Introduction
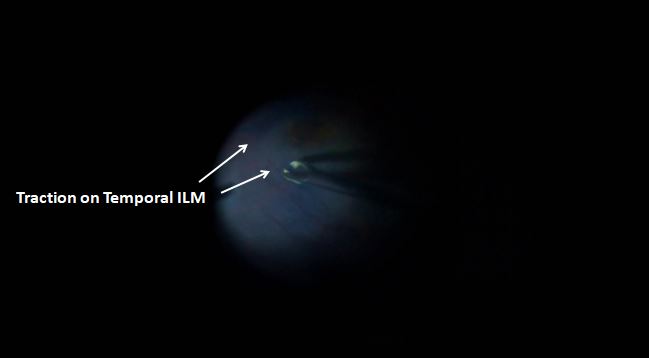
Myopic foveoschisis, also known as myopic traction maculopathy (MTM) or myopic macular schisis, is a relatively rare entity that affects eyes with pathological myopia (-6.00 diopters or more of myopia).[1] These eyes often have a posterior staphyloma, which has been implicated as an etiologic factor in the pathogenesis of MTM. Patients of myopic foveoschisis usually present with a gradual, often progressive, painless diminution of vision in either or both eyes, which may be affected simultaneously or sequentially (see Image. Traction on the Temporal Internal Limiting Membrane [ILM]).[2]
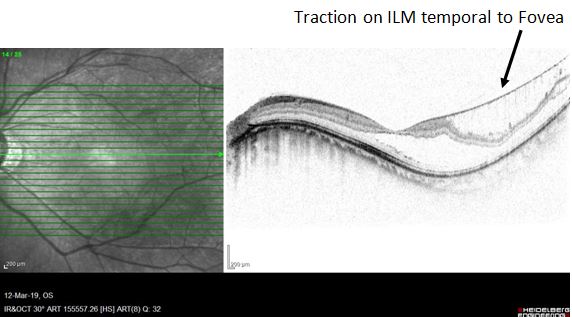
Foveoschisis was first described by Takano and Kishi in 1999 as a split in the layers of the retina at the fovea in eyes with posterior staphyloma.[3] In the past, the diagnosis of MTM was difficult because of high myopic eyes offering a poor contrast when being examined by an ophthalmoscope. With the addition of spectral-domain optical coherence tomography (OCT), the early recognition and prompt treatment of myopic foveoschisis have been made possible (see Image. Traction on the ILM: Temporal to Fovea).[1][4]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Myopic foveoschisis, as the name suggests, is almost always seen in eyes with high myopia and posterior staphyloma.[5] Antonio Scarpa, a skilled anatomist, was the first to describe "posterior staphyloma" as an abnormal outward protrusion of the eyeball owing to changes in the collagen of the scleral wall.[6] However, the ectasia in the choroid and the retina is not similar, and this differential stretching of the retina is primarily responsible for causing myopic traction maculopathy. The ectasia causes tractional forces on the retina in a plane parallel to its surface and also perpendicular to it, which in turn causes the layers of the retina to split up. This tractional force acting along the surface of the retina because of the increase in the size of the eyeball is also responsible for the formation of a macular hole.[7][8]
Apart from posterior staphyloma and elongation of the eyeball, other factors contributing to etiology may be traction exerted by premacular cortical vitreous, stiff retinal vasculature, or internal limiting membrane rigidity. Multiple consequences of high myopia, such as lacquer cracks produced by breaks in the choroid due to ectasia, choroidal atrophy, and choroidal neovascularization, can also coexist in eyes with myopic foveoschisis.[9][10]
Epidemiology
Pathologic myopia is defined as myopia of more than -6 D or if the axial length is more than 26 mm.[11] About 2% of the total world population is known to have pathologic myopia. Of these, 8% to 34% have myopic traction maculopathy. Females are more commonly affected than males (3:1). Myopic eyes are known to increase in size at varied rates for many years. Therefore, myopic foveoschisis may manifest from early adulthood to even later years. There is no known geographical predisposition. The progression of this disease is gradual, and the severity increases with time. However, there may be intermittent phases when the disease may become static.
Pathophysiology
The pathophysiology of myopic traction maculopathy is complex. In the eyes, pathologic myopia and ectasia of the posterior scleral wall lead to posterior staphyloma, also called scleroconus. This ectasia causes a stretch in the posterior scleral wall, choroid, and the retina. Due to different stretch responses of the retina and choroid, differential stretching causes the retina to split up into layers. The elongation of the myopic eye not only occurs along the axial length but also in a plane tangential to the retina at the posterior pole.[12] This anteroposterior stretch causes a perpendicular tractional force on the surface of the retina and leads to layers of the retina splitting up. The tractional force acting along the surface of the retina because of the increase in the size of the eyeball is responsible for the formation of a macular hole.[7][8]
Myopic traction maculopathy is a dynamic disease that gradually progresses over the years. The disease begins with schisis in the inner retinal layers, slowly progressing over the years, leading to schisis in the outer retinal layers. With the disease's progression, an inner or outer lamellar macular hole can develop in many cases.[13] A full-thickness macular hole is also reported in several advanced cases of myopic traction maculopathy.[14] Advanced stages of myopic foveoschisis usually involve macular detachment. In a few cases, there may be a resolution of schisis, but this resolution is usually temporary.
Myopic foveoschisis is believed to be multifactorial in origin, and other causes of anteroposterior traction on the retina include ILM rigidity, vitreomacular traction, and the presence of epiretinal membranes. Based on this etiology, some people believe that a mere peel of ILM in the temporal part of the macula might resolve the foveoschisis. However, reports on such management are quite limited (see Image. Traction on the ILM: Temporal to Fovea).[15]
Myopic foveoschisis is not a single entity but a broad spectrum consisting of many clinical pictures. To date, no single comprehensive classification is available that fits all scenarios. Based on the OCT picture, 4 retinal "types" of MTM are recognized:
- Inner schisis or inner-outer schisis
- Outer schisis
- Schisis detachment
- Detachment
OCT also delineates features of the fovea, and 3 foveal patterns are recognized:
- Intact fovea
- Inner lamellar macular hole
- Full-thickness macular hole
There may be present associated epiretinal anomalies or outer lamellar macular holes.[16]
History and Physical
Patients with myopic traction maculopathy can present from early adulthood to the later years of life. Most commonly, they present in the 5th or 6th decade of their life. But presentation in the 3rd or even 2nd decade of life is not unusual. The chief complaint is usually a unilateral or bilateral diminution of vision in a highly myopic patient. Rarely, the patients may be completely asymptomatic with no visual complaints.
The onset of symptoms is insidious, and the progression is gradual. Both eyes may be affected simultaneously or at a gap of a few months or years. Patients may also have a history of floaters, light flashes, metamorphopsia, or scotoma.
On examination, there may be visible areas of scleral thinning. If the patient has undergone any, the cornea may have tell-tale signs of prior refractive surgery. Myopic eyes usually have a deep anterior chamber. The crystalline lens may have signs of cataractogenesis, especially in older patients. On indirect ophthalmoscopy examination of the fundus with a 20D lens, posterior vitreous detachment may be seen. There may be evidence of lattice degeneration in the retinal periphery, with or without holes.[2]
The disc is generally large with a physiologically large cup. Background tesselations due to chorioretinal degeneration are noted. The macula may not show anything remarkable other than prominent tesselations on examination. Lacquer cracks, Forster-Fuch spots, myopic choroidal neovascular membrane (CNVM), and posterior staphyloma may be noted to be coexistent in these eyes.[17]
The clinician may note diffuse atrophic patches in the retina, which appear whitish-yellow. Peripapillary atrophy may also be noted, which appears as well-defined atrophic areas around the disc or at the posterior pole. Fundus examination with a 90D lens can make the clinician suspicious of myopic foveoschisis. Shallow macular retinal detachments and macular holes may be picked up on slit-lamp biomicroscopy.
Evaluation
A good fundus evaluation with a 20-D and a 90-D lens is indispensable. A detailed ophthalmoscopic examination elicits the various pathological lesions present in a myopic eye. Slit-lamp biomicroscopy with a 90D lens alerts the clinician to the possibility of myopic traction maculopathy and makes them probe thoroughly into the disease.
Spectral-domain OCT (SD-OCT) is essential for diagnosing myopic traction maculopathy. It delineates the retina layer by layer and provides detailed information about the pathology. The OCT shows the formation of cystic cavities within the retina, which causes a split in the layers of the retina, giving rise to a thicker inner layer and a thinner outer layer.[18][19] If the schitic cavities are present in the retina's inner and outer layers, it is called compound foveoschisis. A localized posterior detachment of the fovea is delineated. Other features noted on OCT are epiretinal membrane, lamellar or full-thickness macular hole, inner segment/outer segment (IS/OS) junction disruption, paravascular microholes, and retinal micro folds.[20] These scans must be interpreted carefully to determine the presence of lamellar or full-thickness macular holes.
Since myopic eyes are large, large OCT scans (12 mm) are preferred. It is imperative to scan the entire posterior pole. OCT scans should be done along the horizontal and vertical axis. Myopic traction maculopathy cannot be judged solely based on OCT. Fundus photographs are essential for the evaluation of the eye. Conventional 30-degree photographs do not help properly evaluate the staphyloma; therefore, wide-field fundus photos are preferred. They give the full extent and the location of the staphyloma.
Treatment / Management
Myopic traction maculopathy can only be managed surgically. However, there is no consensus on the management plan. Owing to the rarity of the disease, there are very few studies in the literature quoting specific principles of treatment. Surgeons in different parts of the world have been developing protocols of management based on their clinical experience.
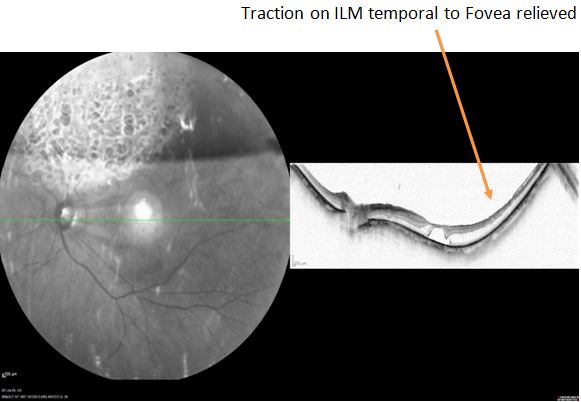
Pars plana vitrectomy, along with internal limiting membrane (ILM) peeling, has shown good results in eyes with myopic traction maculopathy. It has been hypothesized that peeling the ILM not only relieves traction on the macula but decreases recurrence by removing the scaffold for the proliferation of cells. However, due to the need for superior surgical skills and complications of an iatrogenic macular hole, it may not be a preferred entity for some surgeons. Scleral buckling is another modality that helps relieve traction on the macula by providing external compressive force. It has the advantage of being extraocular, decreasing the risk of cataract formation and iatrogenic holes. It might also help in early resolution compared to ILM peeling (see Image. Relieved Traction on the ILM After Surgery).
Before deciding on the treatment plan, the surgeon needs to identify the tractional forces operating on the patient's retina. Both forces, tangential or perpendicular to the plane of the retina, need to be taken into account. Once the nature of force has been ascertained, a surgical modality that applies the force parallel to it but in the opposite direction needs to be chosen.
For eyes having a macular hole, due to tangential traction applied by the internal limiting membrane, the surgery of choice is pars plana vitrectomy with internal limiting membrane peeling.[21] Other eyes having the clinical picture of schisis and detachment should undergo a macular buckle to counter the perpendicular tractional force.[22][23] A macular buckle is a device created to cause shortening in the axial length of the eye at the posterior pole.[24][25] Different models of macular buckles have been put into use.(B3)
Sometimes, myopic eyes may have a combination of perpendicular and tangential tractional forces, as in the case of macular detachment with a macular hole. The surgery of choice in such cases is a pars plana vitrectomy with internal limiting membrane peeling and macular buckling.[26][27]
Suppose the force isn't correctly identified and countered, like providing counter perpendicular traction in an eye with tangential traction, or vice versa. In that case, the problem may not only persist, but it may also get aggravated, and the surgeon may end up doing more harm than providing benefit to the patient. Therefore, the MTM retinal pattern (schisis and detachment) should be treated with a buckle. In contrast, the MTM foveal pattern (macular hole) should be treated with a vitrectomy and ILM peeling, and the MTM retinal with foveal pattern (schisis and detachment combined with macular hole) should be given a combined treatment of vitrectomy with macular buckle.
Differential Diagnosis
Myopic foveoschisis is a cause of gradual progressive loss of vision in high myopic eyes. Other causes of such diminution of vision in myopic eyes need to be ruled out. Such causes include:
- Myopic choroidal neovascularization
- Retinal detachment
- Lamellar or full-thickness macular hole
- Forster-Fuch spots at the fovea
Elaborate history-taking, detailed clinical examination, and judicious use of investigative modalities usually help to make the correct diagnosis.
Staging
To date, there is no universally accepted classification system for myopic foveoschisis. Two classification systems are commonly used.
International Photographic Classification and Grading System for Myopic Maculopathy
In 2015, the meta-analysis for pathologic myopia study group published the International Photographic Classification and Grading System for Myopic Maculopathy based on the color view of the fundus. The classification system is as follows:
- Category 1: Tessellated fundus (well-defined choroidal vessels)
- Category 2: Diffuse atrophy (whitish-yellow appearance)
- Category 3: Patchy atrophy (well-defined atrophic areas around the disc or at the posterior pole)
- Category 4: Macular atrophy
MTM Staging System
Recently, Barabara Parolini and colleagues have presented the MTM staging system (MSS) based on the OCT features of the retina.[28] This classification system considers the tractional forces operating perpendicular and parallel to the retina's surface, as seen on the OCT. Based on the MTM staging system (MSS), the disease can be classified as follows:
- Stage1a: Inner-outer macular schisis with a normal foveal profile
- Stage1b: Inner-outer macular schisis with a lamellar macular hole
- Stage1c: Inner-outer macular schisis with a full-thickness macular hole
- Stage2a: Predominantly outer macular schisis with a normal foveal profile
- Stage2b: Predominantly outer macular schisis with a lamellar macular hole
- Stage2c: Predominantly outer macular schisis with a full-thickness macular hole
- Stage3a: Macular schisis detachment with a normal foveal profile
- Stage3b: Macular schisis detachment with a lamellar macular hole
- Stage3c: Macular schisis detachment with a full-thickness macular hole
- Stage4a: Macular detachment with a normal foveal profile
- Stage4b: Macular detachment with a lamellar macular hole
- Stage4c: Macular detachment with a full-thickness macular hole
Each stage can have epiretinal abnormalities, and a "+" sign can be added to indicate its presence. This classification system standardizes the nomenclature, gives an insight into the natural progression of the disease, and helps plan its management.
Prognosis
The prognosis of surgery depends on the patient's preoperative visual acuity. The lower the visual acuity, the poorer the surgical outcome. If the visual symptoms have been present for a long time, the likelihood of complete visual recovery is remote. The presence of foveal detachment and a full-thickness macular hole worsens the prognosis. High axial length, deep staphyloma, and the presence of choroidal thinning all make the surgical outcome less favorable, with poor recovery of visual acuity and incomplete anatomical resolution.[29]
Complications
Surgery for MTM is technically challenging. The visual contrast in high myopic eyes is poor, and ILM peeling becomes difficult. The thin ILM in such eyes is extremely fragile and difficult to grasp and peel. Therefore, an iatrogenic tear of ILM and the formation of a macular hole post-surgery is not uncommon. For fear of forming a macular hole, some surgeons advocate fovea-sparing ILM peeling. Cataract formation is quite common following pars plana vitrectomy and gas tamponade. Macular buckling is technically challenging and may have complications like an incomplete resolution or recurrence of MTM, choroidal detachment, subretinal hemorrhage, or extrusion of the buckle in the post-operative phase.
Deterrence and Patient Education
High myopic eyes are plagued with many complications, and such patients are urged to have regular check-ups with a retina specialist. Any new visual complaints, such as diminution of vision, floaters, flashes of light, metamorphosis, or scotoma, should be immediately reported to a qualified retina specialist. Annual or biannual retina screening for myopic patients is mandatory to look for the development of new lattice degeneration or a break in the retina.
Enhancing Healthcare Team Outcomes
Patients with high myopia are prone to develop eye complications that may happen at any age. These patients are best managed by an interprofessional team that includes optometrists, ophthalmologists, and retinal specialists.[30][31] Patients with high myopia must be in regular touch with their retina specialists, even if they don't have any visual complaints. While screening, the retina specialist ought to be highly vigilant and search for lesions in the eye, which could go on to cause a visual catastrophe. All lattice degeneration, retinal holes, lacquer cracks, and neovascularization need to be given due attention to prevent the decrease in the patient's vision in a well-timed manner.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Dolar-Szczasny J, Święch-Zubilewicz A, Mackiewicz J. A Review of Current Myopic Foveoschisis Management Strategies. Seminars in ophthalmology. 2019:34(3):146-156. doi: 10.1080/08820538.2019.1610180. Epub 2019 May 6 [PubMed PMID: 31060414]
Nebbioso M, Lambiase A, Gharbiya M, Bruscolini A, Alisi L, Bonfiglio V. High myopic patients with and without foveoschisis: morphological and functional characteristics. Documenta ophthalmologica. Advances in ophthalmology. 2020 Dec:141(3):227-236. doi: 10.1007/s10633-020-09767-y. Epub 2020 Apr 22 [PubMed PMID: 32323040]
Level 3 (low-level) evidenceTakano M, Kishi S. Foveal retinoschisis and retinal detachment in severely myopic eyes with posterior staphyloma. American journal of ophthalmology. 1999 Oct:128(4):472-6 [PubMed PMID: 10577588]
Lee DH, Moon I, Kang HG, Choi EY, Kim SS, Byeon SH, Koh HJ, Lee SC, Kim M. Surgical outcome and prognostic factors influencing visual acuity in myopic foveoschisis patients. Eye (London, England). 2019 Oct:33(10):1642-1648. doi: 10.1038/s41433-019-0462-7. Epub 2019 May 16 [PubMed PMID: 31097818]
Frisina R, Baldi A, Cesana BM, Semeraro F, Parolini B. Morphological and clinical characteristics of myopic posterior staphyloma in Caucasians. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2016 Nov:254(11):2119-2129 [PubMed PMID: 27106626]
Zheng F, Wong CW, Sabanayagam C, Cheung YB, Matsumura S, Chua J, Man REK, Ohno-Matsui K, Wong TY, Cheng CY, Tai ES, Lamoureux ELED, Schmetterer L, Kuo A, Hoang QV, Saw SM. Prevalence, risk factors and impact of posterior staphyloma diagnosed from wide-field optical coherence tomography in Singapore adults with high myopia. Acta ophthalmologica. 2021 Mar:99(2):e144-e153. doi: 10.1111/aos.14527. Epub 2020 Jun 29 [PubMed PMID: 32602252]
Frisina R, Zampedri E, Marchesoni I, Bosio P, Parolini B, Romanelli F. Erratum to: Lamellar macular hole in high myopic eyes with posterior staphyloma: morphological and functional characteristics. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2016 Nov:254(11):2289 [PubMed PMID: 27169805]
dell'Omo R, Virgili G, Bottoni F, Parolini B, De Turris S, Di Salvatore A, dell'Omo E, Costagliola C. Lamellar macular holes in the eyes with pathological myopia. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2018 Jul:256(7):1281-1290. doi: 10.1007/s00417-018-3995-8. Epub 2018 May 3 [PubMed PMID: 29725825]
Leveziel N, Marillet S, Dufour Q, Lichtwitz O, Bentaleb Y, Pelen F, Ingrand P, Bourne R. Prevalence of macular complications related to myopia - Results of a multicenter evaluation of myopic patients in eye clinics in France. Acta ophthalmologica. 2020 Mar:98(2):e245-e251. doi: 10.1111/aos.14246. Epub 2019 Sep 10 [PubMed PMID: 31503418]
Ren P, Lu L, Tang X, Lu H, Zhao Y, Lou D, Han W. Clinical features of simple hemorrhage and myopic choroidal neovascularization associated with lacquer cracks in pathologic myopia. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2020 Dec:258(12):2661-2669. doi: 10.1007/s00417-020-04778-6. Epub 2020 Jul 9 [PubMed PMID: 32648154]
Guo X, Chen X, Li M, Li S, You R, Wang Y. Association between morphological characteristics of the optic disc and other anatomical features of the fundus in highly myopic eyes. European journal of ophthalmology. 2021 Sep:31(5):2329-2338. doi: 10.1177/1120672120945901. Epub 2020 Aug 5 [PubMed PMID: 32757632]
Pugazhendhi S, Ambati B, Hunter AA. Pathogenesis and Prevention of Worsening Axial Elongation in Pathological Myopia. Clinical ophthalmology (Auckland, N.Z.). 2020:14():853-873. doi: 10.2147/OPTH.S241435. Epub 2020 Mar 18 [PubMed PMID: 32256044]
Rino F, Elena Z, Ivan M, Paolo B, Barbara P, Federica R. Lamellar macular hole in high myopic eyes with posterior staphyloma: morphological and functional characteristics. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2016 Nov:254(11):2141-2150 [PubMed PMID: 27122245]
Ono T, Terada Y, Mori Y, Kataoka Y, Nakahara M, Miyata K. Spontaneous resolution of myopic foveoschisis and a macular hole with retinal detachment. American journal of ophthalmology case reports. 2019 Mar:13():143-146. doi: 10.1016/j.ajoc.2019.01.002. Epub 2019 Jan 9 [PubMed PMID: 30705999]
Level 3 (low-level) evidenceXie ZG, He QY, Zhu J, Du W, Tong J, Chen F. A Modified Surgical Technique of Fovea-Sparing Internal Limiting Membrane Peeling: Continuous Arc-Shaped Foldback Peeling. Journal of ophthalmology. 2020:2020():3568938. doi: 10.1155/2020/3568938. Epub 2020 Mar 23 [PubMed PMID: 32280519]
Vogt D, Stefanov S, Guenther SR, Hagenau F, Wolf A, Priglinger SG, Schumann RG. Comparison of Vitreomacular Interface Changes in Myopic Foveoschisis and Idiopathic Epiretinal Membrane Foveoschisis. American journal of ophthalmology. 2020 Sep:217():152-161. doi: 10.1016/j.ajo.2020.04.023. Epub 2020 Apr 29 [PubMed PMID: 32360335]
Al-Dhibi H, Chaudhry IA, Al-Assiri A, Shamsi FA. Development of early choroidal neovascular membrane in a young myope after LASIK. European journal of ophthalmology. 2007 Mar-Apr:17(2):262-5 [PubMed PMID: 17415702]
Level 3 (low-level) evidenceLee GW, Kim JH, Kang SW, Kim J. Structural profile of dome-shaped macula in degenerative myopia and its association with macular disorders. BMC ophthalmology. 2020 May 24:20(1):202. doi: 10.1186/s12886-020-01473-2. Epub 2020 May 24 [PubMed PMID: 32448138]
Sborgia G, Boscia F, Niro A, Giancipoli E, D'Amico Ricci G, Sborgia A, Sborgia L, Recchimurzo N, Romano MR, Addabbo G, Alessio G. Morphologic and functional outcomes of different optical coherence tomography patterns of myopic foveoschisis after vitrectomy and inner limiting membrane peeling. Eye (London, England). 2019 Nov:33(11):1768-1775. doi: 10.1038/s41433-019-0490-3. Epub 2019 Jun 17 [PubMed PMID: 31209260]
AttaAllah HR, Omar IAN, Abdelhalim AS. Assessment of Posterior Segment Using Spectral Domain OCT in Highly Myopic Eyes. The open ophthalmology journal. 2017:11():334-345. doi: 10.2174/1874364101711010334. Epub 2017 Nov 22 [PubMed PMID: 29299081]
Gui J, Ai L, Huang T. Vitrectomy with or without internal limiting membrane peeling for myopic foveoschisis. BMC ophthalmology. 2020 Mar 4:20(1):83. doi: 10.1186/s12886-020-01354-8. Epub 2020 Mar 4 [PubMed PMID: 32131776]
Grewal PS, Seamone M, Greve M, Deveau A, Gupta RR. INTERNAL CHANDELIER-ASSISTED MACULAR BUCKLING FOR MYOPIC FOVEOSCHISIS. Retinal cases & brief reports. 2022 Jul 1:16(4):532-535. doi: 10.1097/ICB.0000000000001024. Epub [PubMed PMID: 32541430]
Level 3 (low-level) evidenceZhao X, Ma W, Lian P, Tanumiharjo S, Lin Y, Ding X, Stewart JM, Liu B, Lu L. Three-year outcomes of macular buckling for macular holes and foveoschisis in highly myopic eyes. Acta ophthalmologica. 2020 Jun:98(4):e470-e478. doi: 10.1111/aos.14305. Epub 2019 Nov 19 [PubMed PMID: 31742899]
Parolini B, Frisina R, Pinackatt S, Gasparotti R, Gatti E, Baldi A, Penzani R, Lucente A, Semeraro F. INDICATIONS AND RESULTS OF A NEW L-SHAPED MACULAR BUCKLE TO SUPPORT A POSTERIOR STAPHYLOMA IN HIGH MYOPIA. Retina (Philadelphia, Pa.). 2015 Dec:35(12):2469-82. doi: 10.1097/IAE.0000000000000613. Epub [PubMed PMID: 26079474]
Parolini B, Frisina R, Pinackatt S, Mete M. A new L-shaped design of macular buckle to support a posterior staphyloma in high myopia. Retina (Philadelphia, Pa.). 2013 Jul-Aug:33(7):1466-70. doi: 10.1097/IAE.0b013e31828e69ea. Epub [PubMed PMID: 23619638]
Wang JL, Wang YL. Long-Term Outcome of Vitrectomy with Suitable Internal Limiting Membrane Peeling and Air Tamponade for Highly Myopic Foveoschisis-Associated Lamellar Macular Hole. Journal of ophthalmology. 2020:2020():2074037. doi: 10.1155/2020/2074037. Epub 2020 Feb 21 [PubMed PMID: 32148935]
Alkabes M, Mateo C. Macular buckle technique in myopic traction maculopathy: a 16-year review of the literature and a comparison with vitreous surgery. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2018 May:256(5):863-877. doi: 10.1007/s00417-018-3947-3. Epub 2018 Mar 28 [PubMed PMID: 29589106]
Parolini B, Palmieri M, Finzi A, Besozzi G, Lucente A, Nava U, Pinackatt S, Adelman R, Frisina R. The new Myopic Traction Maculopathy Staging System. European journal of ophthalmology. 2021 May:31(3):1299-1312. doi: 10.1177/1120672120930590. Epub 2020 Jun 8 [PubMed PMID: 32506945]
Kim CY, Kim MS, Kim KL, Woo SJ, Park KH. Prognostic Factors Related with Surgical Outcome of Vitrectomy in Myopic Traction Maculopathy. Korean journal of ophthalmology : KJO. 2020 Feb:34(1):67-75. doi: 10.3341/kjo.2019.0115. Epub [PubMed PMID: 32037751]
Gaucher D, Haouchine B, Tadayoni R, Massin P, Erginay A, Benhamou N, Gaudric A. Long-term follow-up of high myopic foveoschisis: natural course and surgical outcome. American journal of ophthalmology. 2007 Mar:143(3):455-62 [PubMed PMID: 17222382]
Level 2 (mid-level) evidenceGohil R, Sivaprasad S, Han LT, Mathew R, Kiousis G, Yang Y. Myopic foveoschisis: a clinical review. Eye (London, England). 2015 May:29(5):593-601. doi: 10.1038/eye.2014.311. Epub 2015 Mar 6 [PubMed PMID: 25744445]