Fluoroscopic Angiography Assessment, Protocols, and Interpretation
Fluoroscopic Angiography Assessment, Protocols, and Interpretation
Introduction
Fluoroscopy-guided catheter angiography is an interventional procedure that uses percutaneous access of arteries with needles and catheters to inject contrast for vessel opacification.[1] This procedure may be diagnostic or therapeutic. While some providers use angiography as a general term to include visualization of arteries, veins, or lymphatics, this article uses the term angiography to refer solely to the visualization of arteries, also known as arteriography. The terms venography and lymphangiography refer to veins and lymphatic vessels, respectively. Nevertheless, the principles behind angiography are widely applicable to other vessel types.
Sven Ivar Seldinger’s discovery of a technique in 1953 which described the substitution of a needle or trocar by a percutaneous catheter, since called the Seldinger technique, allowed for the possibility of catheter angiography and the birth of Interventional Radiology as a specialty.[2] The following decades gave rise to several non-invasive angiographic advancements, including computed tomographic and magnetic resonance angiography.[1]
Catheter angiography remains the gold-standard for a wide variety of pathologies. Today, catheter angiography is used to interrogate arteries in nearly every part of the human body, including the brain, neck, heart, chest, abdomen, pelvis, and extremities. Applications of catheter angiography are immense and include identification of arteriovenous malformations, aneurysms, atherosclerosis, embolisms, dissections, congenital abnormalities, stenosis, hemorrhage, and other arterial pathologies. Angiography may help guide implantation of stents, grafts, or provide assessment before surgery, chemoembolization, or internal radiation therapy. Fluoroscopy is undoubtedly the most important tool for an interventionalist. Herein, the general principles of fluoroscopy-guided catheter angiography are described, with a focus on the appropriate use of fluoroscopy-guidance for the diagnosis of arterial pathology.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
A review of the complete arterial anatomy is beyond the scope of this article. However, there are several important anatomical considerations that the interventionalist must be aware of when performing fluoroscopy-guided angiography. Every fluoroscopy machine contains an x-ray source beneath the patient and an intensifier/receptor unit above the patient.[3]
The utilization of oblique images is relevant to fluoroscopic anatomy. When the C-arm is positioned in a left anterior oblique (LAO) orientation, the receptor moves to the patient’s left. The interventionalist must learn how oblique orientation relates to the anterior-posterior anatomical relationship amongst structures. For a supine patient, an LAO projection will cause anterior structures to move to the left side of the display while posterior structures move to the right. Likewise, in a right anterior oblique projection (RAO), anterior structures move to the right side of the display while posterior structures move to the left. An example of this technique's importance is the determination of whether a wire and catheter placed by trans-radial access are pointing to the ascending or descending aorta.
Fluoroscopy-guided angiographic images are a two-dimensional representation of three-dimensional structures. This simplification leads to several difficulties for new interventionalists. The anterior-posterior orientation of a catheter inside the artery may be difficult to discern, especially for images taken in AP view. Via a femoral approach, movement of the catheter in a clockwise direction would result in the catheter moving from patient left to right if the catheter is pointing posteriorly.[3] Similarly, if the catheter moves to the patient’s left when rotated in a clockwise direction, then the catheter is pointing anteriorly. The opposite of this is true when procedures are performed via the radial artery, as the catheter passes over the fulcrum of the arm as it descends the aorta.[3]
Indications
Cerebral Angiography
- Aneurysm
- Brain tumors
- Embolism or thrombosis
- Dissection
- Vasculitis
- Arteriovenous malformation
Spinal Angiography[1]
- Tumors of the spine and spinal cord
- Traumatic spine injury
- Preoperative evaluation
Pulmonary Angiography
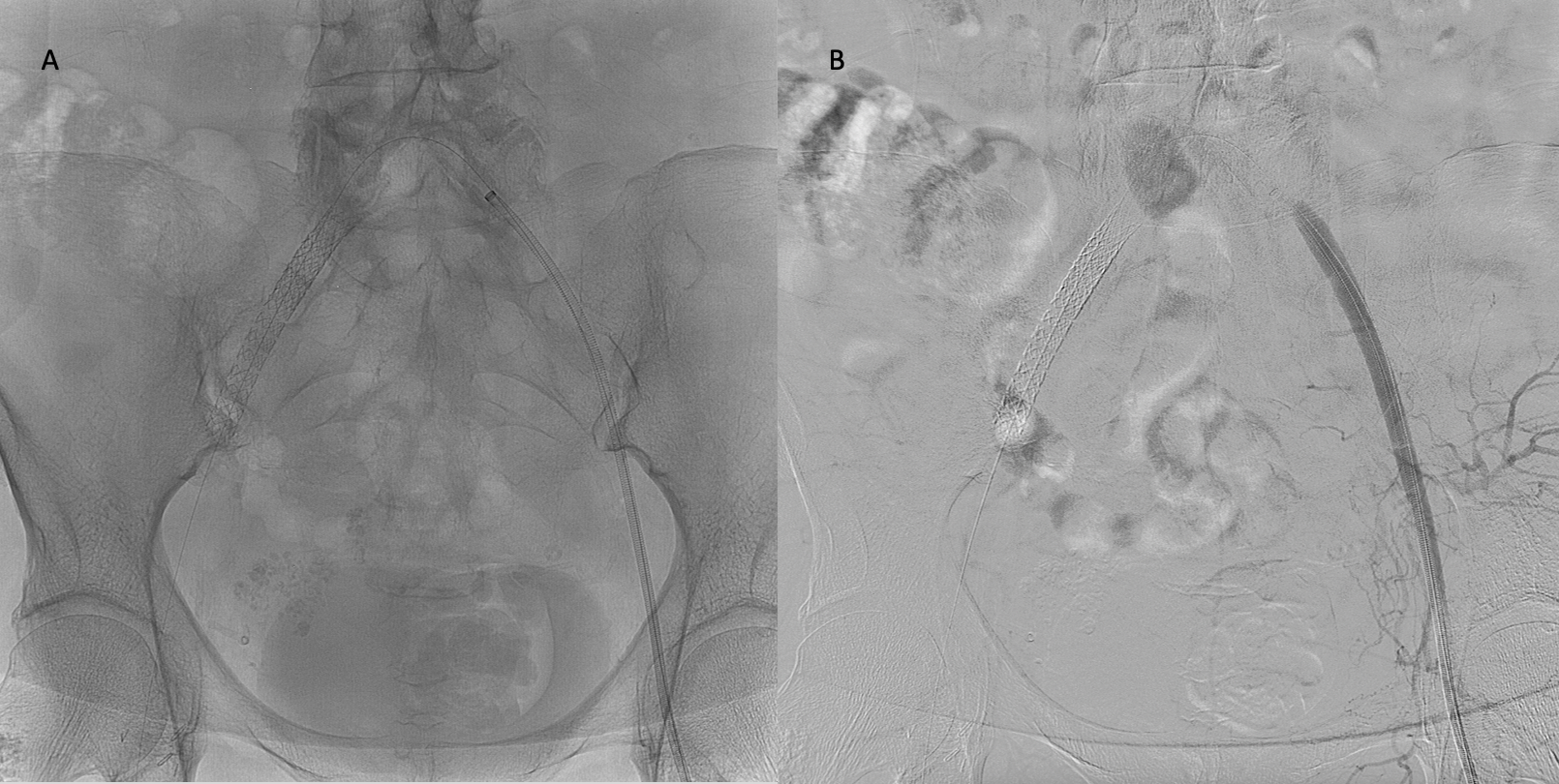
- Non-diagnostic CT angiography in the setting of suspected acute pulmonary embolism (see Image. Pulmonary Angiography Indicating Pulmonary Embolism)
- Vasculitis, congenital and/or acquired vessel abnormalities
- Pre-interventional evaluation before thrombolysis or thrombectomy
Bronchial Angiography[1]
- Pre-embolization assessment
- Assessment of collateral pulmonary artery circulations
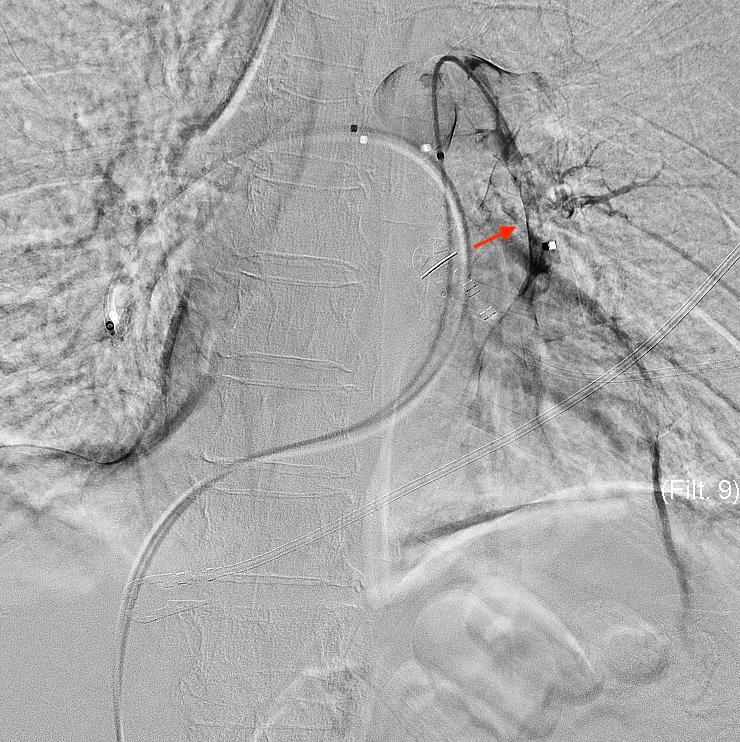
- Congenital anomalies (see Image. Selected Catheterization Angiography, Right Bronchial Artery)
Aortography
- Aortography is the initial step before many interventional procedures
Abdominal Visceral Angiography[1]
- Chronic or acute gastrointestinal hemorrhage, especially in a hemodynamically unstable patient who fails to respond to colonoscopy
- Abdominal tumors
- Pre-operative or post-operative assessment for transplantation
Renal Angiography
- Connective tissue disorders
- Vasculitis
- Pre-interventional therapy assessment
Pelvic Angiography
- Pelvic or gastrointestinal bleeding
- Arterial occlusive disease leading to male impotence
- Pre-interventional therapy assessment
Extremity Angiography
- Assessment of peripheral arterial disease
- Aneurysms, emboli, thrombosis
- Assessment of surgical bypass grafts
- Assessment of fistulas
- Vascular malformations
- Vasculitis
- Extremity tumors
- Subclavian steal syndrome
- Thoracic outlet syndrome
Contraindications
Specific contraindications depend upon the type of angiography being performed. In general, angiography is contraindicated if the diagnostic information obtained will not alter clinical management or obtainable information via a less invasive procedure. Contrast sensitivity, hypotension, coagulopathy, renal disease, and heart failure are relative contraindications that should be considered before the intervention.
Equipment
Fluoroscopy-guided catheter angiography occurs in an interventional suite. The suite should be equipped with the necessary radiographic equipment, with sufficient parameters to perform procedures.[4] Parameters to consider include receptor size, recording modes such as digital subtraction or cine, X-ray focal spot, heat load, cooling capacity, generator capacity, storage capacity, and software packages.[4] For interventional neuroradiology, biplane imaging and three-dimensional capabilities are recommended.[4]
Other equipment includes patient physiologic monitors for oxygen saturation, blood pressure, heart rate, arterial pressures, medication storage space, resuscitation cart, scrub sink, and ceiling-mounted surgical light.[1] The type of access kits, wires, catheters, and other interventional devices depend on the specific type and location of angiography being performed.
Personnel
A minimum of one nurse with critical care, patient monitoring, and intravenous sedation experience should be assigned to each interventional suite. Catheter angiography requires at least one radiology technologist with experience in preparing the angiography suite, assisting in the procedure, taking inventory management, and handling post-procedure image processing and archiving.[1]
Preparation
The physician must make sure that they are informed about patient allergies, particularly those involving iodinated contrast material. A nurse or technologist must make sure that appropriate intravenous access is maintained. The area to be utilized for vascular arterial access should be shaved, cleaned, and appropriately anesthetized. Fluoroscopy-guided angiography is a sterile procedure, and the appropriate hats, gowns, gloves, and masks should be utilized. Physicians and nurses should work together to ensure that the patient is not pregnant so that fetal radiation exposure does not take place.
Technique or Treatment
Obtaining appropriate diagnostic image quality while decreasing both operator and patient radiation exposure is critical to fluoroscopy-guided angiography. The main source of radiation to the patient is direct exposure from the beam.[5] Exposure to the operator primarily occurs from radiation scatter.[5]
Collimation should be performed whenever possible. Collimation decreases the cross-sectional area of the X-ray beam, thereby decreasing the radiation dose to the patient. The reduction in irradiative volume decreases patient scatter and results in lower operator dose and improved image quality via improved contrast and a reduction of noise.[3]
An air gap is defined as the distance from the image receptor to the patient.[3] This gap should be decreased as much as possible during fluoroscopy-guided angiography. Automatic brightness control automatically adjusts current (mAs) and voltage (kVp) to ensure consistent clarity and brightness of images.[6] By reducing the air gap, X-ray beams have less distance to travel before hitting the image receptor. Therefore, the receptor receives less scatter. With less scatter hitting the imaging receptor, the automatic brightness control will not have to increase voltage or current to maintain high-quality images.[3]
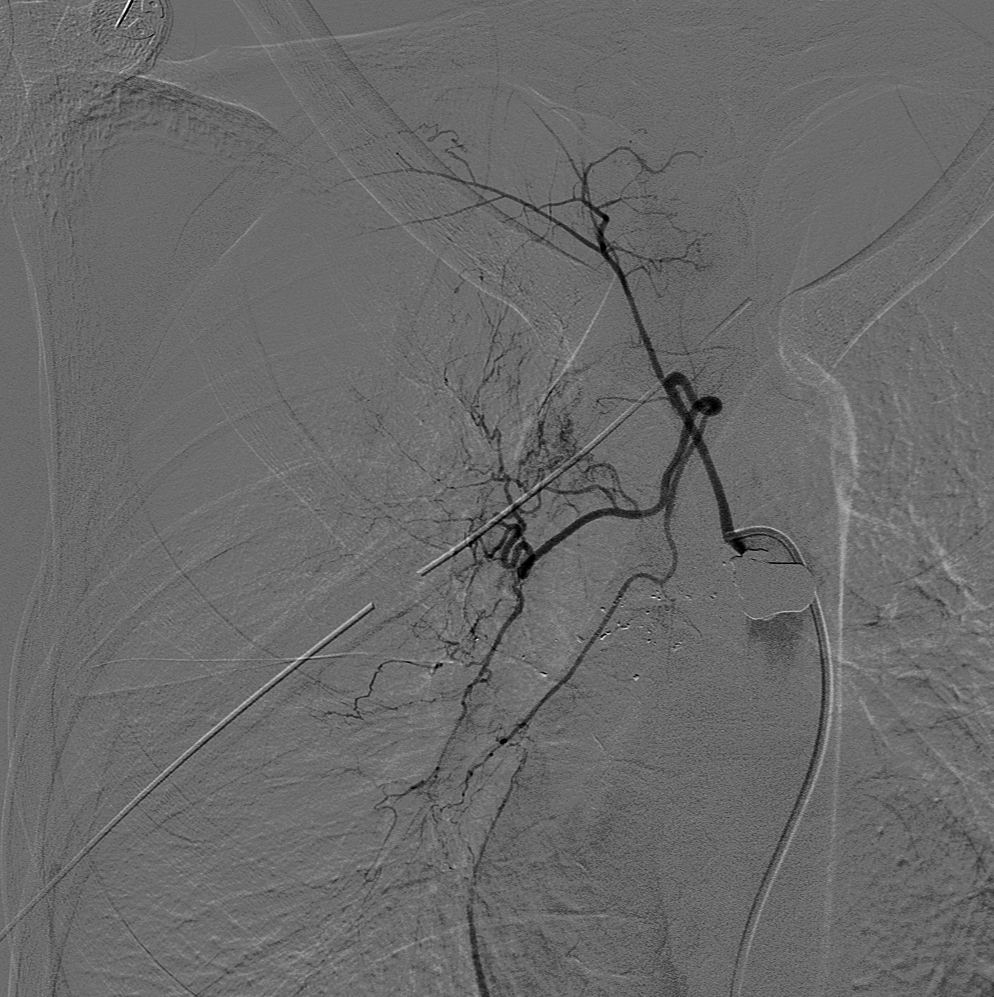
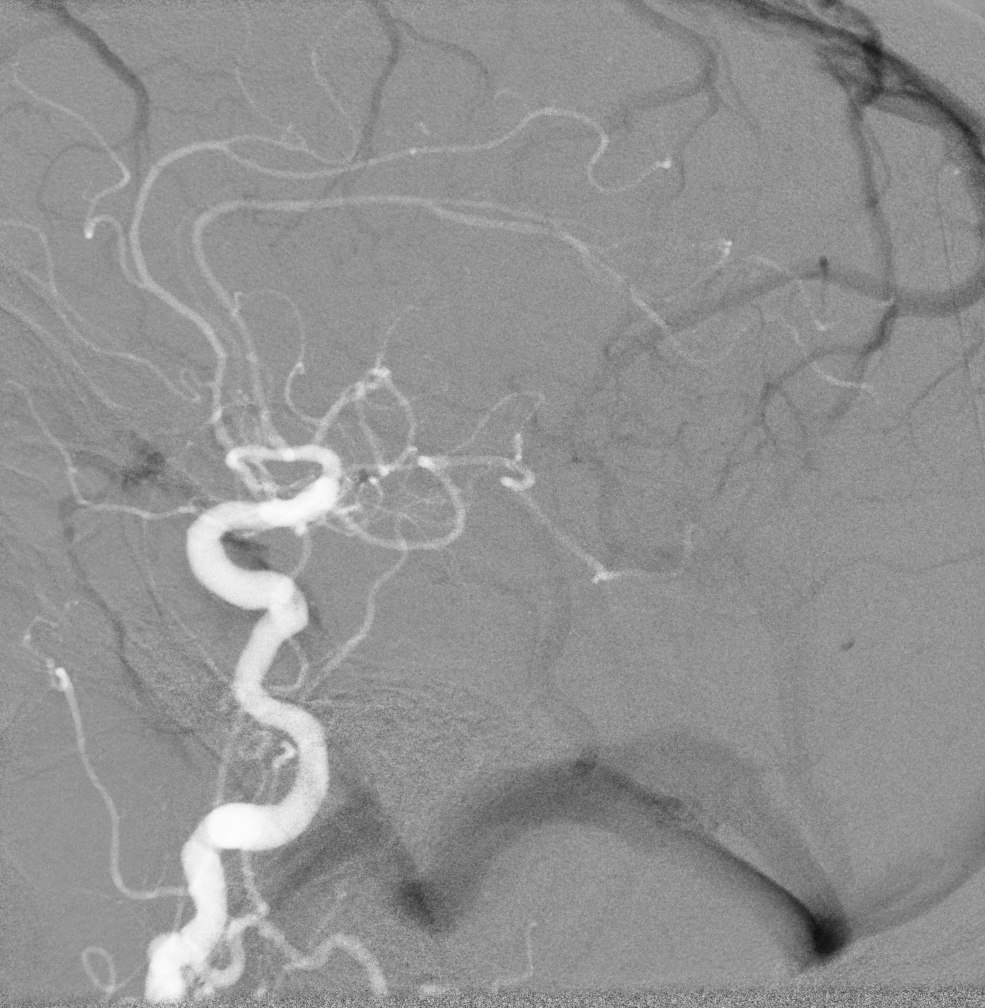
Digital subtraction angiography (DSA) is real-time subtraction between pre-contrast and postcontrast images to visualize vessels (see Image. Normal Fluoroscopy, Digital Subtraction Angiography). By removing superimposed distracting tissues from the image, DSA can be used to better visualize vessels throughout the entire body.[7] Another often-used technique in fluoroscopy-guided angiography is road mapping. First, a DSA image with maximum vessel opacification is obtained to create a road map mask. This mask is then subtracted from real-time fluoroscopic images to create live fluoroscopy images superimposed on a static image of the vessel of interest. Road-mapping allows for better visualization of small wires and improves maneuvering through the arterial system.[7] While both road mapping and DSA are useful, they result in increased radiation dose via the fluoroscopy machine.[3] Thus, DSA and road mapping should be limited. See Image. Roadmapping, Cerebral Angiography.
The entire peripheral arterial system can be imaged with a single injection of contrast via the use of a stepping gantry or table DSA technique. In both stepping table and stepping gantry, pre-contrast and post-contrast images are taken. In the stepping table technique, the patient table moves the arterial anatomy over the field of view in synchrony with the contrast bolus. The stepping gantry technique is similar, except the patient table is fixed while the mobile gantry moves over the arterial anatomy of interest.[7]
Fluoroscopy-guided angiography requires injection of contrast medium into the arteries, as appropriate contrast opacification of arteries is essential for high-quality images. The rate of contrast injection depends on the vessel catheterized and is described as x milliliters per second for a total of y milliliters. For example, the aortic arch can be injected at 20 to 25 for 30 to 50, meaning 20 to 25 milliliters per second for a total volume of 30 to 50 milliliters. Below are some commonly used injection rates. Note that these are rough estimates and that weight-based contrast injection rates may be used.
- Aortic arch: 20 to 25 mL per second for 30 to 50 mL total
- Abdominal aorta: 20 to 25 mL per second for 50 mL total
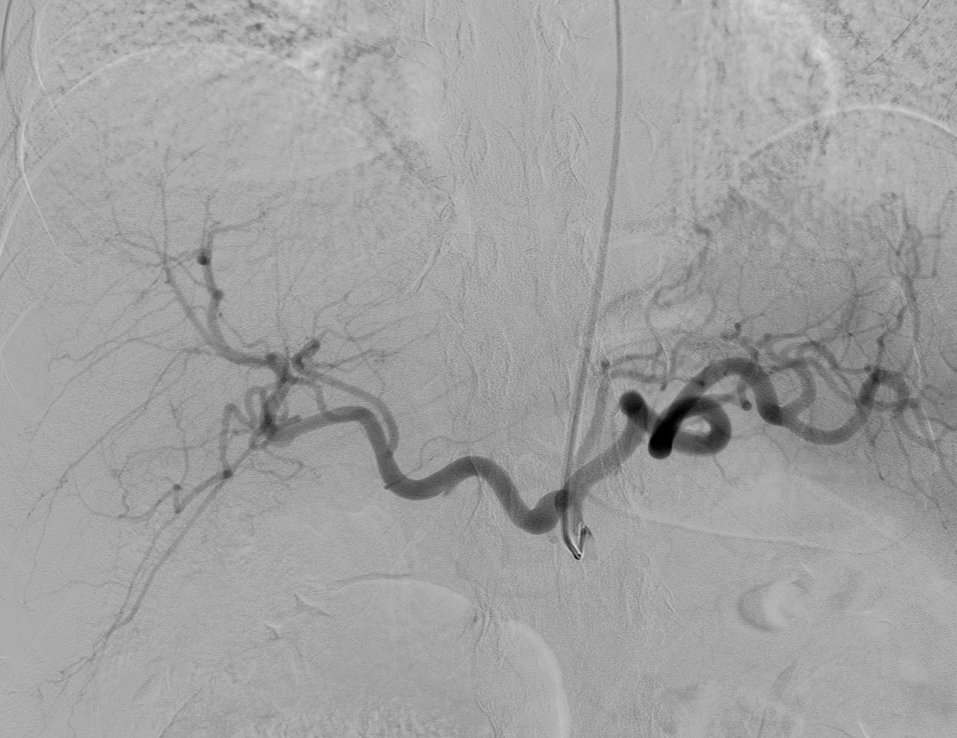
- Celiac: 5 to 7 mL per second for 30 to 60 mL total (see Image. Fluoroscopy-Guided Angiography With Selective Catheterization of the Celiac Artery)
- Splenic: 5 to 6 mL per second for 30 to 50 mL total
- Hepatic: 4 to 15 mL per second for 15 to 30 mL total
- Left gastric: 3 to 4 mL per second for 6 to 16 mL total
- Gastroduodenal: 3 to 4 mL per second for 6 to 16 mL total
- Superior mesenteric: 5 to 7 mL per second for 30 to 60 mL total
- Inferior mesenteric: 3 to 5 mL per second for 9 to 20 mL total
- Pulmonary artery: 25 to 30 mL per second for a total of 2 seconds
- Pelvic angiography: 7 to 15 mL per second for a total of 2 to 4 seconds
Injection rates may need to be increased to opacify abnormalities such as aneurysms. Ensuring a sufficient flow rate and sufficient dilution of contrast material is oftentimes difficult to do by hand. Therefore, dedicated automatic injection systems that can be configured to specific parameters have been produced. Automatic injectors ensure that sufficient vessel opacification occurs, and the images produced are usually of higher quality than those that can be produced by hand.
Complications
Fluoroscopy-guided catheter angiography complications are uncommon and can be divided into four groups: percutaneous access-site, catheter-related, systemic, and radiation-related. Access-site hematoma is unusual but may occur in up to 10% of patients.[8] Major hematoma requiring further intervention occurs in approximately 1.7% of axillary punctures and 0.5% of femoral punctures.[8] Dissection or thrombus may occlude the access site.[1] Pseudoaneurysm and arteriovenous fistula also occur infrequently. Ultrasound-guided arterial access has decreased the frequency of these access-related complications. Antibiotic prophylaxis is generally not required for catheter angiography, given the rarity of puncture site infection.[1]
Catheter-related complications include subintimal guidance of guidewires or catheters, dissections, or embolization. These complications occur in less than 0.5% of cases.[1]
Systemic complications are seen in less than 1% of angiographic cases.[1] Nausea, vomiting, and vasovagal reactions are most frequent. A vasovagal reaction should not be mistaken for an allergic reaction to contrast material. Allergic reactions occur in approximately 4% of angiographic procedures and include urticaria, edema, and wheezing.[1] True allergic reactions occur more frequently with higher-osmolar contrast agents. Contrast-induced nephropathy (CIN) is characterized by a 50% increase or greater than 0.3mg/dL increase in serum creatinine that occurs within 48 hours after contrast administration. The incidence of CIN ranges from 0.3% to 2.3% in angiography.[1] CIN has a higher incidence in patients with renal insufficiency, diabetes, and dehydration.[9]
Reports of fluoroscopy-related skin injuries began to increase in the early 1990s and coincided with the increased utilization of fluoroscopy-guided interventions.[10] Skin reactions may range from mild to severe. Radiation dose, size of the irradiated skin surface, and the interval between irradiations affect the degree of dermal damage.[10] Interventionalists must reduce both patient and operator radiation exposure whenever possible.
Clinical Significance
While the full breadth of arterial pathologies diagnosable by angiography is beyond the scope of this article, commonly encountered pathologies such as embolism, dissection, aneurysm, extravasation, and arteriovenous malformation will be reviewed.
Embolism
Arterial embolism is most commonly thrombotic, although gas, amniotic, tumor, foreign body, and septic embolism may also occur. Pulmonary embolism and thromboembolic stroke occur as thrombotic material is carried distally downstream from its original location. On angiography, embolism may be diagnosed when a partial luminal filling defect or an abrupt cut-off of an artery is visualized.
Dissection
On angiography, vessel wall irregularity or a linear non-filling defect within the lumen is diagnostic for dissection. Dissection may occur as a consequence of arterial access, thus, an initial angiogram after percutaneous access should always be taken.
Aneurysm
Arterial aneurysms may be true or false. All three layers of the vessel, including the intima, media, and adventitia are involved in a true aneurysm. False aneurysms or pseudoaneurysms are bounded by the tunica adventitia. The risk of arterial rupture within a pseudoaneurysm is higher than that of true aneurysms. Aneurysms may be saccular, involving only a portion of the vessel wall, or fusiform, involving the entire vessel wall.
Bleeding
Active contrast extravasation is diagnostic of bleeding. Angiography is routinely used to detect pelvic, gastrointestinal, and traumatic hemorrhages. When used for gastrointestinal hemorrhage, angiography can detect bleeding as small as 0.5 mL/minute.[11] The ability to intervene in a minimally invasive manner makes angiography an excellent diagnostic modality. Not only can a diagnosis be made, but interventional embolization of arterial bleeding can take place in real-time. The amount of contrast extravasation can be exceptionally small, and in pelvic trauma or gastrointestinal hemorrhage, prophylactic embolization is often used when the source of bleeding cannot be localized.
Arteriovenous Malformation
On angiography, arteriovenous malformations will appear as a collection of enlarged and irregular feeding arteries supplying a central nidus. The draining veins may be dilated and will show opacification in the early arterial phase. Cerebral angiography is the gold standard for the diagnosis of brain arteriovenous malformations. This should be performed using a bi-plane with a high acquisition rate due to rapid shunting.
Enhancing Healthcare Team Outcomes
Angiography requires an interventional suite with the appropriate staffing. Staff training and an interventional physician are critical to the standards of practice. The 2016 Consensus statement on staffing guidelines for interventional suites states that all interventional radiology staff members must work together as a unit. Each member is not interchangeable with nurses from other departments or other areas of radiology.[12](Level 1) Interventional procedures are a team-based effort, and thus the enhancement of healthcare outcomes depends on appropriate communication and appropriate staffing.[12]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Dariushnia SR, Gill AE, Martin LG, Saad WE, Baskin KM, Caplin DM, Kalva SP, Hogan MJ, Midia M, Siddiqi NH, Walker TG, Nikolic B, Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for diagnostic arteriography. Journal of vascular and interventional radiology : JVIR. 2014 Dec:25(12):1873-81. doi: 10.1016/j.jvir.2014.07.020. Epub 2014 Sep 18 [PubMed PMID: 25241301]
Level 2 (mid-level) evidenceBaum RA, Baum S. Interventional radiology: a half century of innovation. Radiology. 2014 Nov:273(2 Suppl):S75-91. doi: 10.1148/radiol.14140534. Epub [PubMed PMID: 25340439]
Taslakian B, Ingber R, Aaltonen E, Horn J, Hickey R. Interventional Radiology Suite: A Primer for Trainees. Journal of clinical medicine. 2019 Aug 30:8(9):. doi: 10.3390/jcm8091347. Epub 2019 Aug 30 [PubMed PMID: 31480308]
American College of Radiology.,American Society of Interventional and Therapeutic Neuroradiology.,Society of Interventional Radiology., Practice Guideline for Interventional Clinical Practice. Journal of vascular and interventional radiology : JVIR. 2005 Feb; [PubMed PMID: 15713914]
Level 1 (high-level) evidenceMiller DL, Vañó E, Bartal G, Balter S, Dixon R, Padovani R, Schueler B, Cardella JF, de Baère T, Cardiovascular and Interventional Radiology Society of Europe, Society of Interventional Radiology. Occupational radiation protection in interventional radiology: a joint guideline of the Cardiovascular and Interventional Radiology Society of Europe and the Society of Interventional Radiology. Journal of vascular and interventional radiology : JVIR. 2010 May:21(5):607-15. doi: 10.1016/j.jvir.2010.01.007. Epub [PubMed PMID: 20430294]
Lin PJ. Technical advances of interventional fluoroscopy and flat panel image receptor. Health physics. 2008 Nov:95(5):650-7. doi: 10.1097/01.HP.0000326336.40775.94. Epub [PubMed PMID: 18849699]
Level 3 (low-level) evidencePooley RA, McKinney JM, Miller DA. The AAPM/RSNA physics tutorial for residents: digital fluoroscopy. Radiographics : a review publication of the Radiological Society of North America, Inc. 2001 Mar-Apr:21(2):521-34 [PubMed PMID: 11259716]
Cragg AH,Nakagawa N,Smith TP,Berbaum KS, Hematoma formation after diagnostic angiography: effect of catheter size. Journal of vascular and interventional radiology : JVIR. 1991 May; [PubMed PMID: 1799761]
Level 1 (high-level) evidenceMcCullough PA, Wolyn R, Rocher LL, Levin RN, O'Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. The American journal of medicine. 1997 Nov:103(5):368-75 [PubMed PMID: 9375704]
Balter S, Hopewell JW, Miller DL, Wagner LK, Zelefsky MJ. Fluoroscopically guided interventional procedures: a review of radiation effects on patients' skin and hair. Radiology. 2010 Feb:254(2):326-41. doi: 10.1148/radiol.2542082312. Epub [PubMed PMID: 20093507]
Syed MI, Shaikh A. Accurate localization of life threatening colonic hemorrhage during nuclear medicine bleeding scan as an aid to selective angiography. World journal of emergency surgery : WJES. 2009 May 27:4():20. doi: 10.1186/1749-7922-4-20. Epub 2009 May 27 [PubMed PMID: 19580686]
Baerlocher MO, Kennedy SA, Ward TJ, Nikolic B, Bakal CW, Lewis CA, Winick AB, Niedzwiecki GA, Haskal ZJ, Matsumoto AH. Society of Interventional Radiology Position Statement: Staffing Guidelines for the Interventional Radiology Suite. Journal of vascular and interventional radiology : JVIR. 2016 May:27(5):618-22. doi: 10.1016/j.jvir.2016.02.010. Epub 2016 Mar 4 [PubMed PMID: 26952124]