Indications
As of January 24, 2022, the United States Food and Drug Associated (FDA) fact-sheet for the drug etesevimab states that "due to the high frequency of the Omicron variant, bamlanivimab and etesevimab, administered together, are not currently authorized for use in any U.S. region because of markedly reduced activity against the Omicron variant. These drugs may not be administered for treatment or post-exposure prevention of COVID-19 under the Emergency Use Authorization until further notice by the Agency." This article is for historical purposes only to review the indications, mechanism, and administration of this therapy when it was first developed.
Several neutralizing monoclonal antibodies to SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) are under clinical trials. Neutralizing monoclonal antibodies are recombinant proteins derived from the B cells of convalescent patients or humanized mice. Before SARS-CoV-2, monoclonal antibodies were developed to treat several viral infections, such as Ebola, Rabies, and Middle Eastern Respiratory Syndrome Coronavirus (MERS-CoV).[1][2][3][4] Etesevimab, bamlanivimab, casirivimab, and imdevimab are some of the monoclonal antibodies that specifically target SARS-CoV-2 undergoing current clinical studies. Etesevimab is a human recombinant monoclonal antibody that targets the SARS-CoV-2 surface spike protein receptor-binding domain. Etesevimab is commonly labeled as LY-CoV016 or as JS016.
Neutralizing monoclonal antibodies against SARS-CoV2 are available as two combination products.[5][6]
- Etesevimab and bamlanivimab
- Imdevimab and casirivimab.
The FDA (Food and Drug Administration) has approved both combination products through EUA (Emergency Use Authorizations), which can be used for mild to moderate SARS-CoV-2 in non-hospitalized patients at high risk for progressing to severe disease. FDA has revoked the use of bamlanivimab alone for SARS-COV-2 because of a high number of resistant SARS-CoV-2 strains. The combination product (etesevimab and bamlanivimab) can be used in adults and pediatrics if they are at least 12 years of age and weigh 40 kg or more with mild to moderate SARS CoV-2. The patients are considered high risk of progressing to severe COVID-19 or hospitalization if they satisfy one of the criteria below:
- Body mass index (BMI) 35 and above
- Age above 65 years
- Chronic Renal disease
- Pregnancy
- Diabetes Mellitus
- Immunosuppressive disease or immunosuppressive treatment
- Cardiovascular disease, including congenital heart disease
- Hypertension
- Chronic respiratory disorders, e.g., COPD (chronic obstructive pulmonary disease), Moderate to severe asthma, ILD (interstitial lung disease), and pulmonary hypertension
- Sickle cell disease
- Neurodevelopmental disorders, e.g., cerebral palsy
- Individuals who are medical-related technology-dependent (not related to COVID-19) such as tracheostomy, gastrostomy, or PPV (positive pressure ventilation)
The combination product (bamlanivimab and etesevimab) needs to be given as soon as possible after a positive test result and within ten days of symptom onset. The combination product (etesevimab and bamlanivimab) is not authorized for hospitalized patients with SARS CoV-2 or those patients requiring oxygen therapy.
Mechanism of Action
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Mechanism of Action
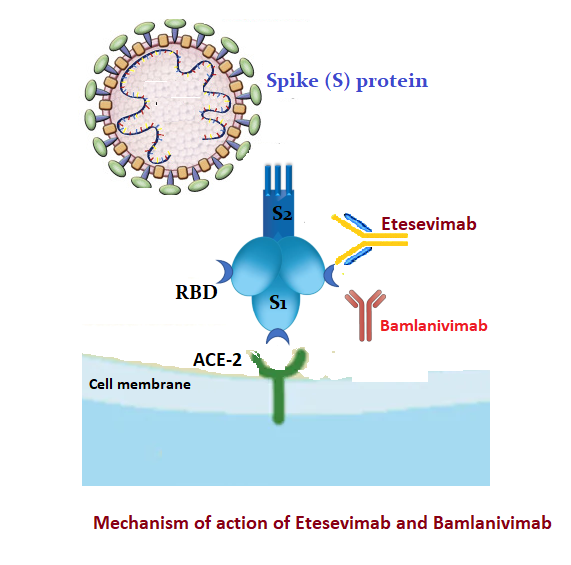
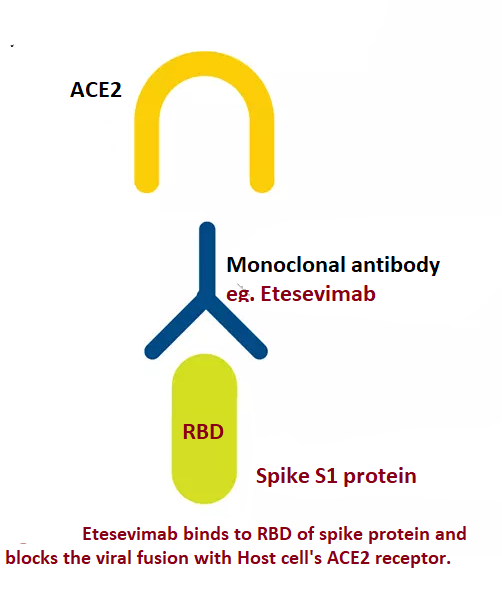
Many glycosylated S proteins layer the surface of SARS-CoV-2, which helps the virus bind to the ACE2 (Angiotensin-converting enzyme-2 receptor) of the host cell, facilitating viral entry. S protein has two subunits - S1 and S2. S1 subunit comprises NTD (N-terminal domain) and RBD (receptor-binding domain).[7] RBD of the S1 subunit binds to the host cell's ACE2. The RBD is a primary target for producing neutralizing monoclonal antibodies.
Etesevimab, bamlanivimab, casirivimab, and imdevimab are neutralizing monoclonal antibodies available against SARS-COV-2. Etesevimab and bamlanivimab bind to the overlapping epitopes in RBD (see Figures. Mechanism of Action of Etesevimab, Bamlanivimab, Mechanism of Action of Etesevimab). Casirivimab and imdevimab bind to non-overlapping epitopes of RBD.[8]
Administration
The authorized dose in adults and pediatric patients (12 years age and above; and weighing 40 kg or more) is 700 mg of bamlanivimab and 1400 mg etesevimab. The combination product comes in a package with the following.
- Bamlanivimab (700 mg) - 1 vial
- Etesevimab (700 mg) - 2 vials
- Sterile prefilled infusion bag - 0.9% sodium chloride (50 mL, 100 mL, 150 mL, or 250 mL)
The package should be stored in the refrigerator. The vials must be warmed to room temperature for 20 minutes before preparation without being exposed to direct heat. Withdraw 20 ml and 40 ml respectively of bamlanivimab and etesevimab from vials, then inject into a prefilled infusion bag. The preparation should not be shaken; instead, gently invert the infusion bag ten times to mix. The prepared solution can stay at room temperature for 7 hours, including the administration time, and it can be kept in the refrigerator for 24 hours. If refrigerated, the solution must be warmed to room temperature for 20 minutes before use.[9]
The compatibility of the combination product with any other medications and IV solutions other than 0.9% sodium chloride is not known. So it is advisable not to administer the prepared solution with any other medication. After the infusion is complete, flush the tubing with 0.9% sodium chloride to ensure the correct total dose is given.
Adverse Effects
The BLAZE-1 trial (randomized, double-blind trial) has witnessed a significant decrease in SARS-CoV-2 viral load among the patients who took bamlanivimab plus etesevimab compared to the placebo group. The hospitalization and death rate were significantly lower with the antibody group compared to the placebo group.[10][11]
- The most frequently reported adverse events were nausea and diarrhea.
- Infusion-related reactions can be possible with combination therapy. Signs and symptoms of infusion-related reactions include fever, chills, headache, myalgia, confusion, chest pain, breathing difficulty, wheezing, hypoxia, arrhythmia, hypotension, hypertension, nausea, angioedema, throat irritation, and urticaria. If an infusion-related reaction is suspected, consider slowing or stopping the infusion. Supportive care may be given.
- Anaphylaxis or severe hypersensitivity reactions can be possible with bamlanivimab monotherapy or combination therapy. Immediately discontinue the infusion and initiate supportive care.
- Clinical worsening of SARS-CoV2 symptoms like fever, hypoxia, respiratory distress after bamlanivimab monotherapy has been reported. Some of these events required hospitalization.
The following adverse events need to be reported within seven days to the FDA.
- Death
- A life-threatening adverse event
- Inpatient hospitalization
- A significant incapacity
- Medical or surgical intervention to prevent death
- A congenital anomaly
Contraindications
There are no strict contraindications for the usage of etesevimab. The following is the list of precautions or warnings while using Etesevimab and Bamlanivimab.
- Pregnant patients: Etesevimab and bamlanivimab can only be prescribed for pregnant patients with SARS-CoV-2 only if the benefit overbalances the risk for the duo (fetus and mother). Being an IgG antibody, both bamlanivimab and etesevimab can cross the placenta and reach the fetus. However, it is unclear if this placental transfer is beneficial or risk for the growing fetus.
- Lactating mother: No data so far has shown any potential adverse effects on the breastfed child.[12] Lactating mothers with SARS-CoV-2 should follow routine practices like wearing masks to avoid exposing the infant to SARS-CoV-2.
- Pediatric patients: Both etesevimab and bamlanivimab are not recommended for children less than 12 years or children weighing < 40 Kg.[13] The same adult dose regimen is followed for adolescents weighing >40 kg or >12 years age group.
- Geriatric patients: The pharmacokinetics of etesevimab and bamlanivimab in elderly patients is similar to young adults, so no dose adjustment is required.
- Hepatic and Renal Impairment: The dose adjustment is not required in renal impairment and mild hepatic impairment. However, more studies are required to study the safety among moderate or severe liver impairment.
Monitoring
Etesevimab and bamlanivimab infusion should be given only by a qualified healthcare professional in an outpatient facility with the ability to manage hypersensitive reactions. The patient should be monitored for anaphylaxis for at least 1 hour after completing the infusion.
Toxicity
Doses up to 7000 mg of bamlanivimab (10 times the FDA recommended dose) or 7000 mg of etesevimab (5 times the FDA recommended dose ) have been studied in clinical trials, which did not show any of the dose-limiting toxicity. If an overdose of bamlanivimab and etesevimab happens, treatment comprises supportive measures, including vitals and clinical status monitoring. As of now, there is no specific antidote available for the overdose.[9]
Enhancing Healthcare Team Outcomes
The interprofessional healthcare team must communicate to the patient, parent, or caregiver and provide the fact sheet copy before administering the combination therapy (bamlanivimab and etesevimab). The fact sheet includes the following information:
- The FDA has approved both etesevimab and bamlanivimab for emergency use to treat mild to moderate SARS CoV-2.
- It can be used in adults and pediatrics if they are 12 years old and above or weighing 40 kg or more and are at high risk of progressing to severe COVID-19 or hospitalization.
- The patient or parent/caregiver has the option to accept or refuse.
- The potential risks and benefits of medications are unknown.
- Patients treated with bamlanivimab and etesevimab should continue following the CDC guidelines (wear a mask, social distance, avoid sharing personal items, and frequent handwashing).
Etesevimab and bamlanivimab infusion should be given only by a qualified healthcare professional, and the patient should be monitored for anaphylaxis for at least 1 hour after completing the infusion. The health care team comprising the physician, nurse practitioner, and nurse are jointly responsible for reporting the medication errors and serious adverse events related to etesevimab and bamlanivimab treatment within seven days from the onset of the event.
The Secretary of the HHS (Department of Health and Human Services) has announced a public health emergency that justifies the EUA of etesevimab and bamlanivimab during the SARS CoV-2 pandemic. This EUA will be terminated when the HHS secretary finds that the situation justifying the EUA no longer exists.
When administering this novel therapy, all interprofessional team members must be in constant communication and operate as a functional unit, collaborating and coordinating their activities and information collection. This includes clinicians (including mid-level practitioners), specialists, nurses, and pharmacists, all operating as a cohesive unit to optimize patient outcomes. [Level 5]
Media
(Click Image to Enlarge)
References
Sizikova TE, Borisevich GV, Shcheblyakov DV, Burmistrova DA, Lebedev VN. [The use of monoclonal antibodies for the treatment of Ebola virus disease.]. Voprosy virusologii. 2018:63(6):245-249. doi: 10.18821/0507-4088-2018-63-6-245-249. Epub [PubMed PMID: 30641019]
Rijal P, Elias SC, Machado SR, Xiao J, Schimanski L, O'Dowd V, Baker T, Barry E, Mendelsohn SC, Cherry CJ, Jin J, Labbé GM, Donnellan FR, Rampling T, Dowall S, Rayner E, Findlay-Wilson S, Carroll M, Guo J, Xu XN, Huang KA, Takada A, Burgess G, McMillan D, Popplewell A, Lightwood DJ, Draper SJ, Townsend AR. Therapeutic Monoclonal Antibodies for Ebola Virus Infection Derived from Vaccinated Humans. Cell reports. 2019 Apr 2:27(1):172-186.e7. doi: 10.1016/j.celrep.2019.03.020. Epub [PubMed PMID: 30943399]
Ilina EN, Larina MV, Aliev TK, Dolgikh DA, Kirpichnikov MP. Recombinant Monoclonal Antibodies for Rabies Post-exposure Prophylaxis. Biochemistry. Biokhimiia. 2018 Jan:83(1):1-12. doi: 10.1134/S0006297918010017. Epub [PubMed PMID: 29534663]
Sivapalasingam S, Saviolakis GA, Kulcsar K, Nakamura A, Conrad T, Hassanein M, Sumner G, Elango C, Kamal MA, Eng S, Kyratsous CA, Musser BJ, Frieman M, Kantrowitz J, Weinreich DM, Yancopoulos G, Stahl N, Lipsich L. Human Monoclonal Antibody Cocktail for the Treatment or Prophylaxis of Middle East Respiratory Syndrome Coronavirus. The Journal of infectious diseases. 2022 May 16:225(10):1765-1772. doi: 10.1093/infdis/jiab036. Epub [PubMed PMID: 33507266]
Hurt AC, Wheatley AK. Neutralizing Antibody Therapeutics for COVID-19. Viruses. 2021 Apr 7:13(4):. doi: 10.3390/v13040628. Epub 2021 Apr 7 [PubMed PMID: 33916927]
Ning L, Abagna HB, Jiang Q, Liu S, Huang J. Development and application of therapeutic antibodies against COVID-19. International journal of biological sciences. 2021:17(6):1486-1496. doi: 10.7150/ijbs.59149. Epub 2021 Apr 10 [PubMed PMID: 33907512]
Xia S, Zhu Y, Liu M, Lan Q, Xu W, Wu Y, Ying T, Liu S, Shi Z, Jiang S, Lu L. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cellular & molecular immunology. 2020 Jul:17(7):765-767. doi: 10.1038/s41423-020-0374-2. Epub 2020 Feb 11 [PubMed PMID: 32047258]
Focosi D, Maggi F. Neutralising antibody escape of SARS-CoV-2 spike protein: Risk assessment for antibody-based Covid-19 therapeutics and vaccines. Reviews in medical virology. 2021 Nov:31(6):e2231. doi: 10.1002/rmv.2231. Epub 2021 Mar 16 [PubMed PMID: 33724631]
. An EUA for bamlanivimab and etesevimab for COVID-19. The Medical letter on drugs and therapeutics. 2021 Apr 5:63(1621):49-50 [PubMed PMID: 33830966]
Level 3 (low-level) evidenceChen P, Nirula A, Heller B, Gottlieb RL, Boscia J, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, Shawa I, Adams AC, Van Naarden J, Custer KL, Shen L, Durante M, Oakley G, Schade AE, Sabo J, Patel DR, Klekotka P, Skovronsky DM, BLAZE-1 Investigators. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. The New England journal of medicine. 2021 Jan 21:384(3):229-237. doi: 10.1056/NEJMoa2029849. Epub 2020 Oct 28 [PubMed PMID: 33113295]
Gottlieb RL, Nirula A, Chen P, Boscia J, Heller B, Morris J, Huhn G, Cardona J, Mocherla B, Stosor V, Shawa I, Kumar P, Adams AC, Van Naarden J, Custer KL, Durante M, Oakley G, Schade AE, Holzer TR, Ebert PJ, Higgs RE, Kallewaard NL, Sabo J, Patel DR, Klekotka P, Shen L, Skovronsky DM. Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA. 2021 Feb 16:325(7):632-644. doi: 10.1001/jama.2021.0202. Epub [PubMed PMID: 33475701]
Level 1 (high-level) evidence. Etesevimab and Bamlanivimab. Drugs and Lactation Database (LactMed®). 2006:(): [PubMed PMID: 33630482]
Wolf J, Abzug MJ, Wattier RL, Sue PK, Vora SB, Zachariah P, Dulek DE, Waghmare A, Olivero R, Downes KJ, James SH, Pinninti SG, Yarbrough A, Aldrich ML, MacBrayne CE, Soma VL, Grapentine SP, Oliveira CR, Hayes M, Kimberlin DW, Jones SB, Bio LL, Morton TH, Hankins JS, Maron GM, Timberlake K, Young JL, Orscheln RC, Schwenk HT, Goldman DL, Groves HE, Huskins WC, Rajapakse NS, Lamb GS, Tribble AC, Lloyd EC, Hersh AL, Thorell EA, Ratner AJ, Chiotos K, Nakamura MM. Initial Guidance on Use of Monoclonal Antibody Therapy for Treatment of Coronavirus Disease 2019 in Children and Adolescents. Journal of the Pediatric Infectious Diseases Society. 2021 May 28:10(5):629-634. doi: 10.1093/jpids/piaa175. Epub [PubMed PMID: 33388760]