Continuing Education Activity
Ear microtia is a congenital ear deformity characterized by external ear malformation and underdevelopment. The more extreme microtia cases involve aural atresia or ear canal absence. Conductive or mixed hearing loss commonly accompanies the condition, often in syndromes involving multiple craniofacial components. Individuals without associated congenital syndromes typically lead normal, complete, and productive lives.
However, children born with microtia, particularly those with craniofacial anomalies, face an increased risk of low self-esteem and bullying. Therapeutic efforts historically aimed to restore normal form and function by school age, but contemporary approaches involve observation, bone-anchored hearing aids, prosthetic ear application, and reconstructive procedures using alloplastic implantation or costal cartilage autografting.
Surgical interventions have evolved. Additionally, the timing of procedures, especially with autologous costal cartilage reconstruction, is often deferred until the patient is at least 10 years old, allowing for adequate cartilage stock and meaningful patient participation in postoperative care. Factors such as infection and extrusion risk associated with high-density porous polyethylene implantation in younger children must be carefully considered during surgical planning. Given the challenges and potential complications, auricular reconstruction should be undertaken by experienced, high-volume surgical teams to ensure optimal outcomes for individuals with microtia.
This activity for healthcare professionals is designed to enhance the learner's competence in evaluating and managing ear microtia. After participation, learners gain refined skills in navigating the complexities of classifying microtia, treatment decision-making, and managing possible comorbidities like aural atresia, leading to improved decision-making, patient discussions, and enhanced outcomes in this specialized field of craniofacial care.
Objectives:
Improve understanding of ear microtia and the required evaluation approach.
Identify the psychosocial considerations for patients with ear microtia.
Differentiate between the various techniques of surgical ear microtia repair.
Improve interprofessional communication and coordination strategies when caring for individuals with ear microtia, especially when determining immediate and long-term management plans that can improve outcomes.
Introduction
Ear Microtia Overview
Ear microtia is a congenital hypoplastic malformation of the pinna, ranging in severity from slight auricular diminution to total external ear absence. Anotia is the most severe form, characterized by complete auricular and lobular formation failure. Ear microtia is often unilateral and creates a noticeable asymmetry.
This condition is often associated with congenital syndromes and is thought to be caused by vascular insults or exposure to medications during pregnancy. External ear malformation or absence can cause conductive hearing loss. Additionally, aural atresia—or failure of the middle ear and external auditory canal to form—can exacerbate conductive hearing loss in patients with microtia.
The severity of auricular deformity often correlates with middle-ear deformity. Careful collaboration between plastic surgeons and otologists is required when considering the repair timing if these 2 conditions coexist.
Children born with craniofacial anomalies are at increased risk of low self-esteem and getting bullied.[1] Historically, attempts have been made to restore normal form and function by school age to prevent such social stigma. Management options include observation, treating hearing loss with devices such as bone-anchored hearing aids, applying a prosthetic ear for cosmetic improvement, and reconstructing the auricle with alloplastic implantation or costal cartilage autografting.[2][3][4][5]
As operative techniques have evolved, particularly reconstruction with autologous costal cartilage (ACC), many surgeons can now postpone interventions until the patient is 10 years old. This strategy ensures adequate costal cartilage stock for reconstruction and patient consent and participation in postoperative care. The high-density porous polyethylene (HDPE) implantation procedure may be performed on younger candidates, typically 3 to 5 years. However, the infection and implant extrusion rate in this cohort is higher than in individuals who undergo ACC implantation later.[6] Auricular reconstruction is challenging, often causing complications. The procedure should only be performed by experienced surgery teams.
External Ear Anatomy
Understanding the external ear's anatomy is crucial to microtia evaluation and management (see Image. External Ear). The auricle or pinna is the external ear's visible part, consisting mainly of elastic cartilage covered by skin. The auricle collects and funnels sound waves into the ear canal. The external ear cartilage is the cartilaginous framework that gives shape and structure to the auricle. This cartilage may be underdeveloped or malformed in microtia, leading to external ear deformity.
The helix is the pinna's prominent outer rim. The scapha is a shallow groove on the auricle's outer surface, running parallel to the helix. The antihelix is the pinna's inner curved ridge. The triangular fossa is a small triangular hollow near the helix-antihelix junction. The helical crus is the portion where the helix begins to curve inward toward the ear canal. The superior antihelical crus is the antihelix's upper part, forming a curved ridge on the inner auricular surface. This structure helps define the auricle's shape and structure, contributing to its overall appearance. The inferior antihelical crus is the antihelix's lower portion, extending downward and supporting the auricle's inferior segment.
The tragus is a small, triangular-shaped cartilaginous projection anterolateral to the ear canal. The lobule is the earlobe's soft, fleshy lower part. The antitragus is a cartilaginous prominence lateral to the tragus, above the lobule, and below the helix. The intertragal notch (intertragal incisure or incisura) is a small groove between the tragus and antitragus.
The ear canal (external auditory canal or meatus) is a tube-like structure that extends from the auricle to the tympanic membrane. The ear canal conducts sound waves to the eardrum. The concha is a hollow, bowl-shaped depression located just inside the ear canal. The concha is divided into the superior and inferior conchae, both playing a role in directing sound waves into the ear canal and enhancing sound localization.
The tympanic membrane (eardrum) is a thin, fibrous structure separating the external auditory canal from the middle ear. This membrane vibrates with sound waves and transmits these vibrations to the middle ear.
In microtia, external ear structures may have varying degrees of underdevelopment or absence, leading to a range of deformities. Surgical reconstruction techniques aim to rebuild these structures to improve both the ear's appearance and function. Understanding the anatomy helps surgeons plan and execute reconstructive procedures effectively.
Etiology
Ear development is complex, with tissues originating from the neural crest and the endoderm, mesoderm, and ectoderm layers to form the inner, middle, and external ear.[7] The outer ear develops from the ectoderm and arises from the 1st and 2nd branchial (pharyngeal) arches, which form 6 hillocks of His at 6 weeks gestational age. While authors have debated the hillocks of His' exact contributions to the auricle's final form, recent research suggests that the 1st branchial arch (mandibular arch or Meckel Cartilage) gives rise to hillocks 1 to 3 and forms the tragus, helical crus, and helix. The antihelical crura, antihelix, lobule, and antitragus complex arise from the 2nd branchial arch, also known as the hyoid arch or Reichert cartilage.[8]
The stapedial artery also arises from the 2nd branchial arch. This vessel's early degeneration results in microtia. Ultimately, the 6 hillocks fuse to form the auricle by week 22 of gestation. The auricle grows to 85% of its adult size by age 5 and is fully developed by age 8. This early development is why some surgeons elect to perform microtia reconstruction in very young patients, particularly if they are not using autologous rib grafting.
While microtia may occur in isolation, the condition is sometimes associated with genetic craniofacial conditions, including Goldenhar, Treacher Collins, Nager, Crouzon, Pierre-Robin sequence, and CHARGE syndromes.[9][10][11] Teratogens, including isotretinoin, thalidomide, alcohol, and mycophenolate mofetil, can also give rise to microtia.[12][13] Other risk factors include first or high maternal parity, advanced maternal or paternal age, high altitude (above 8,200 feet), and low birth weight.[14][15]
Epidemiology
Microtia is usually unilateral (77% to 93%) and right-sided (60%). The condition also occurs more frequently in males (2.5:1). Microtia's prevalence per 10,000 births in the United States ranges from 1.8 to 3.5 and worldwide from 0.4 to 8.3. The condition is more common among Asians, Pacific Islanders, and Hispanic individuals in the United States.[16]
History and Physical
History
Patients may have been diagnosed with microtia shortly after birth or during infancy. The condition is typically present at birth and may be identified during routine newborn screenings or physical examinations. A family history of microtia or other congenital anomalies may be elicited, indicating a potential genetic component. However, microtia can also occur sporadically without a family history.
Microtia may occur in isolation or be associated with other congenital anomalies, such as hearing loss, craniofacial asymmetry, inner ear abnormalities, heart defects, or kidney malformations. The presence of these associated conditions may impact treatment decisions and prognosis. Patients may have undergone various medical tests and interventions. Patients may report challenges related to body image, self-confidence, and peer acceptance.
Physical Examination
The normal, fully developed human ear is approximately 6 cm in height, sloped 20° posteriorly from vertical, and has an auriculocephalic angle of 20° to 30° with a 2 to 2.5 cm distance from the helical rim to the mastoid. Microtia is a condition involving ear underdevelopment. In some cases, only portions of the lobule and helix are evident. While examining patients, a complete head and neck examination must be performed, including evaluation of the mandible, oral cavity, palate, eyes, facial nerve, and skin color and quality. The temporoparietal hairline level and auricular remnant's position must also be noted.
Association with genetic syndromes warrants examination and documentation of the patient's syndromic features. Hemifacial microsomia, preauricular pits, accessory auricular appendages, or aural atresia may be present. The ear's auricular components should be examined and compared to the contralateral side.
Conductive hearing loss is frequent in patients with microtia. Tuning fork tests may be performed to confirm this condition and guide further management if appropriate for age.
Evaluation
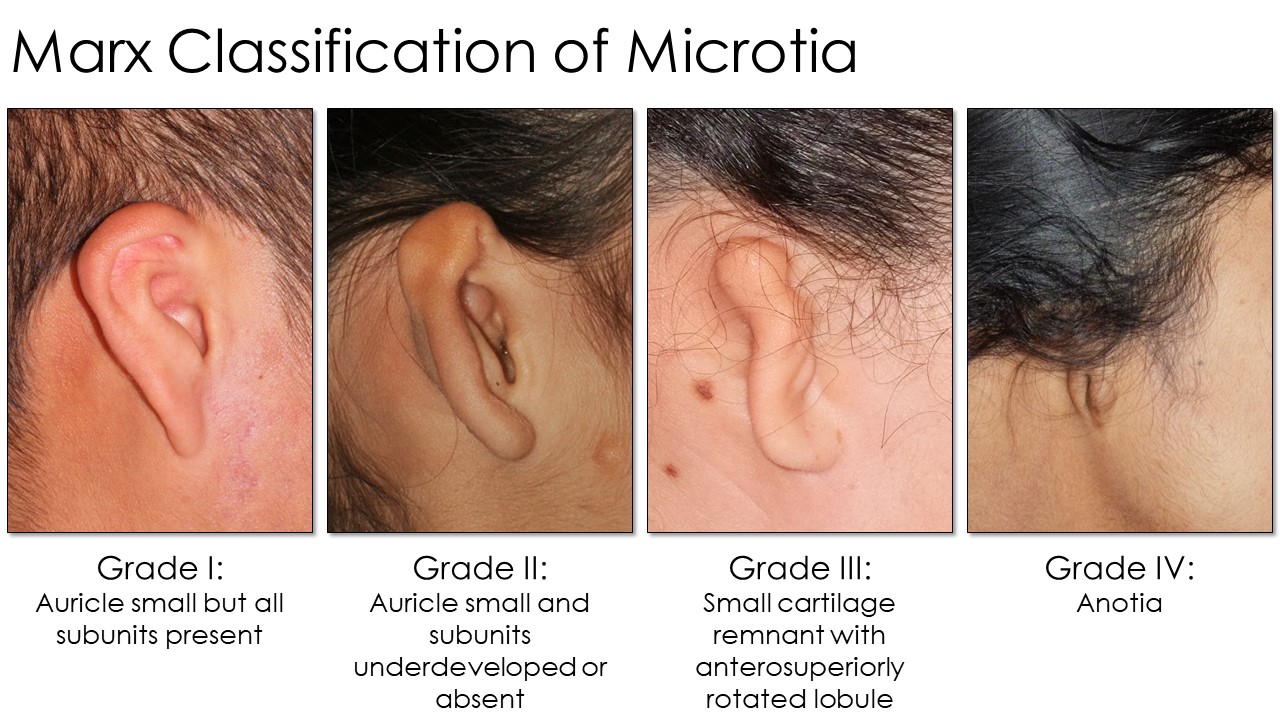
Several grading systems for microtia exist, but the Marx classification is widely used (see Image. Marx Classification Table). This grading system classifies the condition as follows:
- Grade I: The auricle is slightly smaller, by at least 2 standard deviations below the normal, but all subunits are present.
- Grade II: The auricle is smaller than usual, and subunits are severely underdeveloped or absent. The auricle's superior half is often less developed than the inferior half.
- Grade III: Only a small piece of cartilage is present in the ear's superior remnant. The lobule is rotated anterosuperiorly. This configuration is the most common, often colloquially called "peanut ear."
- Grade IV: The auricle and lobule are completely absent (anotia).
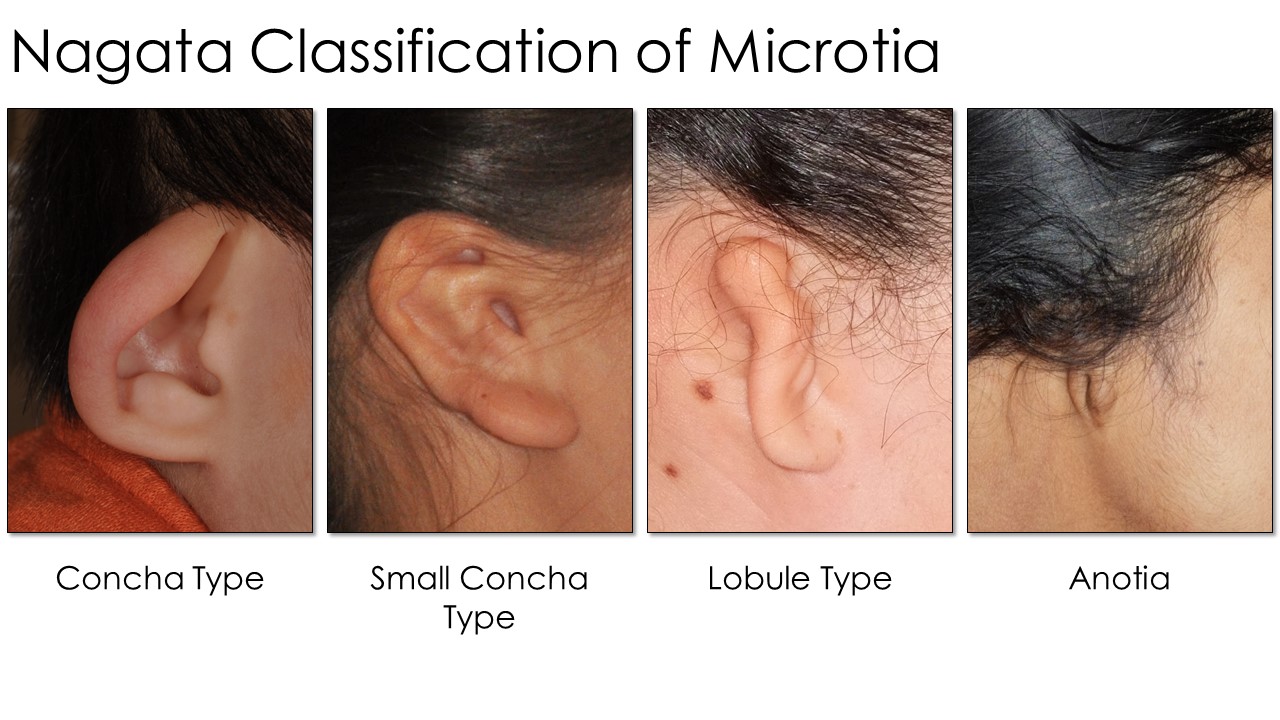
Nagata developed another scale used by practitioners of his reconstructive techniques (see Image. Nagata Classification Table). Each type (lobule, concha, small concha, and anotia) dictates the specific surgical maneuvers and staging used when performing an otoplasty. Lobule-type ears are described as "sausage-shaped" vestiges with no concha, acoustic meatus, or external auditory canal, similar to Marx Grade III. Auricles with concha-type deformity have a lobule, concha, and tragus with the intertragal notch present but a variable absence of the external auditory canal and upper auricular pole. The small-concha type has features of the lobular type but with an additional small indentation representing the concha. As with the Marx classification, Nagata's anotia type is the most severe form, characterized by the auricle's complete absence.
Hearing should be assessed early in an infant with microtia or aural atresia. Auditory brainstem response testing should be performed, especially in young children. A moderate to severe 50 to 65 dB conductive hearing loss can result from unilateral aural atresia, although 10% to 15% may have simultaneous sensorineural hearing loss. Testing of the non-atretic ear is necessary, as clinicians should not assume hearing is normal on that side.[17]
A computed tomography (CT) scan of the temporal bone is necessary to grade aural atresia and assess candidacy for repair using the Jahrsdoerfer criteria. Still, this imaging study should not be performed until the child has reached age 6 years, as atresia repair is not recommended before that age. Exposure to CT radiation may have health risks for young children.[18]
The Jahrsdoerfer grading scale has 10 points. Patients with a score of 7 or more often have better postoperative hearing outcomes. A patient receives 2 points for the presence of a stapes and 1 point each for the following: malleus-incus complex, incus-stapes joint, patent oval window, patent round window, pneumatized middle ear, pneumatized mastoid, normal facial nerve, and normal external ear. A rating of 10 is excellent, 9 is very good, 8 is good, 7 is fair, 6 is marginal, and a score of less than 6 is associated with poor candidacy for surgical repair.
Treatment / Management
Management of the microtic ear occurs along a spectrum, escalating from an observational approach and prosthetic ear placement to surgical reconstruction with an HDPE or ACC implant. A minor auricular abnormality may not need correction. However, parents typically seek intervention when desiring cosmetic and functional improvements, such as wearing glasses or hearing aids.
A prosthetic auricle can be affixed to the head with an adhesive, osseointegrated clips, or magnets. A prosthetic specialist can match the contralateral ear's skin color and appearance and design a realistic prosthesis. Downsides include prohibitive costs and the risk of a young child accidentally losing the prosthesis. Patients with fair skin may require 2 prostheses, as their skin color changes between winter and summer, and prosthetics show wear and tear after a few years of use.
One surgical reconstruction method involves HDPE implants. This newer technique is becoming more popular largely because ACC reconstruction does not consistently achieve excellent cosmesis.[19] With this method, a pedicled temporoparietal fascia (TPF) flap at least 11 cm wide by 12 cm high is harvested through an open scalp incision, a mastoid incision, or an endoscopic technique. The flap covers the entire implant with vascularized tissue and usually includes the superficial temporal artery's anterior and posterior branches. A drain placed under the implant provides suction and effectively shrink-wraps the TPF onto the implant, holding it in place and allowing the implant's complex contours to remain visible. An anteriorly-based skin flap is raised to remove cartilage and skin from the ear remnant and cover the auricle's lateral surface. Full-thickness skin grafting from the contralateral ear's postauricular surface is often required to cover the postauricular sulcus fully.
Cartilage harvesting risks cartilage calcification and pneumothorax. Besides the ability to produce natural-appearing ear contours, HDPE implantation's advantages include non-reliance on cartilage harvesting, shorter operative time, and less patient morbidity. The reconstructive surgery can be performed earlier, as this method does not require costal cartilage maturation time.
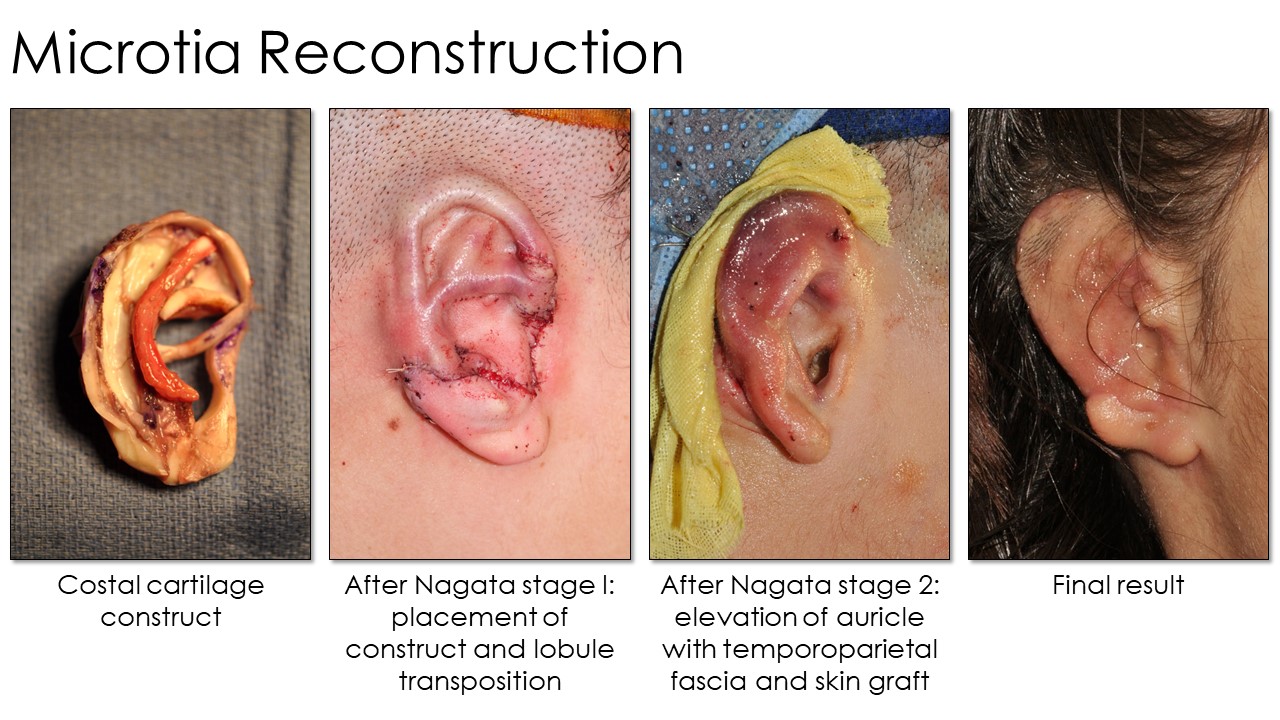
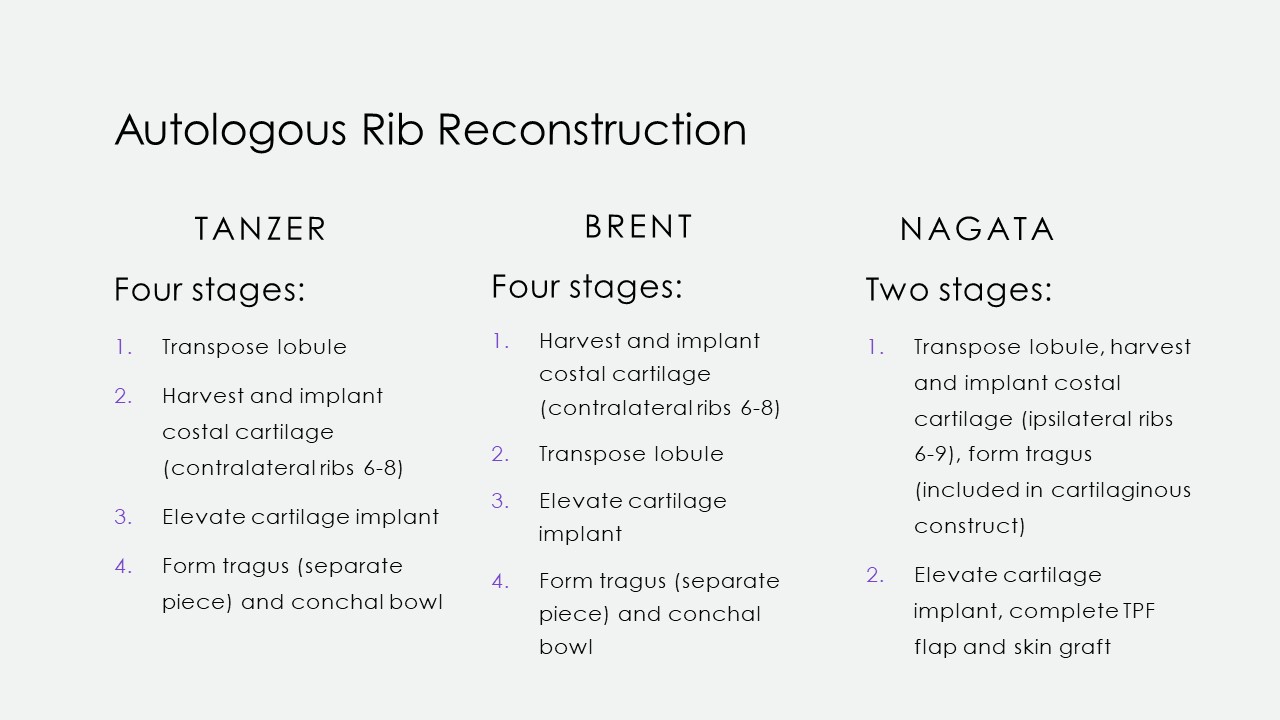
Tanzer first described autologous rib cartilage grafting at Dartmouth in 1959, and subsequent modifications were then reported by Tanzer's pupil, Brent, and later by Satoru Nagata. The operation's concept is consistent: An auricular framework is created from rib cartilage, shaped with scalpels, osteotomes, or biopsy punches, and then fixated with permanent sutures or steel wire. The construct is subsequently covered with overlying scalp tissue in a 2- to 4-stage surgical procedure (see Image. Microtia Reconstruction Stages). However, technique, laterality, and staging differences exist between these procedures (see Image. Microtia Rib Reconstruction Techniques). Additionally, multiple donor sites are required during these complex procedures. Besides costal cartilage, grafts may also be harvested from the groin, scalp, or contralateral ear.
In Tanzer's original technique, his 1st stage transposes useable lobular tissue into a horizontal position and closes the resulting vertical defect. The wound is allowed to heal. A costal cartilage framework is fabricated from the contralateral 6th to 8th ribs when the induration has softened. The 6th and 7th ribs are used together at the synchondrosis. The 8th rib is separated to form the helical rim. The vertical scar from the 1st stage is incised, and the newly constructed cartilage framework is positioned in the wound. The cartilage framework is then covered with skin and sutured into place. The cartilaginous implant is left for 4 months to consolidate. The construct is then elevated in the 3rd stage. The tragus and conchal floor are formed in stage 4. This step is necessary because the tragus and conchal floor are initially separated to diminish the risk of ischemic ear necrosis.
Brent's method differs slightly in that the 1st stage includes the cartilaginous framework's harvesting, carving, and positioning in a subcutaneous pocket at the microtic ear's site. The 2nd stage involves transposing the lobule, reversing the order of Tanzer's first 2 stages. His 3rd and 4th stages again elevate the construct and create a tragus while excavating the conchal bowl, respectively.
Nagata's technique involves autologous ear reconstruction in only 2 stages. In his 1st stage, he harvests and implants the cartilage framework similarly to Tanzer and Brent. However, he simultaneously transposes the lobule and fashions the tragus in this stage because the tragus is part of the cartilaginous construct rather than a separate piece. Nagata's 2nd stage elevates the construct and covers the exposed areas with a TPF flap and a skin graft. Another difference is that Nagata harvests ipsilateral rib cartilage rather than contralateral, as Brent and Tanzer described.
The procedures' specifics are determined based on the Nagata microtia classification system. However, the main differences are in the initial incisions' placement for flap creation. For example, the anterior incision for a small concha-type is placed just posterior to the small indentation, representing the concha (the hallmark of this type). In contrast, this landmark does not exist in lobule-type and must be estimated. From that point forward, the steps for both types are the same. Concha-type ears have a more developed conchal bowl, so the anterior incision is carried through the helix and antihelix to the posterior concha. No cartilaginous structures are present in the anotia type, so the incision is created as an ovoid outline of the future auricular site. Ultra-delicate split-thickness skin and TPF flaps are raised to accommodate the constructed graft.
Further modifications of Nagata's 2-step technique were described by Kurabayashi et al. The technique entails a TPF pocket method, touted to be less invasive and more effective at creating a superior temporoauricular sulcus. Creating a small pocket through a TPF incision avoids complications linked with an extended incision and TPF flap elevation. A stronger temporoauricular sulcus is also simultaneously established. This approach involves inserting costal cartilage blocks into the pocket to reinforce the new pinna, thus offering support to counteract the expected projection reduction caused by postoperative skin shrinkage. Additionally, this technique safeguards the TPF for potential future reconstruction in case of trauma.[20]
Fisher further modified Nagata's technique to create a one-step procedure, eliminating the need for a follow-up surgery while upholding aesthetic excellence. Unlike multistage methods, Fisher carves and affixes the projection block during the initial procedure, thus avoiding the need for subsequent elevation surgery. This step involves extensive skin undermining to accommodate the larger graft. The carved cartilage is then anchored to the mastoid periosteum and covered by skin flaps, using suction to drape and contour the tissue.[21]
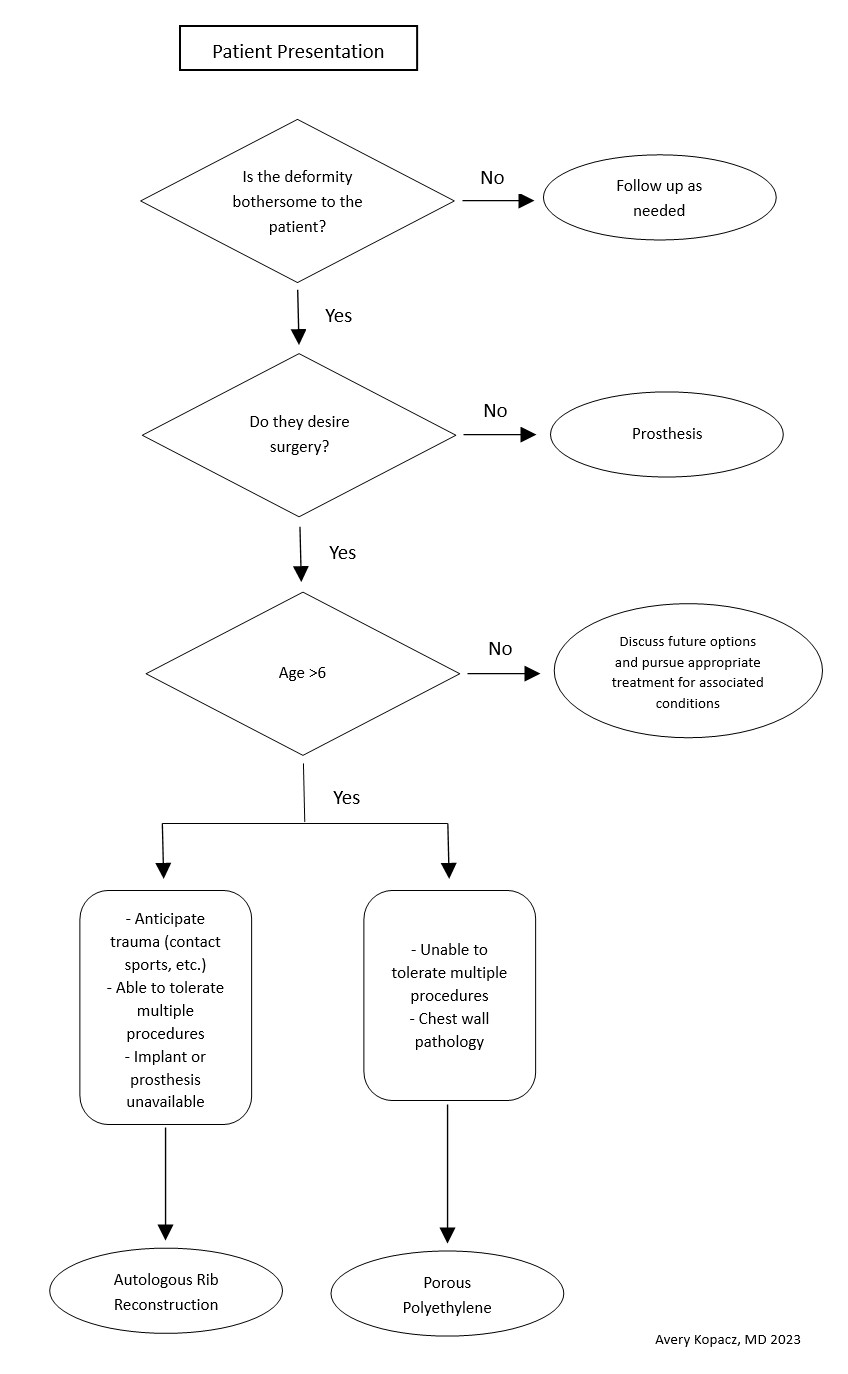
When considering surgical repair, caution is warranted if the patient has had surgery or trauma to the area. Healthy circulation helps avoid reconstruction failure. The surgeons should not feel pressured to operate until the child has adequate costal cartilage and is motivated to participate in postoperative care. Patients with collagen or vascular diseases and limited tolerance for long surgeries might be better suited for prosthetics than autologous reconstruction. Tailored care and shared decision-making are crucial (see Image. Microtia Decision-Making Flowsheet).
Consideration for rib grafting should account for tissue availability and psychological implications in children with ear malformations integrating into society. Brent recommends waiting until age 6 for adequate rib growth, while Nagata prefers waiting until age 10 with a chest circumference exceeding 60 cm at the xiphoid level. These recommendations do not apply to porous polyethylene implants. Generally, ACC surgery is postponed until the child can participate in medical decision-making and follow postoperative instructions.
For patients with congenital aural atresia, the timing of atresiaplasty depends on the reconstruction type. With autologous ribs, reconstruction must be performed 3 to 6 months before atresiaplasty for optimal tissue quality. The auricle can be manipulated subsequently to align with the new bony auditory canal if necessary. Porous polyethylene implants require atresiaplasty before reconstruction to avoid implant exposure from lack of skin coverage. Performing the atresiaplasty beforehand also helps guide the new auricle's placement.
Differential Diagnosis
Microtia is associated with congenital defects. Syndromes such as Goldenhar, Treacher-Collins, and Melnick-Fraser should be considered. Common defects such as prominauris, cryptotia, cup or lop ear deformity, Stahl's ear, and lobule deformities may be mistaken for microtia. However, the neonatal auricular cartilage remains flexible due to circulating maternal estrogens. Thus, these conditions can be treated effectively within 3 weeks of birth using an external ear molding system. Aural atresia is associated with grade 3 and 4 microtia and should be evaluated.
Prognosis
Ear microtia's prognosis depends on the treatment timing and approach and patient response. A bone-anchored hearing aid can be reliably applied to overcome conductive hearing loss. Some microtic auricles may be concealed with long hair. Similarly, applying a prosthesis creates an ear that does not draw social scrutiny and can support glasses.
Chunxiao et al reported that patients were most satisfied with the helix's appearance and least satisfied with the tragus' form after ACC.[22] The comparatively high ACC complication rates make preoperative counseling important in managing expectations and improving patient satisfaction. Nevertheless, most patients are ultimately satisfied with their reconstructed auricles, particularly as many consider the construct to be a part of them, unlike how they typically perceive auricular prostheses.[23]
Complications
ACC reconstruction-specific complications include pneumothorax from costal cartilage harvesting, cartilage infection (often with Pseudomonas aeruginosa), cartilage framework extrusion, framework size changes, lobule necrosis, and construct displacement.[24] Construct extrusion typically happens over the superior helical rim and is treated with TPF or occipitoparietal fascia flap coverage. This complication frequently requires revision because the superior helix tends to have the most tenuous blood supply on the reconstructed auricle.
In contrast, HDPE implantation procedures may produce compression ischemia with loss of skin and TPF flap. While generally durable in the long term, porous polyethylene is more vulnerable to extrusion or infection after minor trauma than autologous cartilage constructs.
In both reconstruction techniques, the usual postsurgical complications can occur, including pain, bleeding, swelling, infection, scarring, damage to surrounding structures, and secondary surgery. Facial nerve injury can also arise from a lack of a predictable anatomical course in a maldeveloped ear, especially when aural atresia repair is performed. Both costal cartilage constructs and porous polyethylene implants can migrate after placement, usually anteroinferiorly, although determining the correct location for initial placement may also be challenging. Many microtia cases are accompanied by hemifacial microsomia, which makes using the normal side of the face as a template less helpful. Most patients also develop a low hairline on the microtic side. Consequently, hair-bearing skin segments arise on top of the reconstructed ear, which may later require laser removal.
As for prosthetic ears, these devices typically have a finite lifespan and may need replacement over time due to wear and tear, skin tone or texture changes, or damage from external factors. Replacement can be costly if the prostheses are damaged or misplaced.
Deterrence and Patient Education
Primary microtia preventive measures are actions taken to prevent the occurrence of microtia in the first place. These measures may include receiving good prenatal care to identify and address potential risk factors during pregnancy, avoiding exposure to known teratogens, and genetic counseling for families with a history of microtia.
Meanwhile, secondary prevention involves efforts to detect and address microtia early in its development or prevent its progression or complications. Preventive measures may include prenatal microtia detection by ultrasound, prompt diagnosis and intervention after birth, and appropriate management to minimize the condition's impact on the individual's health and well-being.
Patients with microtia and their families must be educated regarding the treatment options available. Long-term follow-up with various specialists can help minimize the condition's physical and psychosocial tolls on the patient. Discussion regarding the timing of interventions is crucial. Surgical intervention for comorbid conditions, such as aural atresia, must be considered.
Pearls and Other Issues
Ear microtia is an inborn condition characterized by underdevelopment or absence of the external ear. Microtia may be associated with congenital syndromes or occur in isolation. Patients may have conductive hearing loss and be at risk for psychosocial difficulties due to the condition's cosmetic effects.
Prompt evaluation and interprofessional care are crucial for optimal outcomes. Comprehensive hearing evaluations should be conducted to assess for associated hearing loss, and appropriate interventions should be provided as needed.
Treatment plans should be tailored to each patient's specific needs and circumstances, considering factors such as age, microtia severity, associated anomalies, and patient preferences. Surgical auricular reconstruction or prosthetic ear attachment can significantly improve appearance and function.
Microtia reconstruction is one of the most complex procedures in plastic surgery. An experienced surgery team may avoid ACC reconstruction's potential complications. Understanding the timing of the surgeries and collaboration between plastic and otologic surgeons is essential to produce consistently excellent results. Many of the principles discussed here also apply to auricular reconstruction for ear defects from other etiologies, particularly oncologic resection and avulsive trauma.
Patients with microtia may benefit from psychosocial support and counseling to address any emotional or social challenges associated with the condition. Regular long-term follow-up is essential to monitor outcomes, address complications, and provide ongoing support throughout the patient's life.
Enhancing Healthcare Team Outcomes
In the interprofessional care approach for patients with microtia, collaboration among various specialists is pivotal to ensure comprehensive evaluation, treatment, and support tailored to the individual's needs. Through this collaborative effort, patients receive comprehensive care, enhancing their overall quality of life and optimizing outcomes.
Otologists specialize in diagnosing and managing ear-related conditions, including hearing loss associated with microtia. Plastic surgeons perform surgical reconstruction to improve ear appearance and function. Audiologists assess hearing function, provide rehabilitative services, and monitor auditory progress. Speech pathologists evaluate and treat speech and language disorders associated with microtia and hearing loss. Anaplastologists fabricate custom prostheses, collaborating closely with patients and the surgical team to create lifelike alternatives when surgical reconstruction is not suitable or preferred.
The primary care provider or pediatrician provides comprehensive medical care, overseeing health and development and coordinating referrals to specialists for continuity of care. Patients may have associated conductive hearing loss in the microtic ear, and speech delays are more likely to occur in children with hearing loss. Thus, primary care providers should aggressively treat infections of the contralateral normal ear that can produce these complications. Nurses provide hands-on clinical care, administer medications, monitor patients' vital signs, coordinate care, and educate patients and their families regarding this condition, treatment options, and postoperative care.