Sonography Abdominal Vascular Assessment, Protocols, and Interpretation
Sonography Abdominal Vascular Assessment, Protocols, and Interpretation
Introduction
Several pathological conditions impact the abdominal vasculature, including aneurysms, dissections, atherosclerotic disease, aneurysms, and flow-limiting states. Abdominal aortic aneurysms (AAAs), for instance, are a potentially life-threatening disease process that affects at least 2% of adults over the age of 50.[1] Ultrasound serves as the ideal diagnostic modality for detecting AAAs due to its high sensitivity and specificity.[2][3]
While ultrasound is an excellent method for detecting the presence of AAAs, its role is more limited in assessing other pathologies such as aneurysmal rupture, aortic dissection, abdominal aortic stenosis, major branch pathology, and IVC pathology.
There is no universal protocol for abdominal vascular imaging. This article's proposed protocol is a general guideline that can be used to evaluate the aorta, inferior vena cava, mesenteric arteries, and iliac arteries for sonographic findings consistent with pathology.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
The abdominal aorta is the primary vessel that delivers oxygenated blood to our lower extremities and abdominal organs. It begins just beneath the diaphragm and terminates at the bifurcation of the common iliac arteries.[4] The IVC is the major vessel that carries deoxygenated blood from its tributaries within the abdomen, pelvis, and lower extremities to the heart. The IVC courses parallel and to the right of the aorta. Its course begins at the confluence of the iliac veins and terminates at the right atrium.
Indications
- Screening for abdominal aortic aneurysms
- Suspected iliac aneurysms
- Hypotensive patients
- Absent pulses in the distal extremities
- Suspected mesenteric ischemia
Contraindications
There are no absolute contraindications to performing this procedure.
Equipment
Transducer Selection and Orientation
Transducers with frequencies ranging from 1.0 to 5.0 MHz will provide adequate images for the typical adult patient. Note that the frequency should be adjusted according to the body habitus of the patient being examined.
Personnel
This procedure can be performed by registered diagnostic medical sonographers (RDMS) or by ultrasound-trained physicians.
Preparation
The patient should be in a fasting state, usually 4 to 6 hours, before imaging. During the exam, the patient should ideally be placed in the supine position. If the sonographer cannot obtain adequate images, the patient can be repositioned in the right or left lateral decubitus position. The exam should be performed in a darkened room.
Technique or Treatment
Image Acquisition
The depth should be set so that the vertebrae can be visualized posterior to the aorta.
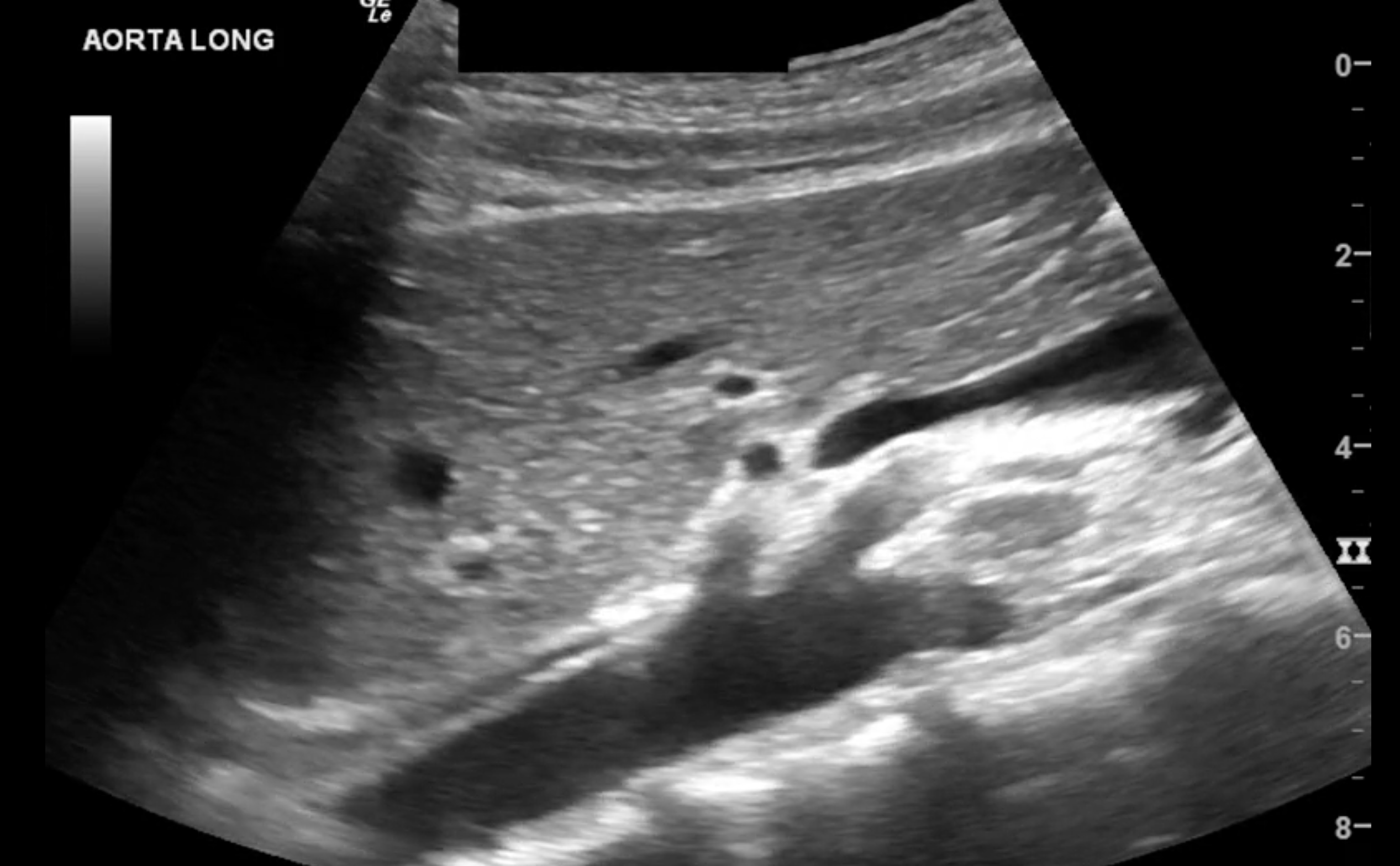
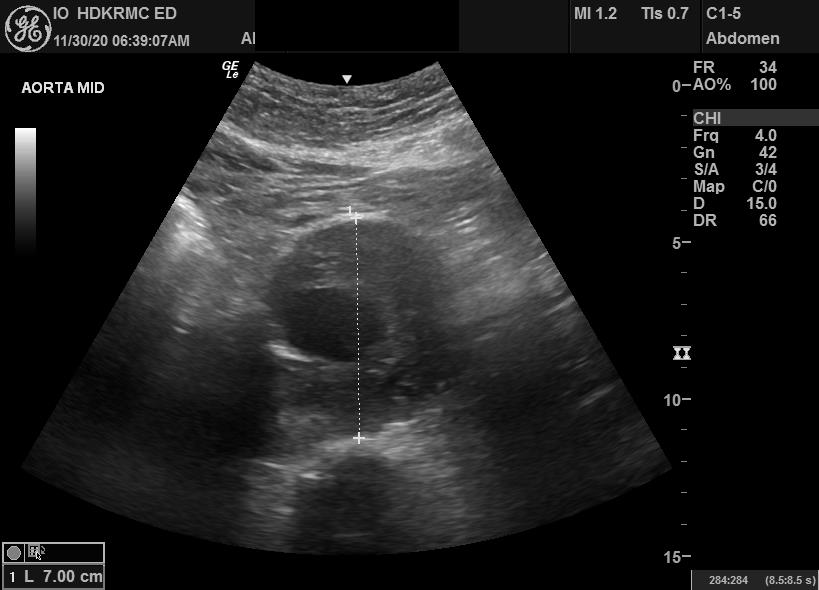
In the short axis, record a sweep of the entire length of the abdominal aorta spanning from the diaphragm to the bifurcation of the common iliac vessels. The widest portion of the aorta should be identified and measured from the anterior leading edge to the posterior leading edge of the vessel.
In the long axis, record a sweep of the entire length of the abdominal aorta spanning from the diaphragm to the bifurcation of the common iliac vessels. The widest portion of the aorta should be identified in the long axis. It should then be measured from the anterior leading edge to the posterior leading edge of the vessel.
Whenever an aneurysm is identified, its geographic relationship to the renal arteries (e.g., infrarenal or suprarenal) should be documented along with the distance from the renal arteries and the aneurysm’s diameter.
The right and left common iliac arteries should be visualized. Their greatest anterior-posterior diameter should be measured in the short and long axis from leading edge to leading edge.
Any pathological finding that the sonographer discovers, such as thrombi, flaps, should be recorded. If an endograft is present, its proximal and distal attachment points should be noted. The sonographer should also apply color and spectral doppler within the lumen of the graft and the aneurysmal sac.
Additional Images of the Mesenteric Arteries, If Clinically Indicated
The sonographer can attempt to obtain spectral doppler tracings of the mesenteric arteries. A doppler angle less than or equal to 60 degrees should be utilized to maximize the accuracy of the Doppler velocities.[5] Peak systolic velocities of the celiac artery, superior mesenteric artery, and inferior mesenteric artery should be measured.
Additional Images of the Inferior Vena Cava (IVC), If Clinically Indicated
The sonographer should identify the inferior vena cava in its short and long axes. In the long axis, the anteroposterior diameter should be measured 2 to 3 cm caudal from the vessel’s confluence at the right atrium. The diameter should be measured during both inspiration and expiration.
Image Optimization and Accuracy
Bowel gas can often preclude the sonographer from obtaining adequate images. The sonographer can apply steady pressure to the abdomen to attempt to displace bowel gas.[6] Another method to displace bowel gas is to have the patient lay in a lateral decubitus position. Lastly, if images obtained from the anterior abdominal wall are inadequate, the sonographer can attempt to visualize the aorta from a coronal view between the anterior axillary line and the midaxillary line.
The sonographer should visualize and measure the aorta in both short and long axes to ensure that aneurysms are not missed and ensure accurate measurements. Measuring in the long axis can lead to the cylinder tangent effect, which refers to the possibility of underestimating the diameter of a cylindrical structure when accidentally measuring lateral to the vessel’s midline.[6] Measuring in the short plane places the sonographer at theoretical risk of overestimating the aortic diameter if the measurement is accidentally taken at an oblique angle.[7]
In settings where it is not possible to store video clips of sonographic images, still, images of the abdominal aorta should be obtained in two planes at the following levels: the celiac trunk, the superior mesenteric artery, and proximal to the iliac bifurcation.
Complications
There are no complications associated with this procedure.
Clinical Significance
Pathological Findings of the Abdominal Aorta
Abdominal Aortic Aneurysm
Ultrasound is highly sensitive and specific for the detection of abdominal aortic aneurysms.[3] Note that there is some variability in the accepted normal values of the abdominal aortic diameter.
Following the American College of Radiology, this author recommends that abdominal aortic diameters ≥ 3 cm should be considered aneurysmal.[8] Note that 3 cm is derived from the assumption that the normal adult abdominal aortic diameter is approximately 2 cm. A vessel is considered aneurysmal once its diameter is 1.5 times larger than its contiguous aortic segment of normal diameter.[8] Diameters between 2 to 3 cm are classified as ectactic because their dilatation is less than 1.5 times larger than the normal diameter.[8] The annual risk of rupture of abdominal aortic aneurysms rises exponentially with increasing diameter.
Aneurysms are commonly classified as either fusiform (involving the entire diameter of the vessel) or saccular (a limited portion of the diameter is involved). This distinction is useful because the differential varies depending on the type of aneurysm.
Intraluminal Pathology
The abdominal aorta lumen is anechoic sonographically. Any other intraluminal appearance is likely pathological.
Hypoechoic structures adjacent to the aortic wall are likely intraluminal thrombi. Intraluminal thrombi can make the aortic lumen appear smaller; for this reason, the aortic diameter must be measured from the leading edge to the leading edge. There have also been case reports in which point-of-care ultrasound was used to identify complete aortic occlusion.[9]
A linear echogenic structure within the lumen of the aorta, called an intimal flap, separates the aorta into two distinct lumens is thought to be highly specific for aortic dissection.[10] Given the high morbidity and mortality associated with aortic dissections, the sonographer must be able to recognize this important clinical finding.
Note that the aorta becomes increasingly tortuous as people age. Sometimes, the sidewall of a tortuous aorta can appear similar to an intraluminal clot. This is further reason to image the aorta in two orthogonal axes, as pathology is usually visible in both axes.
Post-Endovascular Graft Assessment
Sonographers should be familiar with the normal appearance of endovascular grafts, given this increasingly common procedure. The sonographer should assess for fluid collections at the site of the graft. Sonographers should also evaluate for endoleak by applying color and spectral Doppler and looking for flow within the aneurysm sac outside of the graft.[11]
Five Major Types of Endoleaks[12]
- Type I leaks are those that occur at the distal or proximal attachment sites.
- Type II endoleaks occur secondary to retrograde flow from aortic branch vessels into the sac.
- Type III endoleaks refer to those that occur due to the failure of the structural integrity of the graft itself.
- Type IV endoleaks occur due to the porosity of the stent.
- Type V endoleaks are those that occur when the aneurysm expands.
A 2017 Cochrane review notes that the sensitivity and specificity of color duplex ultrasound (CDUS) range from 82% to 91% and 93% to 96%, respectively.[13] The Cochrane review also notes that contrast-enhanced ultrasonography (CEUS) is an increasingly utilized modality with better sensitivity and specificity than CDUS and may be useful as a routine surveillance strategy in the future.
Pathological Findings of the Iliac Arteries
Similar to the pathological findings discussed regarding the abdominal aorta, the iliac arteries can be aneurysmal, contain thrombi, or intraluminal flaps. The appearance of these findings is similar to those discussed above regarding the aorta. Note that the common iliac arteries are considered aneurysmal at ≥ 1.5 cm.[14]
Pathological Findings of the Mesenteric Arteries
Variables such as bowel gas, body habitus, and the timing of the patient’s most recent meal can make the interpretation of doppler tracings difficult. A wide range of normal peak systolic velocities has been described for the mesenteric arteries.[15][16] There is little consensus regarding the threshold at which peak systolic velocities correlate with a clinically significant degree of stenosis.[17] Findings should therefore be considered in conjunction with institutional thresholds and the patient’s clinical condition.
Pathological Findings of the IVC
When visualized sonographically, the IVC should be anechoic within its lumen. Hypoechoic thrombi or IVC filters are examples of pathological intraluminal findings.
Note that the anteroposterior diameter of the IVC is often used to attempt to predict fluid responsiveness in critically ill patients. Although IVC diameter and collapsibility estimate CVP, it has not consistently shown utility in predicting a patient’s intravascular volume status or fluid responsiveness.[18][19][20][21]
Enhancing Healthcare Team Outcomes
Should the sonographer have difficulty obtaining adequate images, this requires immediate discussion with the interprofessional care team and radiologist to consider alternative diagnostic modalities. Nurses should also be included in any discussion of the procedure, as well as the findings of the examination. This type of interprofessional healthcare team communication can help ensure more accurate diagnosis and subsequent treatment and improve patient outcomes. [Level 5]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Kent KC, Zwolak RM, Egorova NN, Riles TS, Manganaro A, Moskowitz AJ, Gelijns AC, Greco G. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. Journal of vascular surgery. 2010 Sep:52(3):539-48. doi: 10.1016/j.jvs.2010.05.090. Epub 2010 Jul 13 [PubMed PMID: 20630687]
Level 2 (mid-level) evidenceLaRoy LL, Cormier PJ, Matalon TA, Patel SK, Turner DA, Silver B. Imaging of abdominal aortic aneurysms. AJR. American journal of roentgenology. 1989 Apr:152(4):785-92 [PubMed PMID: 2646870]
Rubano E, Mehta N, Caputo W, Paladino L, Sinert R. Systematic review: emergency department bedside ultrasonography for diagnosing suspected abdominal aortic aneurysm. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2013 Feb:20(2):128-38. doi: 10.1111/acem.12080. Epub [PubMed PMID: 23406071]
Level 3 (low-level) evidenceFELLER I, WOODBURNE RT. Surgical anatomy of the abdominal aorta. Annals of surgery. 1961 Dec:154(6)Suppl(Suppl 6):239-52 [PubMed PMID: 13892214]
Expert Panels on Vascular Imaging and Gastrointestinal Imaging:, Ginsburg M, Obara P, Lambert DL, Hanley M, Steigner ML, Camacho MA, Chandra A, Chang KJ, Gage KL, Peterson CM, Ptak T, Verma N, Kim DH, Carucci LR, Dill KE. ACR Appropriateness Criteria(®) Imaging of Mesenteric Ischemia. Journal of the American College of Radiology : JACR. 2018 Nov:15(11S):S332-S340. doi: 10.1016/j.jacr.2018.09.018. Epub [PubMed PMID: 30392602]
Barkin AZ, Rosen CL. Ultrasound detection of abdominal aortic aneurysm. Emergency medicine clinics of North America. 2004 Aug:22(3):675-82 [PubMed PMID: 15301845]
Manning BJ, Kristmundsson T, Sonesson B, Resch T. Abdominal aortic aneurysm diameter: a comparison of ultrasound measurements with those from standard and three-dimensional computed tomography reconstruction. Journal of vascular surgery. 2009 Aug:50(2):263-8. doi: 10.1016/j.jvs.2009.02.243. Epub [PubMed PMID: 19631858]
Expert Panel on Vascular Imaging:, Reis SP, Majdalany BS, AbuRahma AF, Collins JD, Francois CJ, Ganguli S, Gornik HL, Kendi AT, Khaja MS, Norton PT, Sutphin PD, Rybicki FJ, Kalva SP. ACR Appropriateness Criteria(®) Pulsatile Abdominal Mass Suspected Abdominal Aortic Aneurysm. Journal of the American College of Radiology : JACR. 2017 May:14(5S):S258-S265. doi: 10.1016/j.jacr.2017.01.027. Epub [PubMed PMID: 28473082]
Bloom B, Gibbons R, Brandis D, Costantino TG. Point-of-care Ultrasound Diagnosis of Acute Abdominal Aortic Occlusion. Clinical practice and cases in emergency medicine. 2020 Feb:4(1):79-82. doi: 10.5811/cpcem.2019.11.44311. Epub 2020 Jan 23 [PubMed PMID: 32064433]
Level 3 (low-level) evidenceFojtik JP, Costantino TG, Dean AJ. The diagnosis of aortic dissection by emergency medicine ultrasound. The Journal of emergency medicine. 2007 Feb:32(2):191-6 [PubMed PMID: 17307632]
Level 3 (low-level) evidenceMaximus S, Skelly C, Milner R. Velocities of type II endoleaks on Doppler ultrasonography predict outcome. Journal of vascular surgery. 2020 May:71(5):1719-1725. doi: 10.1016/j.jvs.2019.07.078. Epub 2019 Oct 13 [PubMed PMID: 31619352]
Stavropoulos SW, Charagundla SR. Imaging techniques for detection and management of endoleaks after endovascular aortic aneurysm repair. Radiology. 2007 Jun:243(3):641-55 [PubMed PMID: 17517926]
Abraha I, Luchetta ML, De Florio R, Cozzolino F, Casazza G, Duca P, Parente B, Orso M, Germani A, Eusebi P, Montedori A. Ultrasonography for endoleak detection after endoluminal abdominal aortic aneurysm repair. The Cochrane database of systematic reviews. 2017 Jun 9:6(6):CD010296. doi: 10.1002/14651858.CD010296.pub2. Epub 2017 Jun 9 [PubMed PMID: 28598495]
Level 1 (high-level) evidenceRichards T, Dharmadasa A, Davies R, Murphy M, Perera R, Walton J. Natural history of the common iliac artery in the presence of an abdominal aortic aneurysm. Journal of vascular surgery. 2009 Apr:49(4):881-5. doi: 10.1016/j.jvs.2008.11.025. Epub 2009 Feb 23 [PubMed PMID: 19233599]
Aburahma AF, Mousa AY, Stone PA, Hass SM, Dean LS, Keiffer T. Duplex velocity criteria for native celiac/superior mesenteric artery stenosis vs in-stent stenosis. Journal of vascular surgery. 2012 Mar:55(3):730-8. doi: 10.1016/j.jvs.2011.10.086. Epub 2012 Feb 1 [PubMed PMID: 22301212]
Level 2 (mid-level) evidenceBowersox JC, Zwolak RM, Walsh DB, Schneider JR, Musson A, LaBombard FE, Cronenwett JL. Duplex ultrasonography in the diagnosis of celiac and mesenteric artery occlusive disease. Journal of vascular surgery. 1991 Dec:14(6):780-6; discussion 786-8 [PubMed PMID: 1960808]
Level 2 (mid-level) evidenceAbuRahma AF, Stone PA, Srivastava M, Dean LS, Keiffer T, Hass SM, Mousa AY. Mesenteric/celiac duplex ultrasound interpretation criteria revisited. Journal of vascular surgery. 2012 Feb:55(2):428-436.e6; discussion 435-6. doi: 10.1016/j.jvs.2011.08.052. Epub 2011 Dec 21 [PubMed PMID: 22195765]
Level 2 (mid-level) evidenceCorl K, Napoli AM, Gardiner F. Bedside sonographic measurement of the inferior vena cava caval index is a poor predictor of fluid responsiveness in emergency department patients. Emergency medicine Australasia : EMA. 2012 Oct:24(5):534-9. doi: 10.1111/j.1742-6723.2012.01596.x. Epub 2012 Sep 7 [PubMed PMID: 23039295]
Dipti A, Soucy Z, Surana A, Chandra S. Role of inferior vena cava diameter in assessment of volume status: a meta-analysis. The American journal of emergency medicine. 2012 Oct:30(8):1414-1419.e1. doi: 10.1016/j.ajem.2011.10.017. Epub 2012 Jan 4 [PubMed PMID: 22221934]
Level 1 (high-level) evidenceFeissel M, Michard F, Faller JP, Teboul JL. The respiratory variation in inferior vena cava diameter as a guide to fluid therapy. Intensive care medicine. 2004 Sep:30(9):1834-7 [PubMed PMID: 15045170]
Ciozda W, Kedan I, Kehl DW, Zimmer R, Khandwalla R, Kimchi A. The efficacy of sonographic measurement of inferior vena cava diameter as an estimate of central venous pressure. Cardiovascular ultrasound. 2016 Aug 20:14(1):33. doi: 10.1186/s12947-016-0076-1. Epub 2016 Aug 20 [PubMed PMID: 27542597]