Pediatric Echocardiography Assessment, Protocols, and Interpretation
Pediatric Echocardiography Assessment, Protocols, and Interpretation
Introduction
Echocardiography is the first-line, non-invasive approach to management in evaluating anatomical, physiological, and hemodynamic abnormalities of the heart.[1] It is one of many imaging modalities utilized by cardiologists around the world. Before beginning this discussion, we must first address the nomenclature. Echocardiography is an all-encompassing term for cardiac ultrasound. It includes many invasive and non-invasive modalities such as transthoracic echocardiography (TTE), stress echocardiography, transesophageal echocardiography, fetal echocardiography, three-dimensional echocardiography, intracardiac echocardiography, Intra-valvular echocardiography, and Intra-operative echocardiography.
This article will discuss pediatric TTEs, a reliable diagnostic approach that has shown to yield as little as 87 diagnostic errors out of 50,660 total TTEs in a previously published study.[2] Both comprehensive transthoracic echocardiography (cTTE) and functional transthoracic echocardiography (fTTE) will be covered. This discussion will be done with an emphasis on highlighting how Pediatric TTEs differ from general adult TTEs. Lastly, given an incidence of 4 to 12 per every 1000 live births, congenital heart disease (CHD) will undoubtedly be a predominant topic covered below.[3]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
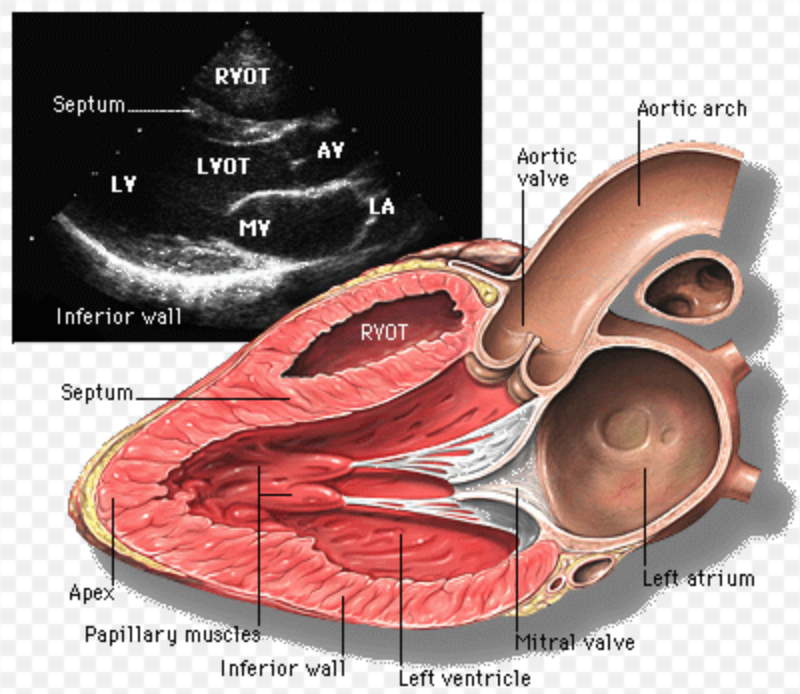
Pediatric TTEs typically resemble Adult TTEs in regards to cardiac structure morphology and to obtaining the basic views. However, one can imagine the added anatomical difficulty of interpreting a pediatric TTE in a patient with a complex CHD malformation. Such atypical anatomical appearances place significance on identifying morphological characteristics, especially in pediatric populations. For this same reason, pediatric TTEs require further in-depth evaluation of the suprasternal notch, right parasternal, and subxiphoid views. Views that are of less significance compared to adult TTEs.
Here is provided a list of distinct morphological characteristics, along with a figure of each view, as well as its anatomical and physiological significance.
Distinct Morphological Characteristics
- Atria – Right atrium (RA) has a wide triangular appendage, is anteriorly located; left atrium (LA) has a thin, long appendage, is posteriorly located.
- Atrial Septum – Right has a Eustachian Valve; left has a foramen ovale flap.
- Ventricles – Right ventricle (RV) has coarse trabeculations, tri-leaflet valve, moderator band presence; left ventricle (LV) has thin trabeculations bi-leaflet valve, absence of a moderator band.
- The tricuspid valve – associated with the RV, has three leaflets, is located toward the apex.
- Mitral valve – associated with the LV, has two leaflets, is attached to the lateral wall by papillary muscles.
- Aorta – will disperse into the coronaries.
- Pulmonary artery – will branch to the right and left.
Anatomical and Physiological Significance by View
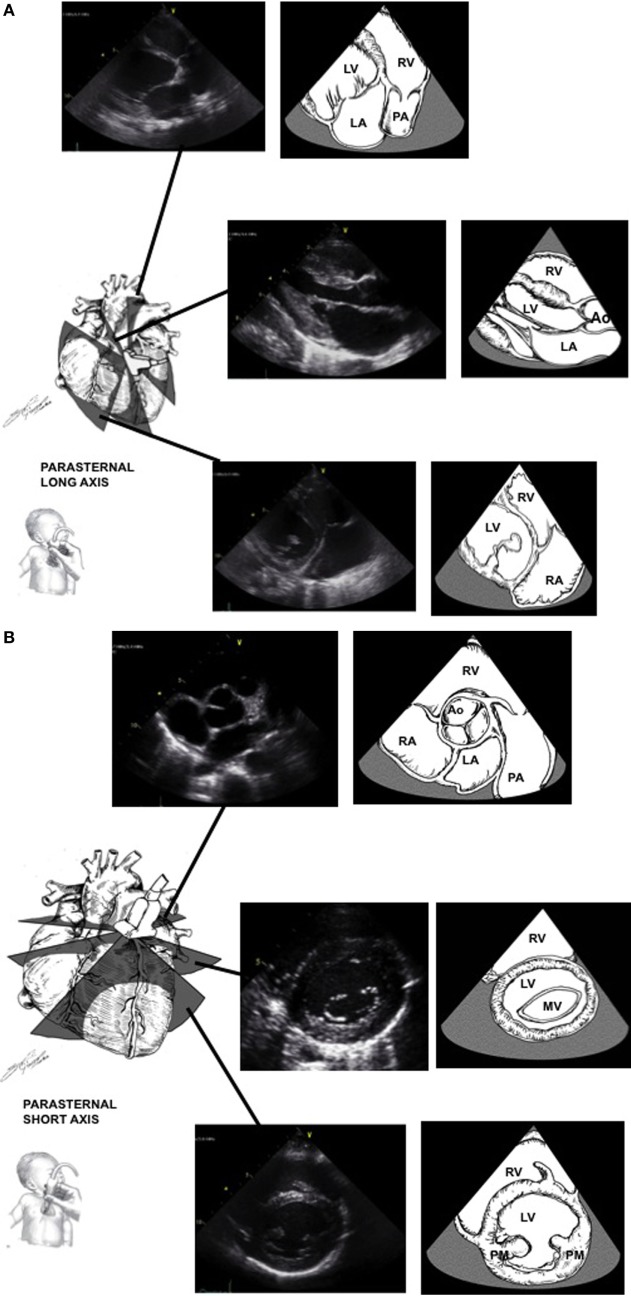
Parasternal Long Axis: (figure)
- LV outflow tract (LVOT)
- RV outflow tract (RVOT)
- Flow across mitral and aortic valves
- Tricuspid and Pulmonic valves
Parasternal Short Axis: (figure)
- Aortic and pulmonary valve architecture
- Aortic valve coronary cusps as well as the RCA and LCA caliber
- Atrial septum
- Main pulmonary artery branches
- RV and LV function and wall size
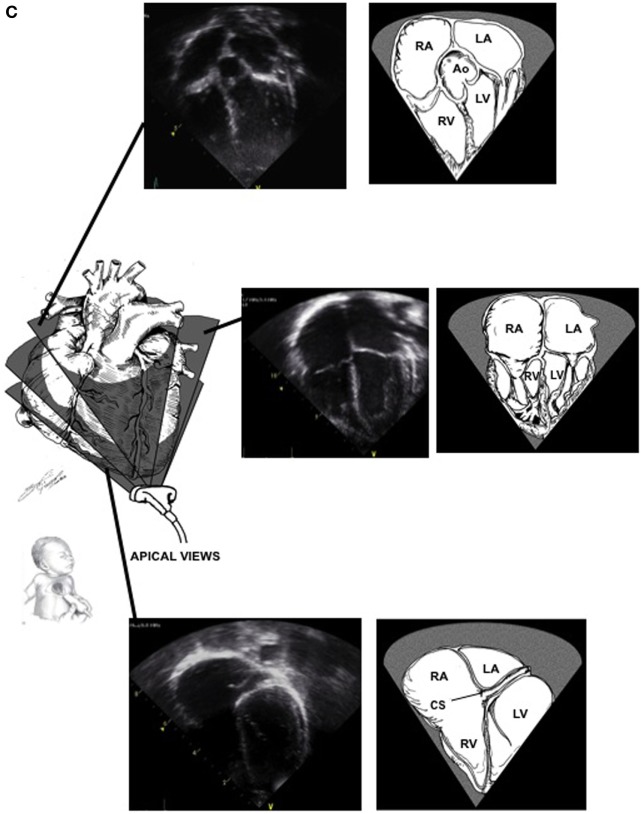
Apical: (figure)
- Assesses all chambers' size and function
- Assess tricuspid and mitral valve function
- LVOT across the aortic valve
- RVOT across the pulmonic valve
- LV Ejection Fraction
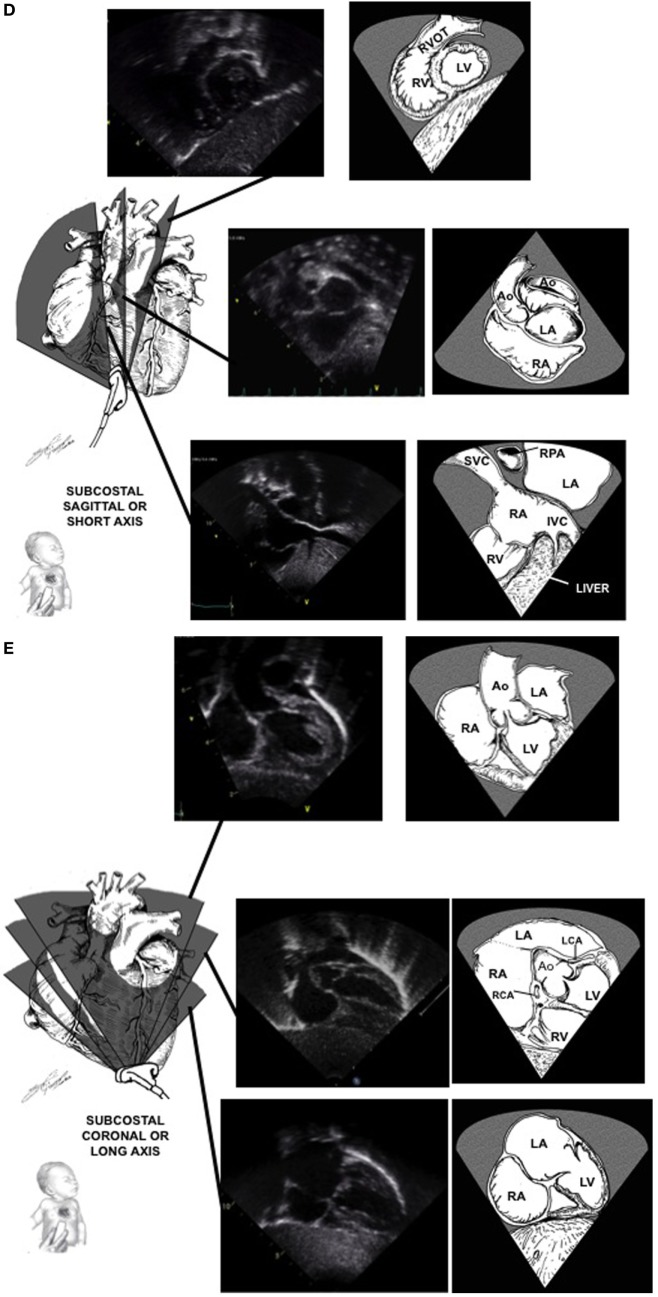
Subcostal Short Axis: (figure)
- Stomach, spleen, abdominal aorta, IVC, SVC
- RV outflow across the pulmonic valve
Subcostal Long Axis: (figure)
- LVOT
- Aortic root
- Atrial septum
- Coronary cusps
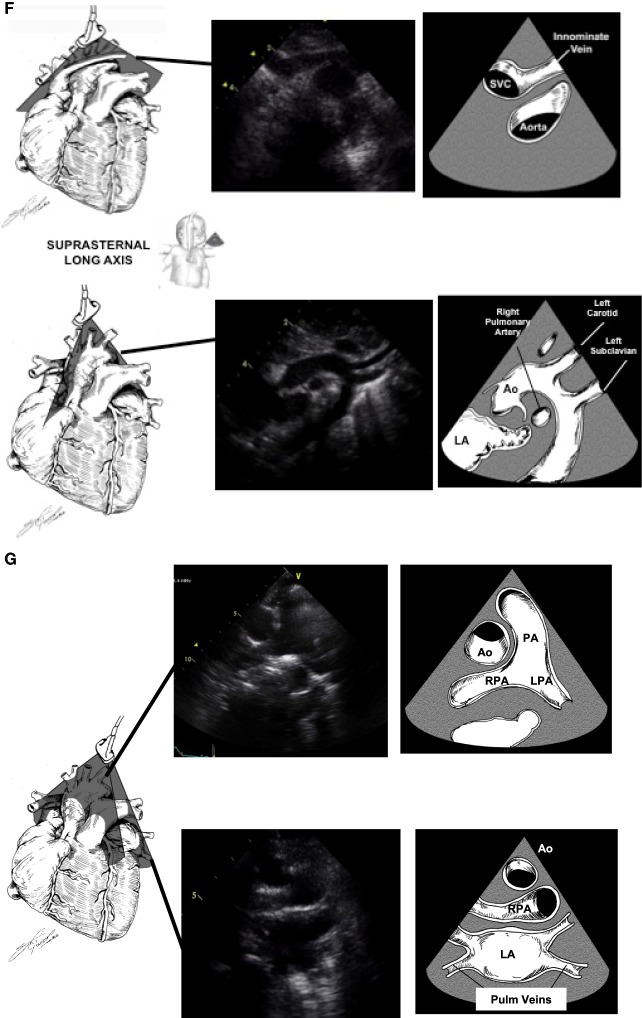
Suprasternal Notch: (figure)
- Brachiocephalic trunk
- Aortic trunk
- Pulmonary veins draining into the left atrium
- Innominate vein connection to SVC
- IVC
Indications
Initial Evaluation
Listed below are the indications for initial outpatient pediatric cTTEs according to the 2014 Appropriate Use Criteria (AUC) Report published by the American College of Cardiology (ACC) AUC Task Force. A total of 113 indications were mentioned, each labeled with an appropriate use rating of “appropriate,” “may be appropriate,” “rarely appropriate.” Only the indications labeled “appropriate” will be mentioned below. Furthermore, in-depth information, including the entire list of indications and symptom-based algorithms, can be found here.[4]
Palpitations with:
- Known cardiomyopathy or family history of cardiomyopathy.
- Family history of sudden death or cardiac arrest and/or implantable defibrillator or pacemaker placed all occurring <50 years of age.
Arrhythmia with:
- electrocardiogram (ECG) findings of supraventricular tachycardia or ventricular tachycardia.
Syncope with:
- Family history of cardiomyopathy.
- Family history of sudden death or cardiac arrest, and/or implantable. Defibrillator or pacemaker placed all occurring <50 years of age.
- Abnormal ECG.
- Exertional and/or following exertion with an unexplained etiology.
Chest Pain with:
- Family history of cardiomyopathy or sudden unexplained death.
- Exertion or abnormal ECG (regardless of the exertional component).
Murmur with:
- History concerning cardiovascular disease (CVD).
- Pathological acoustic characteristics.
Outpatient Neonates without post-natal cardiac evaluation with:
- Maternal phenylketonuria.
- Maternal infection with potential fetal/neonatal cardiac sequelae during pregnancy/delivery.
- Concerning fetal echocardiogram suspicious for cardiovascular abnormality.
Other Signs and Symptoms of:
- Central cyanosis.
- Endocarditis, regardless of blood culture results.
- Congestive heart failure.
Patients without confirmed or suspected cardiac diagnosis and have a family history of:
- Cardiomyopathy (e.g., hypertrophic, non-ischemic dilated).
- High-risk genetic disorder for CVD (e.g., Loeys Dietz, Marfan).
- Heritable pulmonary arterial hypertension.
Prior Test Results of:
- Abnormal chest X-ray or chromosomal pattern suggestive of CVD.
- Abnormal ECG in an asymptomatic patient.
- Abnormal cardiac biomarkers.
- Abnormal bronchoscopy or barium swallow suggesting vascular ring.
- Positive blood cultures concerning infective endocarditis.
- Genotype positive for cardiomyopathy.
- Desaturation demonstrated on pulse oximetry.
- Previously normal TTE with a new family history suggestive of heritable heart disease and/or a change in cardiovascular status.
Systemic Disorders of:
- Suspected or confirmed CHD, connective tissue disorders, Kawasaki disease, Takayasu arteritis, pulmonary hypertension, acute rheumatic fever.
- Abnormalities of cardiac or visceral situs.
- Cancers requiring chemo.
- Human Immunodeficiency Virus infection.
- Confirmed storage diseases, mitochondrial/metabolic disorders, stroke, hemoglobinopathies, autoimmune disorders, Muscular Dystrophy, systemic hypertension, renal failure.
Follow-up Evaluation for CHD
Listed below are the indications for CHD follow-up cTTEs according to the 2020 AUC Report published by the ACC AUC Task Force. A total of 23 CHD defects were mentioned, all with specific unrepaired and post-procedural indications. Similar to above, each indication was labeled with an appropriate use rating of “appropriate,” “may be appropriate,” “rarely appropriate.” Of the 324 indications mentioned, all but 15 received an “appropriate” rating for TTE indication. For simplicity purposes, below, we will list the CHD defects covered with an “appropriate” rating in all scenarios followed by a list of CHDs, only noting the scenarios where the indication was labeled less than “appropriate.” More detailed information, including a full list of all 324 indications can be found here.[5]
CHDs with “appropriate” ratings for cTTE in all pre and post-repair scenarios:
- Single ventricle heart disease
- Tetralogy of fallow
- Truncus arteriosus
- Pulmonary atresia with intact ventricular septum
- Pulmonary stenosis
- Congenitally corrected transposition of the great arteries
- Double outlet RV
- Aortic coarctation and interrupted aortic arch
- Ebstein anomaly and tricuspid valve dysplasia
- Total anomalous pulmonary venous connection
- Atrioventricular septal defects
CHDs with “appropriate” ratings for cTTE in all Pre and Post repair scenarios except listed below:
- Patent Foramen Ovale
- “Rarely Appropriate” for routine surveillance in an asymptomatic patient.
- Atrial Septal Defect (ASD) and Partial Anomalous Pulmonary Venous Connection (PAPVC)
- “Maybe appropriate” for routine surveillance (1-2 years) of an asymptomatic patient with an unrepaired small ASD or PAPVC involving one pulmonary vein.
- “Maybe appropriate” for annual routine surveillance of an asymptomatic patient following surgical PAPVC repair or ASD closure.
- Ventricular Septal Defects (VSD)
- “Rarely appropriate” for routine surveillance (1-2 years) of an unrepaired small muscular VSD in an asymptomatic patient.
- “Maybe appropriate” for annual surveillance of surgical VSD closure in an asymptomatic patient.
- Patent Ductus Arteriosus (PDA)
- “Rarely appropriate” routine surveillance of an unrepaired silent PDA in an asymptomatic patient.
- “Rarely appropriate” for routine surveillance of an asymptomatic patient following surgical PDA closure >2years prior.
- Eisenmenger Syndrome (ES)
- “Maybe appropriate” for routine surveillance every 3 months for ES in a stable child.
- “Rarely Appropriate” for routine surveillance every 3 months for ES in a stable adult.
- Pulmonary Hypertension (PH) Associated with CHD
- “Maybe appropriate “ for routine surveillance every 3 months in a post-repair PH stable adult.
- Mitral valve Disease, i.e., Congenital Mitral Stenosis (MS), Prolapse (MVP), Regurgitation (MR)
- “Maybe appropriate” for routine surveillance every 1-2 years of an unrepaired congenital mitral valve prolapse or congenital mitral valve regurgitation in an asymptomatic child.
- D-loop Transposition of the Great Arteries
- “Rarely appropriate” for routine postoperative surveillance every 6 months in an asymptomatic patient following an Atrial Switch Operation.
- Coronary Anomalies
- “Rarely appropriate” for routine annual surveillance of an unrepaired anomalous right coronary artery (RCA) from the left aortic sinus or a small coronary fistula in an asymptomatic patient.
- LVOT lesion, i.e., Aortic Valve Stenosis (AS), Regurgitation (AR)
- “Rarely appropriate” for routine surveillance of an unrepaired mild aortic valve stenosis (AS) and/or mild aortic valve regurgitation (AR) in an asymptomatic patient without aortic dilation.
Equipment
All Ultrasound (US) devices used for cTTEs should include software to perform the basics of 2D imaging, M-mode, color flow Doppler, and spectral Doppler.[6] The details of each modality are discussed below as well as in the Clinical Significance section.
US probes are used to transmit mechanical longitudinal electrical waves through a medium. This is done by transmitting an electrical current via alternating potential differences amongst electrodes encompassing piezoelectrical crystals. Once electrically stimulated, these crystals will produce sound waves. The sound waves will propagate through the medium at velocities dependent on the material's density. Waves will return to the crystals, at which point the waves will be converted back into an electrical signal and converted into images. Such images are the basis of two-dimensional echocardiography.[7]
US probes are utilized in the same methodology as above to produce doppler images. A color Doppler image varies by color depending on the direction of a moving medium. If an object moves away from the probe, it will produce a longer wavelength, interpreted as a blue color. If an object is moving toward the probe, it will produce a smaller wavelength, interpreted as a red color. A spectral doppler encompasses a pulse and continuous wave Doppler, which varies from color doppler by calculating the mean and peak gradients. This is illustrated along with a graph for the simplicity of comparison. A tissue Doppler can be done via pulse or color mode. It is used to produce peak myocardial velocities.
Diagnostic ultrasounds produce low-frequency currents ranging from 2.5 to 14 MHz. Thus, producing a radiation-free modality safe to use in children, infants, and fetuses. Multiple probes are available, which vary by frequency. To grasp the significance of high vs. low-frequency probes, one must recall the inverse relationship between wavelength and frequency. The higher frequency probes are ideal for small infants, providing the clinician with a smaller wavelength allowing one to obtain a higher resolution image of superficial structures.
Preparation
Prior to the exam, the child could be asked to hold parental oral intake for 6 to 8 hours, as light sedation is required at times for younger children. The patient will be asked to lay supine on the exam table or bed, with the head of the bed at roughly a 30-degree angle. If possible, the lateral decubitus position can provide optimal images given the gravitational pull of the heart towards the surface. In infants, the parent may wish to hold the patient for simplicity. Distractors are provided when needed. A few electrodes will be placed on the child to allow ECG gated images. The gown will be lowered to expose the patient's left breast. A towel is provided to adolescents for privacy purposes. US jelly will then be placed on the tip of the probe, and the exam will start.
Technique or Treatment
Each cTTE should be done in a step-wise fashion. The exam steps are typically defined by the institution, providing the interpreter with a stream-lined systematic approach to all cTTE reads. Such a defined systematic approach provides simplicity to interpreting each individual cTTE. Specifically for pediatric cTTEs, importance is placed on the initial recognition of basic anatomy to rule out or rule any CHD. This is done by starting with the subcostal view to identify the apex, ventricles, and great vessels. Once cardiac orientation is established, the sonographer should transition through windows in a sequential clockwise approach.
An fTTE, also known as a bedside TTE, has no specific technique. It is done strictly for rapid evaluation of structural-functional characteristics. Many fTTE machines do not even possess doppler or ECG gating capabilities. The apical and subcostal acoustic windows are most utilized.
Clinical Significance
The diagnostic ability of TTEs is well known, widely accepted, and has been documented in a multitude of publications.[8][9][10][11][12][13][14] Here we will breakdown the clinical application/diagnostic ability of TTEs into four groups. First, we will discuss the utility of each specific modality included in a pediatric cTTE. Second, we will discuss the predominant pathologies viewed broken down by imaging window. Third, we will provide observed parameters specifically for each structure. Lastly, we will address the utility of an fTTE in the ICU setting.
Clinical Significance by Modality
2D imaging evaluates the real-time structure and motion of the heart. M mode is a still image used to measure wall and chamber size. Doppler evaluates flow through the heart via valves, chambers.
- Color Doppler evaluates the direction of flow via red and blue.
- Provides information on laminar vs turbulent flow through a valve or vessel.
- Utilized to diagnose vessel stenosis.
- Spectral doppler- Continuous and Pulse doppler evaluate intracardiac gradients.
- Provides mean and peak gradients to evaluate valvular or septal defects.
- Utilized to diagnose valvular pathology and VSD/LVOT obstruction/cardiac output.
Clinical Significance by View
Parasternal Long Axis
- All four valve abnormalities
- RV and LV function and size
- PDA
- RVOT obstruction (tetralogy of Fallot)
Parasternal Short Axis
- TV abnormalities (Ebstein anomaly)
- Pulmonary arterial stenosis
- RVOT obstruction (double-chambered RV, tetralogy of Fallot)
- Branch and main pulmonary artery abnormalities
- Interventricular volume and pressure measurements (pulmonary hypertension)
- Coronary cusps (anomalous RCA, LCA)
Apical
- TV, MV, AV abnormalities (congenital bicuspid AV)
- Pulmonary vein abnormalities (total anomalous pulmonary venous return)
- RA and LA function and size
- LV size and function via Simpson biplane method for ejection fraction
Subcostal Short Axis
- SVC stenosis or narrowing
- IVC abnormalities
- Anomalous pulmonary venous drainage
- RV and LV dilation or hypertrophy
- PV stenosis etiology (dysplastic or hypoplastic valve)
Subcostal Long Axis
- Pulmonary venous return (partial and total anomalous pulmonary venous return)
- VSD [see figure]
- ASD (prefered view) [see figure]
- AV and aortic root abnormality
Suprasternal Notch
- Pulmonary venous return (partial and total anomalous pulmonary venous return)
- Aortic arch abnormalities (vascular ring)
- Ascending aorta abnormalities (aortic dilation)
- Descending aorta abnormalities (coarctation of the aorta [see figure])
- Head and neck vessel abnormalities (common brachiocephalic trunk)
- SVC abnormalities
- PDA [see figure]
Clinical Significance of Recorded Values
Multiple structural, functional, and hemodynamic values are required to perform a cTTE. With the most variation from typical Adult TTEs, the former require adjustment according to body surface area.[15][16][17] This structural adjustment is then reported as a Z-score, allowing normalization of the data.[18][19] More information regarding the most up-to-date z-score calculators is available here.[20]
Listed below are the recommended measurements according to a report from the Task Force of the Pediatric Council of the American Society of Echocardiography and a report from the Task Force of a Standardized Echocardiography of the American Society of Echocardiography.[21][22]
TV and MV:
- Valve annulus diameter during diastole, mean gradient, E wave velocity (peak velocity in early diastole), A wave velocity (peak velocity in late diastole), deceleration time, isovolumic relaxation time (IVRT); TV Regurgitant jet velocity.
Main Pulmonary Artery as well as Left and Right Pulmonary Arteries:
- Artery diameter during systole; left or right pulmonary artery mean and peak gradient, velocity-time interval (VTI).
PV, AV, RVOT, LVOT:
- Valve annulus diameter during systole, mean and peak gradients, VTI; PV regurgitant jet velocity, AV pressure half-time.
Aortic arch:
- Mean and peak gradient, VTI.
Aortic root, ascending aorta, transverse aorta arch, descending aorta (Aortic isthmus):
- Vessel diameter.
LV and RV:
- End diastolic volume, end-systolic volume, wall thickness, assessment of systolic function, assessment of diastolic function.
LA and RA:
- Size.
Clinical Significance of fTTEs in Neonatal and Pediatric Intensive Care Units[23][24][25]
- Assessment of pulmonary hypertension
- Assessment of cardiac function
- Assessment of fluid responsiveness resuscitation
- Diagnosis and evaluation of hemodynamics in patent ductus arteriosus
- Diagnosis of pericardial effusion, pericardial tamponade
- Evaluation of hemodynamics in a shock patient
- Evaluation of central line placement, ECMO
- Evaluation of ventricular function in patients with hypoxic-ischemic encephalopathy
Enhancing Healthcare Team Outcomes
The combination of determining an indication, obtaining and interpreting a cTTE certainly requires interprofessional collaboration. For starters, a pediatrician must obtain a good history to clearly offer an appropriate indication. Once the cTTE is ordered, a certified ultrasound technician must perform the cTTE. This task can be difficult, especially in the setting of a young child. Nurses are needed to help distract the patient, and at times, a pharmacist may be consulted to provide mild sedation as the image quality is key for interpretation. Lastly, a cardiologist is needed to actually read and interpret the images.
The need for such an interprofessional team approach has been demonstrated inadvertently. A recent study demonstrated the significance of sedation via comparison of sedated vs. non-sedated pediatric cTTEs regarding providing optimal images. The conclusion was that sedated cTTEs provide a significant improvement in image quality. Without a multidisciplinary approach, obtaining optimal images in pediatric patients would not be possible.[26] [Level 4]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Opfer E, Shah S. Advances in Pediatric Cardiovascular Imaging. Missouri medicine. 2018 Jul-Aug:115(4):354-360 [PubMed PMID: 30228767]
Level 3 (low-level) evidenceBenavidez OJ, Gauvreau K, Jenkins KJ, Geva T. Diagnostic errors in pediatric echocardiography: development of taxonomy and identification of risk factors. Circulation. 2008 Jun 10:117(23):2995-3001. doi: 10.1161/CIRCULATIONAHA.107.758532. Epub 2008 Jun 2 [PubMed PMID: 18519849]
Hoffman JI,Kaplan S, The incidence of congenital heart disease. Journal of the American College of Cardiology. 2002 Jun 19; [PubMed PMID: 12084585]
Campbell RM, Douglas PS, Eidem BW, Lai WW, Lopez L, Sachdeva R. ACC/AAP/AHA/ASE/HRS/SCAI/SCCT/SCMR/SOPE 2014 appropriate use criteria for initial transthoracic echocardiography in outpatient pediatric cardiology: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Academy of Pediatrics, American Heart Association, American Society of Echocardiography, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Pediatric Echocardiography. Journal of the American College of Cardiology. 2014 Nov 11:64(19):2039-60. doi: 10.1016/j.jacc.2014.08.003. Epub 2014 Sep 29 [PubMed PMID: 25277848]
Sachdeva R, Valente AM, Armstrong AK, Cook SC, Han BK, Lopez L, Lui GK, Pickard SS, Powell AJ, Bhave NM, Sachdeva R, Valente AM, Pickard SS, Baffa JM, Banka P, Cohen SB, Glickstein JS, Kanter JP, Kanter RJ, Kim YY, Kipps AK, Latson LA, Lin JP, Parra DA, Rodriguez FH 3rd, Saarel EV, Srivastava S, Stephenson EA, Stout KK, Zaidi AN. ACC/AHA/ASE/HRS/ISACHD/SCAI/SCCT/SCMR/SOPE 2020 Appropriate Use Criteria for Multimodality Imaging During the Follow-Up Care of Patients With Congenital Heart Disease: A Report of the American College of Cardiology Solution Set Oversight Committee and Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Pediatric Echocardiography. Journal of the American College of Cardiology. 2020 Feb 18:75(6):657-703. doi: 10.1016/j.jacc.2019.10.002. Epub 2020 Jan 6 [PubMed PMID: 31918898]
Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, Douglas PS, Faxon DP, Gillam LD, Kimball TR, Kussmaul WG, Pearlman AS, Philbrick JT, Rakowski H, Thys DM, Antman EM, Smith SC Jr, Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, Hunt SA, Fuster V, Jacobs AK, Gibbons RJ, Russell RO, American College of Cardiology, American Heart Association, American Society of Echocardiography. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation. 2003 Sep 2:108(9):1146-62 [PubMed PMID: 12952829]
Level 1 (high-level) evidenceTissot C, Muehlethaler V, Sekarski N. Basics of Functional Echocardiography in Children and Neonates. Frontiers in pediatrics. 2017:5():235. doi: 10.3389/fped.2017.00235. Epub 2017 Dec 1 [PubMed PMID: 29250515]
Ni JR, Yan PJ, Liu SD, Hu Y, Yang KH, Song B, Lei JQ. Diagnostic accuracy of transthoracic echocardiography for pulmonary hypertension: a systematic review and meta-analysis. BMJ open. 2019 Dec 22:9(12):e033084. doi: 10.1136/bmjopen-2019-033084. Epub 2019 Dec 22 [PubMed PMID: 31871259]
Level 1 (high-level) evidenceKitada R, Fukuda S, Watanabe H, Oe H, Abe Y, Yoshiyama M, Song JM, Sitges M, Shiota T, Ito H, Yoshikawa J. Diagnostic accuracy and cost-effectiveness of a pocket-sized transthoracic echocardiographic imaging device. Clinical cardiology. 2013 Oct:36(10):603-10. doi: 10.1002/clc.22171. Epub 2013 Jul 24 [PubMed PMID: 23893844]
Li RJ, Sun Z, Yang J, Yang Y, Li YJ, Leng ZT, Liu GW, Pu LH. Diagnostic Value of Transthoracic Echocardiography in Patients With Anomalous Origin of the Left Coronary Artery From the Pulmonary Artery. Medicine. 2016 Apr:95(15):e3401. doi: 10.1097/MD.0000000000003401. Epub [PubMed PMID: 27082616]
O'Byrne ML, Glatz AC, Goldberg DJ, Shinohara R, Dori Y, Rome JJ, Gillespie MJ. Accuracy of Transthoracic Echocardiography in Assessing Retro-aortic Rim prior to Device Closure of Atrial Septal Defects. Congenital heart disease. 2015 Jul-Aug:10(4):E146-54. doi: 10.1111/chd.12226. Epub 2014 Sep 16 [PubMed PMID: 25227430]
Level 2 (mid-level) evidenceKabirdas D, Scridon C, Brenes JC, Hernandez AV, Novaro GM, Asher CR. Accuracy of transthoracic echocardiography for the measurement of the ascending aorta: comparison with transesophageal echocardiography. Clinical cardiology. 2010 Aug:33(8):502-7. doi: 10.1002/clc.20807. Epub [PubMed PMID: 20734448]
Monin JL, Dehant P, Roiron C, Monchi M, Tabet JY, Clerc P, Fernandez G, Houel R, Garot J, Chauvel C, Gueret P. Functional assessment of mitral regurgitation by transthoracic echocardiography using standardized imaging planes diagnostic accuracy and outcome implications. Journal of the American College of Cardiology. 2005 Jul 19:46(2):302-9 [PubMed PMID: 16022959]
Level 2 (mid-level) evidenceParlakay AO, Karagöz T, Ozkutlu S, Ozen S, Alehan D, Yiğit S. Evaluation of diagnostic accuracy of portable echocardiography in newborns. Anadolu kardiyoloji dergisi : AKD = the Anatolian journal of cardiology. 2011 Nov:11(7):627-32. doi: 10.5152/akd.2011.167. Epub 2011 Sep 29 [PubMed PMID: 21959878]
Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. Journal of applied physiology (Bethesda, Md. : 1985). 2005 Aug:99(2):445-57 [PubMed PMID: 15557009]
Daubeney PE, Blackstone EH, Weintraub RG, Slavik Z, Scanlon J, Webber SA. Relationship of the dimension of cardiac structures to body size: an echocardiographic study in normal infants and children. Cardiology in the young. 1999 Jul:9(4):402-10 [PubMed PMID: 10476831]
Pettersen MD, Du W, Skeens ME, Humes RA. Regression equations for calculation of z scores of cardiac structures in a large cohort of healthy infants, children, and adolescents: an echocardiographic study. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2008 Aug:21(8):922-34. doi: 10.1016/j.echo.2008.02.006. Epub 2008 Apr 11 [PubMed PMID: 18406572]
Level 2 (mid-level) evidenceKaski JP, Daubeney PE. Normalization of echocardiographically derived paediatric cardiac dimensions to body surface area: time for a standardized approach. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2009 Jan:10(1):44-5. doi: 10.1093/ejechocard/jen242. Epub 2008 Sep 30 [PubMed PMID: 18827032]
Gokhroo RK, Anantharaj A, Bisht D, Kishor K, Plakkal N, Mondal N. A pediatric echocardiographic Z-score nomogram for a developing country: Indian pediatric echocardiography study - The Z-score. Annals of pediatric cardiology. 2018 Jan-Apr:11(1):109-111. doi: 10.4103/apc.APC_123_17. Epub [PubMed PMID: 29440845]
Cantinotti M, Scalese M, Giordano R, Assanta N, Marchese P, Franchi E, Viacava C, Koestenberger M, Jani V, Kutty S. A statistical comparison of reproducibility in current pediatric two-dimensional echocardiographic nomograms. Pediatric research. 2021 Feb:89(3):579-590. doi: 10.1038/s41390-020-0900-z. Epub 2020 Apr 24 [PubMed PMID: 32330930]
Lai WW, Geva T, Shirali GS, Frommelt PC, Humes RA, Brook MM, Pignatelli RH, Rychik J, Task Force of the Pediatric Council of the American Society of Echocardiography, Pediatric Council of the American Society of Echocardiography. Guidelines and standards for performance of a pediatric echocardiogram: a report from the Task Force of the Pediatric Council of the American Society of Echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2006 Dec:19(12):1413-30 [PubMed PMID: 17138024]
Gardin JM, Adams DB, Douglas PS, Feigenbaum H, Forst DH, Fraser AG, Grayburn PA, Katz AS, Keller AM, Kerber RE, Khandheria BK, Klein AL, Lang RM, Pierard LA, Quinones MA, Schnittger I, American Society of Echocardiography. Recommendations for a standardized report for adult transthoracic echocardiography: a report from the American Society of Echocardiography's Nomenclature and Standards Committee and Task Force for a Standardized Echocardiography Report. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2002 Mar:15(3):275-90 [PubMed PMID: 11875394]
Level 1 (high-level) evidenceMertens L, Seri I, Marek J, Arlettaz R, Barker P, McNamara P, Moon-Grady AJ, Coon PD, Noori S, Simpson J, Lai WW, Writing Group of the American Society of Echocardiography, European Association of Echocardiography, Association for European Pediatric Cardiologists. Targeted Neonatal Echocardiography in the Neonatal Intensive Care Unit: practice guidelines and recommendations for training. Writing Group of the American Society of Echocardiography (ASE) in collaboration with the European Association of Echocardiography (EAE) and the Association for European Pediatric Cardiologists (AEPC). Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2011 Oct:24(10):1057-78. doi: 10.1016/j.echo.2011.07.014. Epub [PubMed PMID: 21933743]
Level 1 (high-level) evidenceSingh Y, Katheria A, Tissot C. Functional Echocardiography in the Neonatal Intensive Care Unit. Indian pediatrics. 2018 May 15:55(5):417-424 [PubMed PMID: 29845957]
Gaspar HA, Morhy SS. The Role of Focused Echocardiography in Pediatric Intensive Care: A Critical Appraisal. BioMed research international. 2015:2015():596451. doi: 10.1155/2015/596451. Epub 2015 Oct 28 [PubMed PMID: 26605333]
Williams JL, Raees MA, Sunthankar S, Killen SAS, Bichell D, Parra DA, Soslow JH. Sedated Echocardiograms Better Characterize Branch Pulmonary Arteries Following Bidirectional Glenn Palliation with Minimal Risk of Adverse Events. Pediatric cardiology. 2020 Jun:41(5):955-961. doi: 10.1007/s00246-020-02342-x. Epub 2020 Apr 4 [PubMed PMID: 32248280]