangmonkongvoragul C. Concomitant herpes simplex virus and cytomegalovirus endotheliitis in immunocompetent patient. BMJ case reports. 2013 May 9:2013():. doi: 10.1136/bcr-2012-007942. Epub 2013 May 9
[PubMed PMID: 23667217]
Level 3 (low-level) evidence
[65]
Guo X, Chen D, Luo S, Huang J, Li Y. EDI-OCT choroidal thickness in Posner-Schlossman syndrome. International ophthalmology. 2020 Apr:40(4):877-889. doi: 10.1007/s10792-019-01251-0. Epub 2020 Jan 2
[PubMed PMID: 31894459]
[66]
Wu KY. Glaucomatocyclitic crisis and glaucomatous optic neuropathy. Taiwan journal of ophthalmology. 2015 Apr-Jun:5(2):49. doi: 10.1016/j.tjo.2015.04.005. Epub 2015 Jun 10
[PubMed PMID: 29018666]
[67]
Hung PT, Chang JM. Treatment of glaucomatocyclitic crises. American journal of ophthalmology. 1974 Feb:77(2):169-72
[PubMed PMID: 4855827]
[68]
Kim R, Van Stavern G, Juzych M. Nonarteritic anterior ischemic optic neuropathy associated with acute glaucoma secondary to Posner-Schlossman syndrome. Archives of ophthalmology (Chicago, Ill. : 1960). 2003 Jan:121(1):127-8
[PubMed PMID: 12523901]
[69]
Lippert J, Falgiani M, Ganti L. Posner-Schlossman Syndrome. Cureus. 2020 Jan 7:12(1):e6584. doi: 10.7759/cureus.6584. Epub 2020 Jan 7
[PubMed PMID: 32051797]
[70]
Irak I, Katz BJ, Zabriskie NA, Zimmerman PL. Posner-Schlossman syndrome and nonarteritic anterior ischemic optic neuropathy. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2003 Dec:23(4):264-7
[PubMed PMID: 14663306]