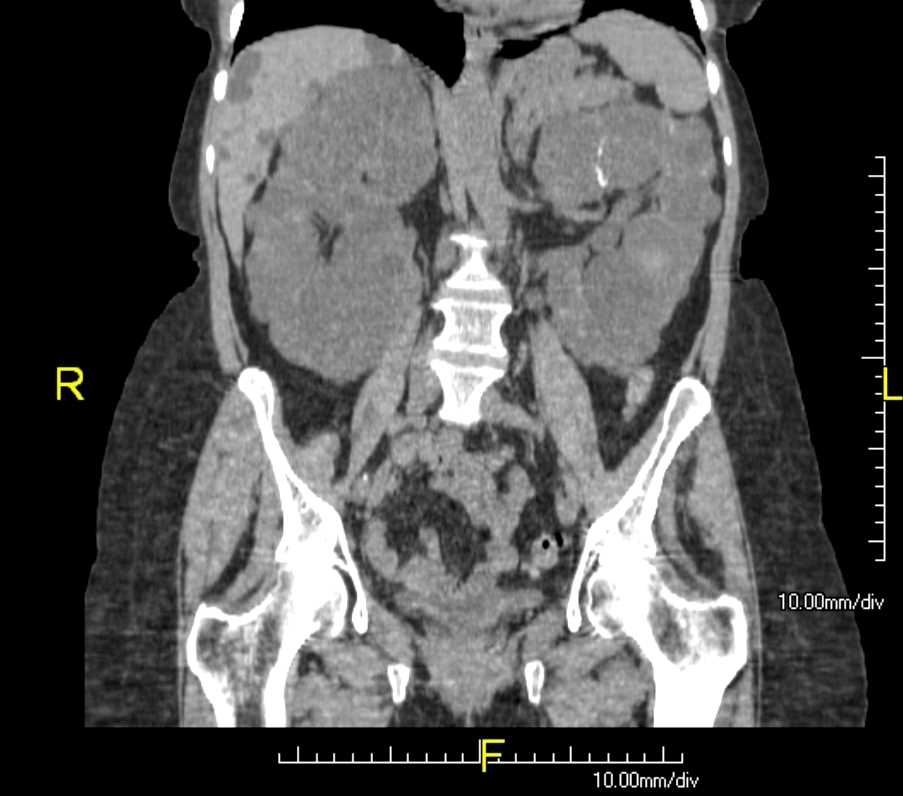
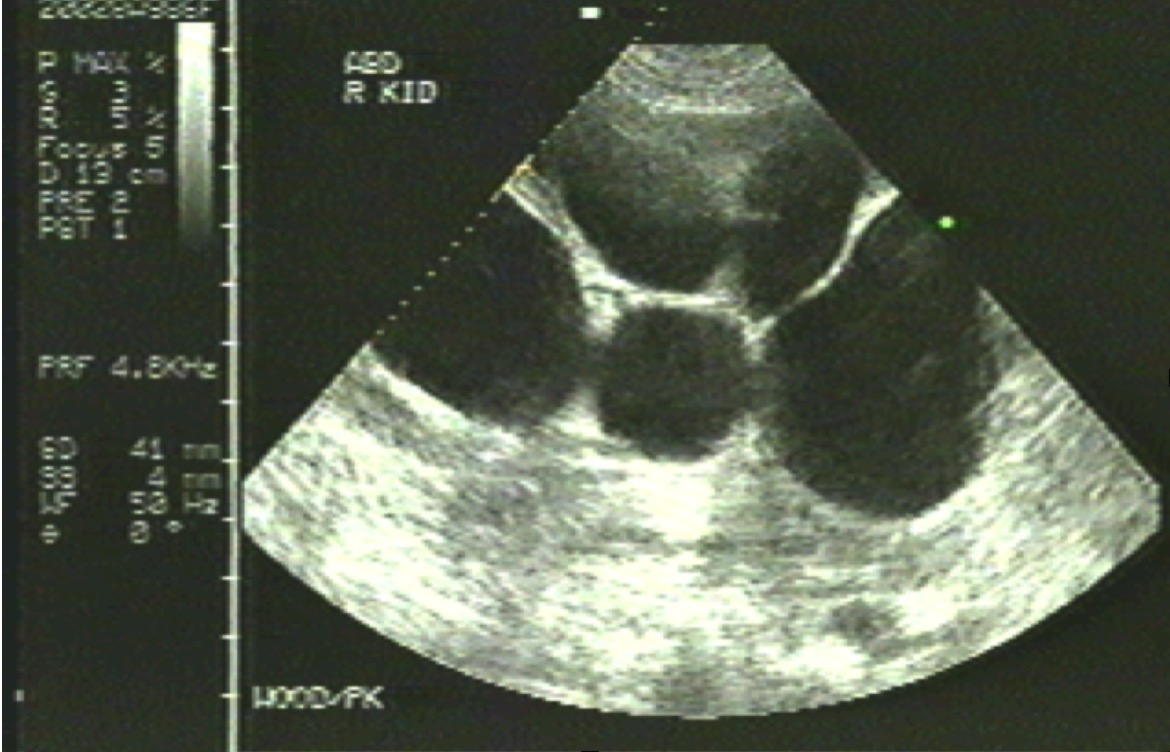
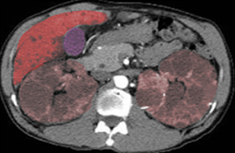
[1]
Subramanian S, Leslie SW, Ahmad T. Autosomal Recessive Polycystic Kidney Disease. StatPearls. 2024 Jan:():
[PubMed PMID: 30725822]
[2]
Porath B, Gainullin VG, Cornec-Le Gall E, Dillinger EK, Heyer CM, Hopp K, Edwards ME, Madsen CD, Mauritz SR, Banks CJ, Baheti S, Reddy B, Herrero JI, Bañales JM, Hogan MC, Tasic V, Watnick TJ, Chapman AB, Vigneau C, Lavainne F, Audrézet MP, Ferec C, Le Meur Y, Torres VE, Genkyst Study Group, HALT Progression of Polycystic Kidney Disease Group, Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease, Harris PC. Mutations in GANAB, Encoding the Glucosidase IIα Subunit, Cause Autosomal-Dominant Polycystic Kidney and Liver Disease. American journal of human genetics. 2016 Jun 2:98(6):1193-1207. doi: 10.1016/j.ajhg.2016.05.004. Epub
[PubMed PMID: 27259053]
[3]
Steele C, You Z, Gitomer BY, Brosnahan GM, Abebe KZ, Braun WE, Chapman AB, Harris PC, Perrone RD, Steinman TI, Torres VE, Yu ASL, Chonchol M, Nowak KL. PKD1 Compared With PK D2 Genotype and Cardiac Hospitalizations in the Halt Progression of Polycystic Kidney Disease Studies. Kidney international reports. 2022 Jan:7(1):117-120. doi: 10.1016/j.ekir.2021.09.013. Epub 2021 Oct 7
[PubMed PMID: 35005320]
[4]
Shaw C, Simms RJ, Pitcher D, Sandford R. Epidemiology of patients in England and Wales with autosomal dominant polycystic kidney disease and end-stage renal failure. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2014 Oct:29(10):1910-8. doi: 10.1093/ndt/gfu087. Epub 2014 Apr 15
[PubMed PMID: 24737444]
[5]
Budhram B, Akbari A, Brown P, Biyani M, Knoll G, Zimmerman D, Edwards C, McCormick B, Bugeja A, Sood MM. End-Stage Kidney Disease in Patients With Autosomal Dominant Polycystic Kidney Disease: A 12-Year Study Based on the Canadian Organ Replacement Registry. Canadian journal of kidney health and disease. 2018:5():2054358118778568. doi: 10.1177/2054358118778568. Epub 2018 Jun 11
[PubMed PMID: 29977583]
[6]
Spithoven EM, Kramer A, Meijer E, Orskov B, Wanner C, Caskey F, Collart F, Finne P, Fogarty DG, Groothoff JW, Hoitsma A, Nogier MB, Postorino M, Ravani P, Zurriaga O, Jager KJ, Gansevoort RT, ERA-EDTA Registry, EuroCYST Consortium, WGIKD, EuroCYST Consortium, WGIKD. Analysis of data from the ERA-EDTA Registry indicates that conventional treatments for chronic kidney disease do not reduce the need for renal replacement therapy in autosomal dominant polycystic kidney disease. Kidney international. 2014 Dec:86(6):1244-52. doi: 10.1038/ki.2014.120. Epub 2014 May 14
[PubMed PMID: 24827775]
[7]
Abbott KC, Agodoa LY. Polycystic kidney disease at end-stage renal disease in the United States: patient characteristics and survival. Clinical nephrology. 2002 Mar:57(3):208-14
[PubMed PMID: 11924752]
[8]
Harris PC, Torres VE. Polycystic kidney disease. Annual review of medicine. 2009:60():321-37. doi: 10.1146/annurev.med.60.101707.125712. Epub
[PubMed PMID: 18947299]
[9]
Liebau MC, Mekahli D, Perrone R, Soyfer B, Fedeles S. Polycystic Kidney Disease Drug Development: A Conference Report. Kidney medicine. 2023 Mar:5(3):100596. doi: 10.1016/j.xkme.2022.100596. Epub 2022 Dec 27
[PubMed PMID: 36698747]
[10]
Bergmann C, Guay-Woodford LM, Harris PC, Horie S, Peters DJM, Torres VE. Polycystic kidney disease. Nature reviews. Disease primers. 2018 Dec 6:4(1):50. doi: 10.1038/s41572-018-0047-y. Epub 2018 Dec 6
[PubMed PMID: 30523303]
[11]
Gallagher AR, Germino GG, Somlo S. Molecular advances in autosomal dominant polycystic kidney disease. Advances in chronic kidney disease. 2010 Mar:17(2):118-30. doi: 10.1053/j.ackd.2010.01.002. Epub
[PubMed PMID: 20219615]
Level 3 (low-level) evidence
[12]
Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000 Dec 21-28:408(6815):990-4
[PubMed PMID: 11140688]
Level 2 (mid-level) evidence
[14]
Olsan EE, Mukherjee S, Wulkersdorfer B, Shillingford JM, Giovannone AJ, Todorov G, Song X, Pei Y, Weimbs T. Signal transducer and activator of transcription-6 (STAT6) inhibition suppresses renal cyst growth in polycystic kidney disease. Proceedings of the National Academy of Sciences of the United States of America. 2011 Nov 1:108(44):18067-72. doi: 10.1073/pnas.1111966108. Epub 2011 Oct 24
[PubMed PMID: 22025716]
[15]
Grantham JJ, Ye M, Davidow C, Holub B, Sharma M. Evidence for a potent lipid secretagogue in the cyst fluids of patients with autosomal dominant polycystic kidney disease. Journal of the American Society of Nephrology : JASN. 1995 Oct:6(4):1242-9
[PubMed PMID: 8589292]
[16]
Davidow CJ, Maser RL, Rome LA, Calvet JP, Grantham JJ. The cystic fibrosis transmembrane conductance regulator mediates transepithelial fluid secretion by human autosomal dominant polycystic kidney disease epithelium in vitro. Kidney international. 1996 Jul:50(1):208-18
[PubMed PMID: 8807590]
[17]
Hanaoka K, Devuyst O, Schwiebert EM, Wilson PD, Guggino WB. A role for CFTR in human autosomal dominant polycystic kidney disease. The American journal of physiology. 1996 Jan:270(1 Pt 1):C389-99
[PubMed PMID: 8772467]
[18]
Grantham JJ, Ye M, Gattone VH 2nd, Sullivan LP. In vitro fluid secretion by epithelium from polycystic kidneys. The Journal of clinical investigation. 1995 Jan:95(1):195-202
[PubMed PMID: 7814614]
[19]
Grantham JJ. Lillian Jean Kaplan International Prize for advancement in the understanding of polycystic kidney disease. Understanding polycystic kidney disease: a systems biology approach. Kidney international. 2003 Oct:64(4):1157-62
[PubMed PMID: 12969132]
Level 3 (low-level) evidence
[20]
Sagar PS, Rangan GK. Cardiovascular Manifestations and Management in ADPKD. Kidney international reports. 2023 Oct:8(10):1924-1940. doi: 10.1016/j.ekir.2023.07.017. Epub 2023 Aug 4
[PubMed PMID: 37850017]
[21]
Bell PE, Hossack KF, Gabow PA, Durr JA, Johnson AM, Schrier RW. Hypertension in autosomal dominant polycystic kidney disease. Kidney international. 1988 Nov:34(5):683-90
[PubMed PMID: 2974094]
[22]
Chapman AB, Johnson A, Gabow PA, Schrier RW. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. The New England journal of medicine. 1990 Oct 18:323(16):1091-6
[PubMed PMID: 2215576]
[23]
Torres VE, Donovan KA, Scicli G, Holley KE, Thibodeau SN, Carretero OA, Inagami T, McAteer JA, Johnson CM. Synthesis of renin by tubulocystic epithelium in autosomal-dominant polycystic kidney disease. Kidney international. 1992 Aug:42(2):364-73
[PubMed PMID: 1405319]
[24]
Onori P, Franchitto A, Mancinelli R, Carpino G, Alvaro D, Francis H, Alpini G, Gaudio E. Polycystic liver diseases. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2010 Apr:42(4):261-71. doi: 10.1016/j.dld.2010.01.006. Epub 2010 Feb 6
[PubMed PMID: 20138815]
[25]
Gabow PA, Chapman AB, Johnson AM, Tangel DJ, Duley IT, Kaehny WD, Manco-Johnson M, Schrier RW. Renal structure and hypertension in autosomal dominant polycystic kidney disease. Kidney international. 1990 Dec:38(6):1177-80
[PubMed PMID: 2074659]
[26]
Perrone RD, Oberdhan D, Ouyang J, Bichet DG, Budde K, Chapman AB, Gitomer BY, Horie S, Ong ACM, Torres VE, Turner AN, Krasa H. OVERTURE: A Worldwide, Prospective, Observational Study of Disease Characteristics in Patients With ADPKD. Kidney international reports. 2023 May:8(5):989-1001. doi: 10.1016/j.ekir.2023.02.1073. Epub 2023 Feb 13
[PubMed PMID: 37180499]
Level 2 (mid-level) evidence
[27]
Caroli A, Antiga L, Conti S, Sonzogni A, Fasolini G, Ondei P, Perico N, Remuzzi G, Remuzzi A. Intermediate volume on computed tomography imaging defines a fibrotic compartment that predicts glomerular filtration rate decline in autosomal dominant polycystic kidney disease patients. The American journal of pathology. 2011 Aug:179(2):619-27. doi: 10.1016/j.ajpath.2011.04.036. Epub 2011 Jun 17
[PubMed PMID: 21683674]
[28]
Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet (London, England). 2007 Apr 14:369(9569):1287-1301. doi: 10.1016/S0140-6736(07)60601-1. Epub
[PubMed PMID: 17434405]
[29]
Galliani M, Chicca S, Vitaliano E, Di Lullo L, Giannakakis K, Paone A. [Renal manifestation of Autosomal Dominant Polycystic Kidney Disease]. Giornale italiano di nefrologia : organo ufficiale della Societa italiana di nefrologia. 2018 Mar:35(2):. pii: 2018-vol2. Epub
[PubMed PMID: 29582957]
[30]
Kalatharan V, Grewal G, Nash DM, Welk B, Sarma S, Pei Y, Garg AX. Stone Prevalence in Autosomal Dominant Polycystic Kidney Disease: A Systematic Review and Meta-Analysis. Canadian journal of kidney health and disease. 2020:7():2054358120934628. doi: 10.1177/2054358120934628. Epub 2020 Jul 4
[PubMed PMID: 35186303]
Level 1 (high-level) evidence
[31]
Mallett A, Patel M, Tunnicliffe DJ, Rangan GK. KHA-CARI Autosomal Dominant Polycystic Kidney Disease Guideline: Management of Renal Stone Disease. Seminars in nephrology. 2015 Nov:35(6):603-606.e3. doi: 10.1016/j.semnephrol.2015.10.012. Epub
[PubMed PMID: 26718165]
[32]
Gabow PA. Autosomal dominant polycystic kidney disease--more than a renal disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1990 Nov:16(5):403-13
[PubMed PMID: 2239929]
[33]
Torres VE, Wilson DM, Hattery RR, Segura JW. Renal stone disease in autosomal dominant polycystic kidney disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1993 Oct:22(4):513-9
[PubMed PMID: 8213789]
[34]
Grampsas SA, Chandhoke PS, Fan J, Glass MA, Townsend R, Johnson AM, Gabow P. Anatomic and metabolic risk factors for nephrolithiasis in patients with autosomal dominant polycystic kidney disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2000 Jul:36(1):53-7
[PubMed PMID: 10873872]
[35]
Chasan O, Mirioglu S, Artan AS, Gursu M, Kazancioglu R, Elcioglu OC. Assessment of metabolic risk factors for nephrolithiasis in patients with autosomal dominant polycystic kidney disease: a cross-sectional study. Clinical and experimental nephrology. 2023 Nov:27(11):912-918. doi: 10.1007/s10157-023-02378-2. Epub 2023 Jul 26
[PubMed PMID: 37493903]
Level 2 (mid-level) evidence
[36]
Nishiura JL, Neves RF, Eloi SR, Cintra SM, Ajzen SA, Heilberg IP. Evaluation of nephrolithiasis in autosomal dominant polycystic kidney disease patients. Clinical journal of the American Society of Nephrology : CJASN. 2009 Apr:4(4):838-44. doi: 10.2215/CJN.03100608. Epub 2009 Apr 1
[PubMed PMID: 19339428]
[37]
Levine E, Grantham JJ. Calcified renal stones and cyst calcifications in autosomal dominant polycystic kidney disease: clinical and CT study in 84 patients. AJR. American journal of roentgenology. 1992 Jul:159(1):77-81
[PubMed PMID: 1609726]
[38]
Gabow PA, Johnson AM, Kaehny WD, Manco-Johnson ML, Duley IT, Everson GT. Risk factors for the development of hepatic cysts in autosomal dominant polycystic kidney disease. Hepatology (Baltimore, Md.). 1990 Jun:11(6):1033-7
[PubMed PMID: 2365280]
[39]
Everson GT. Hepatic cysts in autosomal dominant polycystic kidney disease. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1993 Oct:22(4):520-5
[PubMed PMID: 8213790]
[40]
Kim JA, Blumenfeld JD, Chhabra S, Dutruel SP, Thimmappa ND, Bobb WO, Donahue S, Rennert HE, Tan AY, Giambrone AE, Prince MR. Pancreatic Cysts in Autosomal Dominant Polycystic Kidney Disease: Prevalence and Association with PKD2 Gene Mutations. Radiology. 2016 Sep:280(3):762-70. doi: 10.1148/radiol.2016151650. Epub 2016 Apr 5
[PubMed PMID: 27046073]
[41]
Fick GM, Gabow PA. Hereditary and acquired cystic disease of the kidney. Kidney international. 1994 Oct:46(4):951-64
[PubMed PMID: 7861721]
[42]
Dudley J, Winyard P, Marlais M, Cuthell O, Harris T, Chong J, Sayer J, Gale DP, Moore L, Turner K, Burrows S, Sandford R. Clinical practice guideline monitoring children and young people with, or at risk of developing autosomal dominant polycystic kidney disease (ADPKD). BMC nephrology. 2019 Apr 30:20(1):148. doi: 10.1186/s12882-019-1285-2. Epub 2019 Apr 30
[PubMed PMID: 31039757]
Level 1 (high-level) evidence
[43]
Al Sayyab M, Chapman A. Pregnancy in Autosomal Dominant Polycystic Kidney Disease. Advances in kidney disease and health. 2023 Sep:30(5):454-460. doi: 10.1053/j.akdh.2023.10.006. Epub
[PubMed PMID: 38032583]
Level 3 (low-level) evidence
[44]
Noce EM. Considerations for genetic testing in individuals with autosomal dominant polycystic kidney disease. Journal of the American Association of Nurse Practitioners. 2022 Dec 1:34(12):1249-1251. doi: 10.1097/JXX.0000000000000787. Epub 2022 Dec 1
[PubMed PMID: 36469907]
[45]
Ars E, Bernis C, Fraga G, Furlano M, Martínez V, Martins J, Ortiz A, Pérez-Gómez MV, Rodríguez-Pérez JC, Sans L, Torra R, en nombre del grupo de trabajo de Enfermedades Renales Hereditarias de la Sociedad Española de Nefrología. Consensus document on autosomal dominant polycystic kindey disease from the Spanish Working Group on Inherited Kindey Diseases. Review 2020. Nefrologia. 2022 Jul-Aug:42(4):367-389. doi: 10.1016/j.nefroe.2022.11.011. Epub 2022 Nov 17
[PubMed PMID: 36404270]
Level 3 (low-level) evidence
[46]
Petrucci I, Clementi A, Sessa C, Torrisi I, Meola M. Ultrasound and color Doppler applications in chronic kidney disease. Journal of nephrology. 2018 Dec:31(6):863-879. doi: 10.1007/s40620-018-0531-1. Epub 2018 Sep 6
[PubMed PMID: 30191413]
[47]
Pei Y, Obaji J, Dupuis A, Paterson AD, Magistroni R, Dicks E, Parfrey P, Cramer B, Coto E, Torra R, San Millan JL, Gibson R, Breuning M, Peters D, Ravine D. Unified criteria for ultrasonographic diagnosis of ADPKD. Journal of the American Society of Nephrology : JASN. 2009 Jan:20(1):205-12. doi: 10.1681/ASN.2008050507. Epub 2008 Oct 22
[PubMed PMID: 18945943]
[48]
Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, Bae KT, Chapman AB, Grantham JJ, Mrug M, Hogan MC, El-Zoghby ZM, Harris PC, Erickson BJ, King BF, Torres VE, CRISP Investigators. Imaging classification of autosomal dominant polycystic kidney disease: a simple model for selecting patients for clinical trials. Journal of the American Society of Nephrology : JASN. 2015 Jan:26(1):160-72. doi: 10.1681/ASN.2013101138. Epub 2014 Jun 5
[PubMed PMID: 24904092]
[49]
Bae KT, Sun H, Lee JG, Bae K, Wang J, Tao C, Chapman AB, Torres VE, Grantham JJ, Mrug M, Bennett WM, Flessner MF, Landsittel DP, Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease. Novel methodology to evaluate renal cysts in polycystic kidney disease. American journal of nephrology. 2014:39(3):210-7. doi: 10.1159/000358604. Epub 2014 Feb 22
[PubMed PMID: 24576800]
[50]
Torres VE, Bankir L, Grantham JJ. A case for water in the treatment of polycystic kidney disease. Clinical journal of the American Society of Nephrology : CJASN. 2009 Jun:4(6):1140-50. doi: 10.2215/CJN.00790209. Epub 2009 May 14
[PubMed PMID: 19443627]
Level 3 (low-level) evidence
[51]
Barash I, Ponda MP, Goldfarb DS, Skolnik EY. A pilot clinical study to evaluate changes in urine osmolality and urine cAMP in response to acute and chronic water loading in autosomal dominant polycystic kidney disease. Clinical journal of the American Society of Nephrology : CJASN. 2010 Apr:5(4):693-7. doi: 10.2215/CJN.04180609. Epub 2010 Feb 18
[PubMed PMID: 20167686]
Level 3 (low-level) evidence
[52]
Chapman AB, Guay-Woodford LM, Grantham JJ, Torres VE, Bae KT, Baumgarten DA, Kenney PJ, King BF Jr, Glockner JF, Wetzel LH, Brummer ME, O'Neill WC, Robbin ML, Bennett WM, Klahr S, Hirschman GH, Kimmel PL, Thompson PA, Miller JP, Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease cohort. Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney international. 2003 Sep:64(3):1035-45
[PubMed PMID: 12911554]
[53]
Vikrant S, Parashar A. Autosomal dominant polycystic kidney disease: Study of clinical characteristics in an Indian population. Saudi journal of kidney diseases and transplantation : an official publication of the Saudi Center for Organ Transplantation, Saudi Arabia. 2017 Jan-Feb:28(1):115-124. doi: 10.4103/1319-2442.198163. Epub
[PubMed PMID: 28098112]
[55]
Schrier RW, Abebe KZ, Perrone RD, Torres VE, Braun WE, Steinman TI, Winklhofer FT, Brosnahan G, Czarnecki PG, Hogan MC, Miskulin DC, Rahbari-Oskoui FF, Grantham JJ, Harris PC, Flessner MF, Bae KT, Moore CG, Chapman AB, HALT-PKD Trial Investigators. Blood pressure in early autosomal dominant polycystic kidney disease. The New England journal of medicine. 2014 Dec 11:371(24):2255-66. doi: 10.1056/NEJMoa1402685. Epub 2014 Nov 15
[PubMed PMID: 25399733]
[56]
Arogundade FA, Akinbodewa AA, Sanusi AA, Okunola O, Hassan MO, Akinsola A. Clinical presentation and outcome of autosomal dominant polycystic kidney disease in Nigeria. African health sciences. 2018 Sep:18(3):671-680. doi: 10.4314/ahs.v18i3.25. Epub
[PubMed PMID: 30603000]
[57]
Müller RU, Benzing T. Management of autosomal-dominant polycystic kidney disease-state-of-the-art. Clinical kidney journal. 2018 Dec:11(Suppl 1):i2-i13. doi: 10.1093/ckj/sfy103. Epub 2018 Dec 17
[PubMed PMID: 30581561]
[58]
Müller RU, Messchendorp AL, Birn H, Capasso G, Cornec-Le Gall E, Devuyst O, van Eerde A, Guirchoun P, Harris T, Hoorn EJ, Knoers NVAM, Korst U, Mekahli D, Le Meur Y, Nijenhuis T, Ong ACM, Sayer JA, Schaefer F, Servais A, Tesar V, Torra R, Walsh SB, Gansevoort RT. An update on the use of tolvaptan for autosomal dominant polycystic kidney disease: consensus statement on behalf of the ERA Working Group on Inherited Kidney Disorders, the European Rare Kidney Disease Reference Network and Polycystic Kidney Disease International. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2022 Apr 25:37(5):825-839. doi: 10.1093/ndt/gfab312. Epub
[PubMed PMID: 35134221]
Level 3 (low-level) evidence
[59]
Patel SJ, Sadowski CK. An update on treatments for autosomal dominant polycystic kidney disease. JAAPA : official journal of the American Academy of Physician Assistants. 2023 Jun 1:36(6):11-16. doi: 10.1097/01.JAA.0000931420.46207.82. Epub
[PubMed PMID: 37163712]
[60]
Kim Y, Han S. Recent updates in therapeutic approach using tolvaptan for autosomal dominant polycystic kidney disease. The Korean journal of internal medicine. 2023 May:38(3):322-331. doi: 10.3904/kjim.2022.376. Epub 2023 Apr 25
[PubMed PMID: 37089056]
[61]
Lacquaniti A. [Tolvaptan and autosomal dominant polycystic kidney disease in the adult: let's give time to the "TEMPO" trial (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and Its Outcomes)]. Giornale italiano di nefrologia : organo ufficiale della Societa italiana di nefrologia. 2013 Jan-Feb:30(1):. pii: gin/30.1.10. Epub
[PubMed PMID: 25083527]
[62]
Torres VE, Higashihara E, Devuyst O, Chapman AB, Gansevoort RT, Grantham JJ, Perrone RD, Ouyang J, Blais JD, Czerwiec FS, TEMPO 3:4 Trial Investigators. Effect of Tolvaptan in Autosomal Dominant Polycystic Kidney Disease by CKD Stage: Results from the TEMPO 3:4 Trial. Clinical journal of the American Society of Nephrology : CJASN. 2016 May 6:11(5):803-811. doi: 10.2215/CJN.06300615. Epub 2016 Feb 23
[PubMed PMID: 26912543]
[63]
Chebib FT, Zhou X, Garbinsky D, Davenport E, Nunna S, Oberdhan D, Fernandes A. Tolvaptan and Kidney Function Decline in Older Individuals With Autosomal Dominant Polycystic Kidney Disease: A Pooled Analysis of Randomized Clinical Trials and Observational Studies. Kidney medicine. 2023 Jun:5(6):100639. doi: 10.1016/j.xkme.2023.100639. Epub 2023 Apr 14
[PubMed PMID: 37250503]
Level 1 (high-level) evidence
[64]
Bais T, Gansevoort RT, Meijer E. Drugs in Clinical Development to Treat Autosomal Dominant Polycystic Kidney Disease. Drugs. 2022 Jul:82(10):1095-1115. doi: 10.1007/s40265-022-01745-9. Epub 2022 Jul 19
[PubMed PMID: 35852784]
[65]
Shoaf SE, Ouyang J, Sergeyeva O, Estilo A, Li H, Leung D. A Post Hoc Analysis of Statin Use in Tolvaptan Autosomal Dominant Polycystic Kidney Disease Pivotal Trials. Clinical journal of the American Society of Nephrology : CJASN. 2020 May 7:15(5):643-650. doi: 10.2215/CJN.08170719. Epub 2020 Apr 2
[PubMed PMID: 32241780]
[66]
Lin CH, Chao CT, Wu MY, Lo WC, Lin TC, Wu MS. Use of mammalian target of rapamycin inhibitors in patient with autosomal dominant polycystic kidney disease: an updated meta-analysis. International urology and nephrology. 2019 Nov:51(11):2015-2025. doi: 10.1007/s11255-019-02292-1. Epub 2019 Oct 1
[PubMed PMID: 31578673]
Level 1 (high-level) evidence
[67]
Meijer E, Visser FW, van Aerts RMM, Blijdorp CJ, Casteleijn NF, D'Agnolo HMA, Dekker SEI, Drenth JPH, de Fijter JW, van Gastel MDA, Gevers TJ, Lantinga MA, Losekoot M, Messchendorp AL, Neijenhuis MK, Pena MJ, Peters DJM, Salih M, Soonawala D, Spithoven EM, Wetzels JF, Zietse R, Gansevoort RT, DIPAK-1 Investigators. Effect of Lanreotide on Kidney Function in Patients With Autosomal Dominant Polycystic Kidney Disease: The DIPAK 1 Randomized Clinical Trial. JAMA. 2018 Nov 20:320(19):2010-2019. doi: 10.1001/jama.2018.15870. Epub
[PubMed PMID: 30422235]
Level 1 (high-level) evidence
[68]
Pirson Y, Christophe JL, Goffin E. Outcome of renal replacement therapy in autosomal dominant polycystic kidney disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1996:11 Suppl 6():24-8
[PubMed PMID: 9044324]
[69]
Wang X, Constans MM, Chebib FT, Torres VE, Pellegrini L. Effect of a Vasopressin V2 Receptor Antagonist on Polycystic Kidney Disease Development in a Rat Model. American journal of nephrology. 2019:49(6):487-493. doi: 10.1159/000500667. Epub 2019 May 22
[PubMed PMID: 31117065]
[70]
Natoli TA, Modur V, Ibraghimov-Beskrovnaya O. Glycosphingolipid metabolism and polycystic kidney disease. Cellular signalling. 2020 May:69():109526. doi: 10.1016/j.cellsig.2020.109526. Epub 2020 Jan 10
[PubMed PMID: 31911181]
[71]
Sieben CJ, Harris PC. Experimental Models of Polycystic Kidney Disease: Applications and Therapeutic Testing. Kidney360. 2023 Aug 1:4(8):1155-1173. doi: 10.34067/KID.0000000000000209. Epub 2023 Jul 7
[PubMed PMID: 37418622]
[72]
Nowak KL, Farmer-Bailey H, Wang W, You Z, Steele C, Cadnapaphornchai MA, Klawitter J, Patel N, George D, Jovanovich A, Soranno DE, Gitomer B, Chonchol M. Curcumin Therapy to Treat Vascular Dysfunction in Children and Young Adults with ADPKD: A Randomized Controlled Trial. Clinical journal of the American Society of Nephrology : CJASN. 2022 Feb:17(2):240-250. doi: 10.2215/CJN.08950621. Epub 2021 Dec 14
[PubMed PMID: 34907021]
Level 1 (high-level) evidence
[73]
Ghafouri-Fard S, Shoorei H, Bahroudi Z, Hussen BM, Talebi SF, Taheri M, Ayatollahi SA. Nrf2-Related Therapeutic Effects of Curcumin in Different Disorders. Biomolecules. 2022 Jan 5:12(1):. doi: 10.3390/biom12010082. Epub 2022 Jan 5
[PubMed PMID: 35053230]
[74]
Tomlinson JW. Bardet-Biedl syndrome: A focus on genetics, mechanisms and metabolic dysfunction. Diabetes, obesity & metabolism. 2024 Apr:26 Suppl 2():13-24. doi: 10.1111/dom.15480. Epub 2024 Feb 1
[PubMed PMID: 38302651]
[75]
Melluso A, Secondulfo F, Capolongo G, Capasso G, Zacchia M. Bardet-Biedl Syndrome: Current Perspectives and Clinical Outlook. Therapeutics and clinical risk management. 2023:19():115-132. doi: 10.2147/TCRM.S338653. Epub 2023 Jan 30
[PubMed PMID: 36741589]
Level 3 (low-level) evidence
[76]
Clissold RL, Hamilton AJ, Hattersley AT, Ellard S, Bingham C. HNF1B-associated renal and extra-renal disease-an expanding clinical spectrum. Nature reviews. Nephrology. 2015 Feb:11(2):102-12. doi: 10.1038/nrneph.2014.232. Epub 2014 Dec 23
[PubMed PMID: 25536396]
[78]
Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, Franco B, Bruel AL, Thauvin-Robinet C. Oral-Facial-Digital Syndrome Type I. GeneReviews(®). 1993:():
[PubMed PMID: 20301367]
[79]
Portocarrero LKL, Quental KN, Samorano LP, Oliveira ZNP, Rivitti-Machado MCDM. Tuberous sclerosis complex: review based on new diagnostic criteria. Anais brasileiros de dermatologia. 2018 Jun:93(3):323-331. doi: 10.1590/abd1806-4841.20186972. Epub
[PubMed PMID: 29924239]
[82]
Rozenfeld MN, Ansari SA, Mohan P, Shaibani A, Russell EJ, Hurley MC. Autosomal Dominant Polycystic Kidney Disease and Intracranial Aneurysms: Is There an Increased Risk of Treatment? AJNR. American journal of neuroradiology. 2016 Feb:37(2):290-3. doi: 10.3174/ajnr.A4490. Epub 2015 Sep 3
[PubMed PMID: 26338918]
[83]
Ushio Y, Kataoka H, Akagawa H, Sato M, Manabe S, Kawachi K, Makabe S, Akihisa T, Seki M, Teraoka A, Iwasa N, Yoshida R, Tsuchiya K, Nitta K, Hoshino J, Mochizuki T. Factors associated with early-onset intracranial aneurysms in patients with autosomal dominant polycystic kidney disease. Journal of nephrology. 2024 Feb 5:():. doi: 10.1007/s40620-023-01866-8. Epub 2024 Feb 5
[PubMed PMID: 38315279]
[84]
Bugazia S, Hogan MC. Extrarenal Manifestations: Polycystic Liver Disease and Its Complications. Advances in kidney disease and health. 2023 Sep:30(5):440-453. doi: 10.1053/j.akdh.2023.10.004. Epub
[PubMed PMID: 37943238]
Level 3 (low-level) evidence
[85]
König JC, Titieni A, Konrad M, NEOCYST Consortium. Network for Early Onset Cystic Kidney Diseases-A Comprehensive Multidisciplinary Approach to Hereditary Cystic Kidney Diseases in Childhood. Frontiers in pediatrics. 2018:6():24. doi: 10.3389/fped.2018.00024. Epub 2018 Feb 13
[PubMed PMID: 29497606]
[86]
Chebib FT, Perrone RD, Chapman AB, Dahl NK, Harris PC, Mrug M, Mustafa RA, Rastogi A, Watnick T, Yu ASL, Torres VE. A Practical Guide for Treatment of Rapidly Progressive ADPKD with Tolvaptan. Journal of the American Society of Nephrology : JASN. 2018 Oct:29(10):2458-2470. doi: 10.1681/ASN.2018060590. Epub 2018 Sep 18
[PubMed PMID: 30228150]
[87]
Corradi V, Gastaldon F, Caprara C, Giuliani A, Martino F, Ferrari F, Ronco C. Predictors of rapid disease progression in autosomal dominant polycystic kidney disease. Minerva medica. 2017 Feb:108(1):43-56. doi: 10.23736/S0026-4806.16.04830-8. Epub 2016 Oct 4
[PubMed PMID: 27701376]
[88]
Ryu H, Park HC, Oh YK, Sangadi I, Wong A, Mei C, Ecder T, Wang AY, Kao TW, Huang JW, Rangan GK, Ahn C. RAPID-ADPKD (Retrospective epidemiological study of Asia-Pacific patients with rapId Disease progression of Autosomal Dominant Polycystic Kidney Disease): study protocol for a multinational, retrospective cohort study. BMJ open. 2020 Feb 6:10(2):e034103. doi: 10.1136/bmjopen-2019-034103. Epub 2020 Feb 6
[PubMed PMID: 32034027]
Level 2 (mid-level) evidence
[89]
de Chickera S, Akbari A, Levin A, Tang M, Brown P, Djurdev O, Biyani M, Clark EG, Sood MM. The Risk of Adverse Events in Patients With Polycystic Kidney Disease With Advanced Chronic Kidney Disease. Canadian journal of kidney health and disease. 2018:5():2054358118774537. doi: 10.1177/2054358118774537. Epub 2018 Jun 8
[PubMed PMID: 30186614]
[90]
Wilkinson DA, Heung M, Deol A, Chaudhary N, Gemmete JJ, Thompson BG, Pandey AS. Cerebral Aneurysms in Autosomal Dominant Polycystic Kidney Disease: A Comparison of Management Approaches. Neurosurgery. 2019 Jun 1:84(6):E352-E361. doi: 10.1093/neuros/nyy336. Epub
[PubMed PMID: 30060240]