Indications
Upadacitinib is a new FDA-approved second-line agent for treating moderate to severe active rheumatoid arthritis (RA) in patients who have not shown an adequate response or intolerance to the first-line agent, methotrexate.[1] This agent is a second-generation selective Janus kinase (JAK) inhibitor targeting the JAK1 enzyme.[2] Upadacitinib received FDA approval on August 16, 2019, based on positive and promising results from its multinational phase III trials in subjects with moderate to severe rheumatoid arthritis.[3]
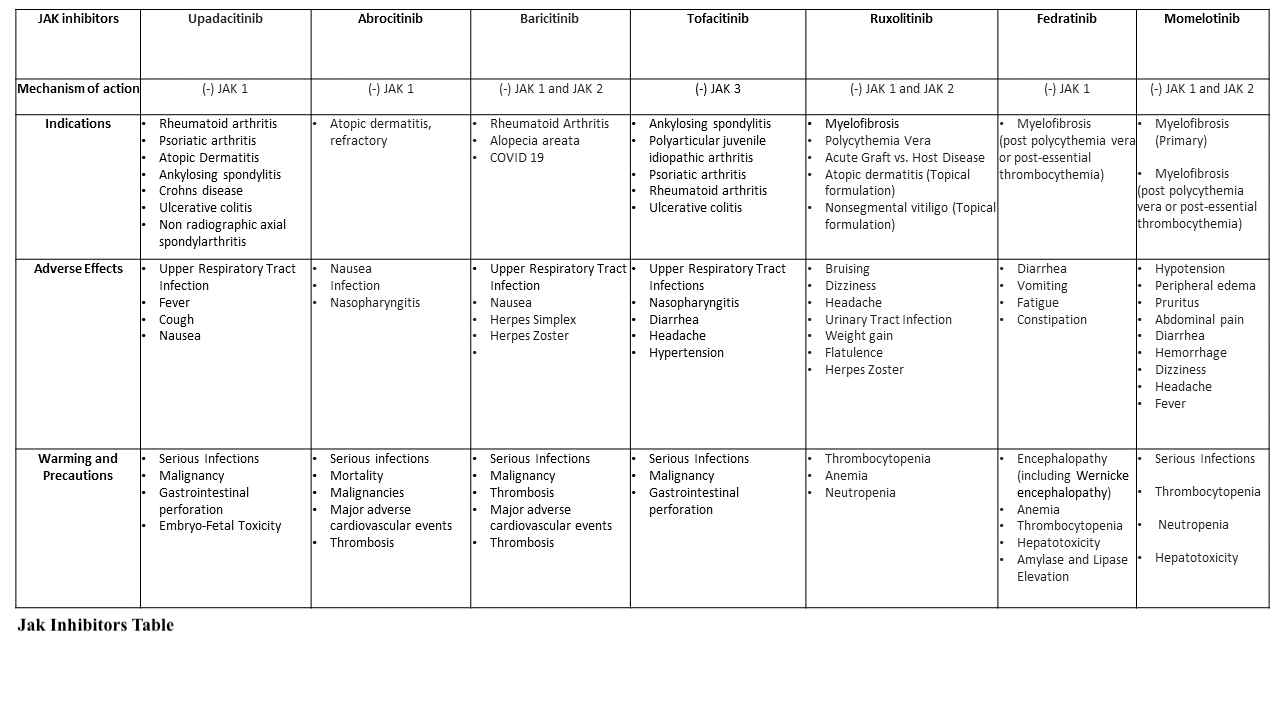
Using upadacitinib with other JAK inhibitors (JAKinibs) or robust immunosuppressants like azathioprine and cyclosporine is not advised. However, its use in combination with nonbiologic disease-modifying antirheumatic drugs (DMARDs) such as methotrexate is supported, while its use with biological DMARDs is not recommended (see Image. Jak Inhibitors Table).[3] When combined with the first-line therapy methotrexate, upadacitinib repressed disease progression on radiographic imaging and maintained clinical efficacy.[1]
Clinical advancements for agents used in other autoimmune diseases, such as psoriatic arthritis (PA), atopic dermatitis (AD), ankylosing spondylitis (AS), giant cell arteritis (GCA), systemic lupus erythematosus (SLE), and inflammatory bowel disease (IBD), Crohn disease (CD) and ulcerative colitis (UC), have shown promising results.[3] Upadacitinib marks a significant milestone as the first oral medication approved by the FDA to treat moderate to severe Crohn disease.
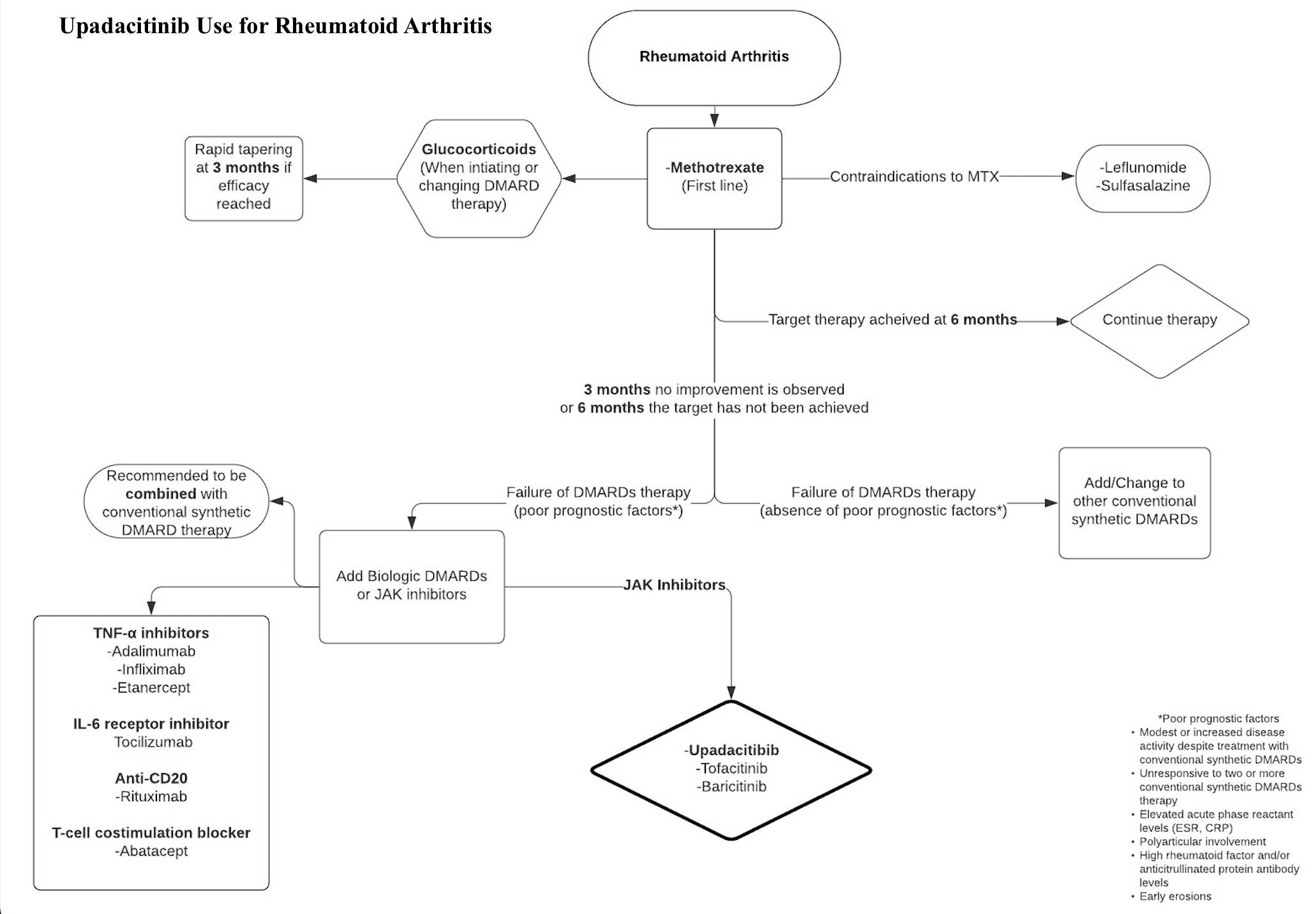
The American Academy of Dermatology (AAD) endorses using upadacitinib for adults with moderate to severe atopic dermatitis (AD). Upadacitinib is FDA-approved for patients with AD who have failed other systemic therapies, including biologics, or when the use of those therapies is inadvisable.[4] For patients with ulcerative colitis (UC) who experience recurrent pouchitis after ileal pouch-anal anastomosis (IPAA), the American Gastroenterological Association (AGA) suggests advanced immunosuppressive therapies like upadacitinib. Upadacitinib can be considered for chronic antibiotic-dependent pouchitis. Endoscopic evaluation is advised to confirm inflammation and rule out other causes. For patients with ulcerative colitis (UC) who have undergone IPAA and develop symptoms due to cuffitis, the AGA suggests using topical therapies approved for UC treatment, such as topical mesalamine and topical corticosteroids. Upadacitinib or other immunosuppressive therapies may be considered for refractory cases.[5] According to the American College of Rheumatology guidelines, targeted synthetic disease-modifying antirheumatic drugs (tsDMARDs), including JAK inhibitors like tofacitinib, baricitinib, and upadacitinib, are recommended as part of a treat-to-target (TTT) approach. This approach is strongly recommended over standard care for patients without prior treatment with biologic DMARDs (bDMARDs) or tsDMARDs. This recommendation extends to optimizing methotrexate dosage and subsequently adding DMARDs as needed. Although the evidence is of low certainty, this recommendation is maintained due to the recognized importance of systematic monitoring and treatment adjustment to minimize inflammation, thereby preventing joint damage and other long-term complications such as cardiovascular disease and osteoporosis. In DMARD-naive patients with moderate-to-high disease activity, methotrexate monotherapy is recommended.[6] The indication algorithm for rheumatoid arthritis is provided in Image. Upadacitinib for Rheumatoid Arthritis Algorithm.[7]
FDA-Approved Indications
The FDA has approved upadacitinib to treat several autoimmune and inflammatory conditions. The approved indications include:
- Rheumatoid arthritis (moderate to severe)
- Psoriatic arthritis
- Atopic dermatitis (for patients older than 12 years)
- Ankylosing spondylitis [8]
- Crohn's disease (moderate to severe) [9]
- Ulcerative colitis (moderate to severe) [10][11]
- Non-radiographic axial spondyloarthritis (active inflammation with inadequate response or intolerance to TNF blockers) [12]
This list reflects the range of conditions for which upadacitinib can be prescribed, highlighting its utility in treating various inflammatory disorders.
Mechanism of Action
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Mechanism of Action
Upadacitinib exerts its mechanism of action by inhibiting intracellular cytoplasmic enzymes Janus kinases (JAK), a group of 4 tyrosine kinases (JAK1, JAK2, JAK3, and TYK2) involved in the process of immune-mediated inflammatory diseases (IMIDs).[13] The inhibition of JAKs further hinders growth factor and cytokine-mediated signals from being transduced intracellularly by the JAK-STAT pathway.[13]
JAKs function by phosphorylating signal transducers and activators of transcription (STATs), regulating gene expression, and influencing hematopoiesis and immune cell function.[13] Upadacitinib prevents the phosphorylation and intracellular activation of STATs, further decreasing their inflammatory effects.[14] Upadacitinib has a selective and more prominent inhibitory influence on JAK1 relative to the JAK2, JAK3, and TYK2 subtypes.[15]
Pharmacokinetics
Absorption: Following oral administration of the extended-release formulation of upadacitinib, absorption occurs within 2 to 4 hours.
Distribution: Upadacitinib exhibits approximately 52% plasma protein binding.
Metabolism: Metabolism of upadacitinib primarily occurs through CYP3A4, with a minor contribution from CYP2D6.[16][17]
Excretion: Upadacitinib is excreted unchanged in feces (38%) and urine (24%). The mean terminal elimination half-life of upadacitinib ranges from 8 to 14 hours.
Administration
Available Dosage Forms and Strengths
Upadacitinib is available as 15 mg and 30 mg extended-release (ER) tablets for oral consumption.
Before initiating treatment with upadacitinib, it is necessary to ensure that immunizations are up-to-date and to assess for active or latent tuberculosis (TB). Additional evaluations include liver function, viral hepatitis panel, and pregnancy status in females of childbearing age. Concomitant administration of upadacitinib with other JAK inhibitors, biologic DMARD agents, or potent immunosuppressants such as azathioprine and cyclosporine is not recommended.
Adult Dosage
Rheumatoid Arthritis
The recommended daily dosage is 15 mg orally as monotherapy.[3] The medication may be taken with or without food and should be consumed as a whole tablet without splitting or crushing it.
Psoriatic Arthritis
The recommended daily dosage is 15 mg orally as monotherapy. The medication can be taken with or without food and should be consumed as a whole tablet without splitting or crushing it.
Atopic Dermatitis
For pediatric patients aged 12 years and older and weighing at least 40 kg, the recommended daily dosage is 15 mg once a day. If the therapeutic response is not reached, the dose can be increased to 30 mg daily. If the desired therapeutic response is not achieved with 30 mg daily, upadacitinib should be discontinued.
The recommended daily dosage for adult patients younger than 65 is 15 mg once daily. If the desired therapeutic response is not achieved, the dosage can be increased to 30 mg daily. If the desired therapeutic response is not attained with 30 mg daily, upadacitinib should be discontinued.
Ulcerative Colitis
The recommended initial dose of upadacitinib for induction therapy is 45 mg once daily for 8 weeks (induction phase). For maintenance therapy, the recommended dosage is 15 mg once daily. In cases of severe, refractory, or extensive ulcerative colitis, a dosage of 30 mg once daily may be considered. If an adequate therapeutic response is not attained with the 30 mg dose, upadacitinib should be discontinued. Using the lowest effective dosage required to sustain the desired response is advised.
Crohn Disease
Providers should initiate treatment with upadacitinib at a dosage of 45 mg once daily for 12 weeks (induction phase). For maintenance therapy, a daily dosage of 15 mg of upadacitinib is recommended. However, physicians may consider increasing the dosage to 30 mg once daily for patients with severe, refractory, or extensive disease. If patients do not achieve an adequate therapeutic response to the 30 mg dose, discontinuation of upadacitinib is advised. Physicians should administer the lowest effective dosage necessary to maintain the desired treatment response.
Ankylosing Spondylitis
The suggested dosage of upadacitinib is 15 mg once daily.
Non-radiographic Axial Spondyloarthritis
The suggested dosage of upadacitinib is 15 mg once daily.
Specific Patient Populations
Hepatic impairment: Upadacitinib is not recommended for severe hepatic impairment. For psoriatic arthritis, atopic dermatitis, rheumatoid arthritis, non-radiographic axial spondyloarthritis, and ankylosing spondylitis, no dosage adjustment of upadacitinib is required for mild or moderate hepatic impairment (Child-Pugh A or B). Patients with mild to moderate hepatic impairment require dose reduction in ulcerative colitis and Crohn disease. In ulcerative colitis, the recommended regimen includes an induction dose of 30 mg once daily for 8 weeks and a maintenance dose of 15 mg once daily. Similarly, patients with Crohn disease should be initiated with an induction dose of 30 mg once daily for 12 weeks, followed by a maintenance dose of 15 mg once daily.
Renal impairment:
For patients diagnosed with rheumatoid arthritis, psoriatic arthritis, or non-radiographic axial spondyloarthritis and ankylosing spondylitis, dosage adjustment is unnecessary for mild (eGFR ≥60 to <90 mL/min/1.73 m²), moderate (eGFR ≥30 to <60 mL/min/1.73 m²), or severe renal impairment (eGFR ≥15 to <30 mL/min/1.73 m²). Upadacitinib has not been investigated for any condition in patients with end-stage renal disease (eGFR <15 mL/min/1.73 m2).
For patients with atopic dermatitis, the maximum recommended dosage for patients with severe renal impairment is 15 mg once daily. No dosage adjustment is necessary for patients with mild or moderate renal impairment.
For patients with ulcerative colitis or Crohn disease, the suggested dosage for severe renal impairment is 30 mg once daily for induction, followed by 15 mg once daily for maintenance. No dosage adjustment of upadacitinib is needed for patients with mild or moderate renal impairment.
Pregnancy considerations: Upadacitinib was found to be teratogenic in animal studies, although no studies on human pregnancy have been reported. Administration of upadacitinib during pregnancy is not recommended. Contraception use is advised during treatment and for 4 weeks post-treatment with upadacitinib.
Breastfeeding considerations: Upadacitinib was also present in breast milk during animal studies, and women are advised not to breastfeed while receiving therapy. Clinicians should advise against breastfeeding while administering upadacitinib. Alternative medications should be considered, particularly for preterm infants. According to the product labeling information, breastfeeding should be avoided for 6 days after the last dose of upadacitinib.[18]
Pediatric patients: For pediatric patients with atopic dermatitis aged 12 years and older and weighing at least 40 kg, the recommended starting dose of upadacitinib is 15 mg once daily. If the desired response is not attained, the dosage of upadacitinib may be increased to 30 mg once daily. Upadacitinib should be discontinued if the 30 mg dose is ineffective. The lowest effective dose required to sustain the desired response should be used.
Older patients: The suggested dosage of upadacitinib is 15 mg once daily for older patients with atopic dermatitis. In older adults (65 years and older) with various conditions, including ulcerative colitis, Crohn disease, ankylosing spondylitis, rheumatoid arthritis, psoriatic arthritis, atopic dermatitis, and non-radiographic axial spondyloarthritis, no difference in effectiveness was observed compared to younger patients. However, older patients are at an increased risk of general adverse events, including infection and malignancy.
Adverse Effects
Upadacitinib has various adverse effects, which are listed below.[19]
- Upper respiratory tract infections (URTI) (14%)
- Nausea (4%)
- Elevated liver enzymes (2%)
- Fever (1%)
- Cough (2%)
Upper respiratory tract infections (URTI) encompass:
- Acute sinusitis
- Laryngitis
- Nasopharyngitis
- Oropharyngeal pain
- Pharyngitis
- Pharyngotonsillitis
- Rhinitis
- Sinusitis
- Tonsillitis
- Viral upper respiratory tract infection
These adverse effects were observed during placebo-controlled studies where subjects were administered 15 mg of oral upadacitinib.[3] More severe adverse effects, such as herpes zoster virus (HZV) and serious infections, were seen in subjects administered 30 mg in a double-blind, randomized, controlled phase 3 clinical trial (<1%).[19] Clinical studies have also reported malignancy, thrombosis, and gastrointestinal (GI) perforations with concomitant non-steroidal anti-inflammatory drugs (NSAIDs) use.[3] Headache, acne, upper respiratory tract infection, and elevated creatine phosphokinase (CPK) levels were common adverse events observed in adolescents receiving upadacitinib for atopic dermatitis.[20] In a study investigating the adverse effects associated with upadacitinib, data from the Food and Drug Administration Adverse Event Reporting System (FAERS) spanning from 2004 to 2023 were analyzed. The study reported adverse drug reactions (ADRs), including vulvar dysplasia, acne, mediastinal neoplasm, eczema herpeticum, lip neoplasm, herpes zoster, eosinopenia, ureteral neoplasm, and Moraxella infection.[21]
Drug-Drug Interactions: Upadacitinib is metabolized in the liver by the cytochrome P450 (CYP) system, primarily through the CYP3A4 enzyme, and is eliminated in the feces and urine as metabolites, with a drug half-life of 8 to 14 hours. The concomitant use of CYP3A4 inhibitors and CYP3A4 inducers should be approached with caution and is generally not recommended, as it may alter the drug's pharmacokinetics, potentially increasing or decreasing drug plasma concentrations. Clinical studies have also reported malignancy, thrombosis, and gastrointestinal (GI) perforations with concomitant use of non-steroidal anti-inflammatory drugs (NSAIDs).[3][19]
- Upadacitinib exposure may increase when used concurrently with strong CYP3A4 inhibitors such as grapefruit, ketoconazole, and clarithromycin, potentially exacerbating the risk of adverse reactions. Patients should avoid consuming grapefruit or grapefruit-containing products during upadacitinib treatment.
- For patients with atopic dermatitis or Crohn disease who are prescribed strong CYP3A4 inhibitors, the induction dosage of upadacitinib should be reduced to 30 mg once daily while maintaining the maintenance dosage at 15 mg once daily. Conversely, strong CYP3A4 inducers such as rifampin may decrease upadacitinib exposure, potentially diminishing its therapeutic effects. Therefore, co-administration of upadacitinib with strong CYP3A4 inducers is not advised.
Contraindications
The use of upadacitinib with biological DMARDs (infliximab, adalimumab, etanercept, abatacept, tofacitinib) and immunosuppressants (azathioprine, tacrolimus, cyclosporine, intravenous (IV) corticosteroids, and 6-mercaptopurine) is not recommended.[22] Administration of a live attenuated vaccine shortly before or during treatment is also not advised.[23]
US Boxed Warning:
Infections, malignancy, thrombosis, tuberculosis, death, and major adverse cardiovascular events (MACE) are significant risks.[24]
Recent updates in upadacitinib use have shown an increased rate of all-cause mortality and sudden cardiovascular-associated mortality when used concurrently with another JAK inhibitor agent versus tumor necrosis factor (TNF) inhibitors in subjects with RA. MACE includes cardiovascular mortality, myocardial infarction (MI), and stroke.
Warnings and Precautions:
Patients taking upadacitinib may notice medication residue in their stool or ostomy output, especially in cases involving a colostomy, ileostomy, intestinal resection, or shortened transit times. If patients observe repeated instances of medication residue, they should promptly notify their healthcare provider. Clinical monitoring is recommended, and alternative treatment options should be considered if there is an inadequate therapeutic response. The predominant serious infections associated with upadacitinib include pneumonia, cellulitis, tuberculosis, herpes zoster, oral and esophageal candidiasis, and cryptococcosis.[25][26] The product labeling notes an increased frequency of serious infections with the higher 30 mg dosage of upadacitinib compared to the 15 mg dosage.
Monitoring
Before initiating treatment with upadacitinib, a negative tuberculosis (TB) test is required as it may reactivate latent TB infection. During treatment with upadacitinib, patients should be routinely tested for TB. A complete blood count (CBC) must be monitored before starting therapy and routinely after that; initiating upadacitinib is not recommended for subjects with an absolute lymphocyte count (ALC) below 500 cells/mm³ or an absolute neutrophil count (ANC) below 1,000 cells/mm³. Reports of lymphopenia, neutropenia, and anemia exist. Liver function tests (LFTs) should be checked before beginning therapy and regularly after that, as patients with severe hepatic impairment are not advised to start treatment.[27] The lipid panel should also be monitored 12 weeks after starting treatment, as increases in total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol may occur.
Patients should be monitored for serious infections during therapy, as severe bacterial, viral, fungal, tuberculous, and opportunistic infections have occurred, leading to hospitalization and mortality in patients receiving upadacitinib. In such cases, the treatment regime must be interrupted and halted. Monitoring for reactivation of prior hepatitis B virus (HBV) or herpes zoster virus (HZV) infections is recommended, as these infections were reported during clinical studies. Upadacitinib should be temporarily discontinued until HZV is resolved.[3]
Subjects at increased risk for skin cancers receiving treatment are advised to undergo routine skin examinations, as they have an increased risk of non-melanoma skin cancers (NMSCs). Patients receiving upadacitinib with concomitant NSAID use should be monitored for new-onset gastrointestinal manifestations, as GI perforations have been reported during clinical studies.
Toxicity
Upadacitinib was found to be teratogenic in animal studies, although no human studies during pregnancy have been reported. Use during pregnancy is not recommended. Contraception is advised during treatment and for 4 weeks after completing treatment with upadacitinib.
Hepatotoxicity
The pattern of liver injury associated with upadacitinib indicates a potential for low-level, direct hepatotoxicity. During the initial weeks of treatment, ALT levels may slightly increase, typically returning to baseline upon discontinuation of the drug. Serum aminotransferase elevations above 5 times the upper limit of normal should prompt consideration of dose reduction or temporary cessation of upadacitinib treatment. Managing potential hepatotoxicity carefully is essential for minimizing further liver injury. Close monitoring of liver function tests is crucial during this period to ensure timely intervention and appropriate management.[17]
There is no information regarding overdose of upadacitinib in the FDA-approved product labeling.
Enhancing Healthcare Team Outcomes
Upadacitinib is a second-generation selective Janus kinase inhibitor targeting the JAK1 enzyme. This medication is FDA-approved to treat active moderate to severe rheumatoid arthritis in patients who are intolerant or unresponsive to first-line therapy with methotrexate (MTX). Managing rheumatoid arthritis requires decisive care from an interprofessional team of healthcare professionals. Early clinical analysis and diagnosis can lead to more effective management strategies, decreasing lifelong complications and improving the quality of life. The interprofessional team can include physicians, nurse practitioners, physician assistants, rheumatologists, nurses, a physical therapist (PT), and a pharmacist. For severe atopic dermatitis, consultation with a dermatologist may be required. Consultation with a gastroenterologist is necessary when using upadacitinib for inflammatory bowel disease. A phase 3 clinical trial, comprising 2 induction trials, U-EXCEL and U-EXCEED, and one maintenance trial, U-ENDURE, was conducted across 43 countries at 277 sites. U-EXCEL and U-EXCEED involved a 12-week double-blind, placebo-controlled induction treatment period, demonstrating the efficacy of upadacitinib in treating moderate-to-severe Crohn disease.[28]
Rheumatologists and primary care physicians (PCPs) should maintain continuous communication regarding their patient's care and updates on the latest treatment guidelines. Patients should be thoroughly educated about rheumatoid arthritis, therapy with upadacitinib, and its potential adverse effects. The interdisciplinary team should monitor the patient's CBC, liver enzymes, and lipid panel before initiating treatment and routinely after that. Particular attention should be paid to the lymphocyte count, absolute neutrophil count, and hemoglobin (Hgb), as lymphopenia, neutropenia, and anemia may occur during therapy. Treatment should be avoided or halted if the patient's absolute lymphocyte count is below 500 cells/mm³, the absolute neutrophil count is below 1,000 cells/mm³, or the Hgb level is below 8 g/dL.
The primary care physician must monitor total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol levels 12 weeks after treatment initiation, given the possibility of elevated levels, which may require prompt management. Every patient initiating treatment should have TB screening with a tuberculin skin test (TST) or interferon-γ release assay (IGRA). Patients with a positive TB test should be treated before starting treatment with upadacitinib.
Patients should be advised about the broad range of adverse effects while on therapy. Routine follow-ups with the PCP and specialist are essential to prevent complications, hospitalization, and mortality. New-onset gastrointestinal symptoms must be thoroughly evaluated, as GI perforation has been reported in clinical studies, particularly with concomitant NSAID use. Patients should also be counseled about the increased risk of severe infections, and any new-onset clinical manifestation should be reported and investigated.
In reproductive animal studies, upadacitinib was shown to cause injury to the developing fetus and be present in breast milk. During pregnancy, women should be advised and counseled about upadacitinib use and the likely effects on the fetus and to discontinue breastfeeding while on therapy. Women should also be counseled on contraception use while being treated and for 4 weeks after discontinuation of treatment. Intercommunication between the healthcare team and their patients is imperative to establish a solid physician-patient relationship. This can further increase medication compliance and decrease disease progression, improving the quality of life. An interprofessional team approach and open communication between clinicians (MDs, DOs, NPs, PAs), pharmacists, rheumatologists, gastroenterologists, and dermatologists are necessary to optimize patient outcomes with upadacitinib therapy.
Media
(Click Image to Enlarge)
References
Serhal L, Edwards CJ. Upadacitinib for the treatment of rheumatoid arthritis. Expert review of clinical immunology. 2019 Jan:15(1):13-25. doi: 10.1080/1744666X.2019.1544892. Epub 2018 Nov 19 [PubMed PMID: 30394138]
Tanaka Y. A review of upadacitinib in rheumatoid arthritis. Modern rheumatology. 2020 Sep:30(5):779-787. doi: 10.1080/14397595.2020.1782049. Epub 2020 Jul 13 [PubMed PMID: 32530345]
Duggan S, Keam SJ. Upadacitinib: First Approval. Drugs. 2019 Nov:79(16):1819-1828. doi: 10.1007/s40265-019-01211-z. Epub [PubMed PMID: 31642025]
Davis DMR, Drucker AM, Alikhan A, Bercovitch L, Cohen DE, Darr JM, Eichenfield LF, Frazer-Green L, Paller AS, Schwarzenberger K, Silverberg JI, Singh AM, Wu PA, Sidbury R. Guidelines of care for the management of atopic dermatitis in adults with phototherapy and systemic therapies. Journal of the American Academy of Dermatology. 2024 Feb:90(2):e43-e56. doi: 10.1016/j.jaad.2023.08.102. Epub 2023 Nov 7 [PubMed PMID: 37943240]
Barnes EL, Agrawal M, Syal G, Ananthakrishnan AN, Cohen BL, Haydek JP, Al Kazzi ES, Eisenstein S, Hashash JG, Sultan SS, Raffals LE, Singh S, AGA Clinical Guidelines Committee. Electronic address: clinicalpractice@gastro.org. AGA Clinical Practice Guideline on the Management of Pouchitis and Inflammatory Pouch Disorders. Gastroenterology. 2024 Jan:166(1):59-85. doi: 10.1053/j.gastro.2023.10.015. Epub [PubMed PMID: 38128971]
Level 1 (high-level) evidenceFraenkel L, Bathon JM, England BR, St Clair EW, Arayssi T, Carandang K, Deane KD, Genovese M, Huston KK, Kerr G, Kremer J, Nakamura MC, Russell LA, Singh JA, Smith BJ, Sparks JA, Venkatachalam S, Weinblatt ME, Al-Gibbawi M, Baker JF, Barbour KE, Barton JL, Cappelli L, Chamseddine F, George M, Johnson SR, Kahale L, Karam BS, Khamis AM, Navarro-Millán I, Mirza R, Schwab P, Singh N, Turgunbaev M, Turner AS, Yaacoub S, Akl EA. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis care & research. 2021 Jul:73(7):924-939. doi: 10.1002/acr.24596. Epub 2021 Jun 8 [PubMed PMID: 34101387]
Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester GR, Dougados M, Kerschbaumer A, McInnes IB, Sepriano A, van Vollenhoven RF, de Wit M, Aletaha D, Aringer M, Askling J, Balsa A, Boers M, den Broeder AA, Buch MH, Buttgereit F, Caporali R, Cardiel MH, De Cock D, Codreanu C, Cutolo M, Edwards CJ, van Eijk-Hustings Y, Emery P, Finckh A, Gossec L, Gottenberg JE, Hetland ML, Huizinga TWJ, Koloumas M, Li Z, Mariette X, Müller-Ladner U, Mysler EF, da Silva JAP, Poór G, Pope JE, Rubbert-Roth A, Ruyssen-Witrand A, Saag KG, Strangfeld A, Takeuchi T, Voshaar M, Westhovens R, van der Heijde D. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Annals of the rheumatic diseases. 2020 Jun:79(6):685-699. doi: 10.1136/annrheumdis-2019-216655. Epub 2020 Jan 22 [PubMed PMID: 31969328]
Raychaudhuri SP, Shah RJ, Banerjee S, Raychaudhuri SK. JAK-STAT Signaling and Beyond in the Pathogenesis of Spondyloarthritis and Their Clinical Significance. Current rheumatology reports. 2024 Jun:26(6):204-213. doi: 10.1007/s11926-024-01144-x. Epub 2024 Mar 16 [PubMed PMID: 38492148]
Dignass A, Esters P, Flauaus C. Upadacitinib in Crohn's disease. Expert opinion on pharmacotherapy. 2024 Mar:25(4):359-370. doi: 10.1080/14656566.2024.2333964. Epub 2024 Mar 29 [PubMed PMID: 38512115]
Irani M, Fan C, Glassner K, Abraham BP. Clinical Evaluation of Upadacitinib in the Treatment of Adults with Moderately to Severely Active Ulcerative Colitis (UC): Patient Selection and Reported Outcomes. Clinical and experimental gastroenterology. 2023:16():21-28. doi: 10.2147/CEG.S367086. Epub 2023 Mar 7 [PubMed PMID: 36915649]
Panaccione R, Danese S, Zhou W, Klaff J, Ilo D, Yao X, Levy G, Higgins PDR, Loftus EV Jr, Chen S, Gonzalez YS, Leonard C, Hébuterne X, Lindsay JO, Cao Q, Nakase H, Colombel JF, Vermeire S. Efficacy and safety of upadacitinib for 16-week extended induction and 52-week maintenance therapy in patients with moderately to severely active ulcerative colitis. Alimentary pharmacology & therapeutics. 2024 Feb:59(3):393-408. doi: 10.1111/apt.17816. Epub 2023 Nov 27 [PubMed PMID: 38010661]
Harrison SR, Marzo-Ortega H. Have Therapeutics Enhanced Our Knowledge of Axial Spondyloarthritis? Current rheumatology reports. 2023 Mar:25(3):56-67. doi: 10.1007/s11926-023-01097-7. Epub [PubMed PMID: 36652160]
Kotyla PJ, Islam MA, Engelmann M. Clinical Aspects of Janus Kinase (JAK) Inhibitors in the Cardiovascular System in Patients with Rheumatoid Arthritis. International journal of molecular sciences. 2020 Oct 7:21(19):. doi: 10.3390/ijms21197390. Epub 2020 Oct 7 [PubMed PMID: 33036382]
Baker KF, Isaacs JD. Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn's disease and ulcerative colitis? Annals of the rheumatic diseases. 2018 Feb:77(2):175-187. doi: 10.1136/annrheumdis-2017-211555. Epub 2017 Aug 1 [PubMed PMID: 28765121]
Lin CM, Cooles FA, Isaacs JD. Basic Mechanisms of JAK Inhibition. Mediterranean journal of rheumatology. 2020 Jun:31(Suppl 1):100-104. doi: 10.31138/mjr.31.1.100. Epub 2020 Jun 11 [PubMed PMID: 32676567]
Mohamed MF, Minocha M, Trueman S, Feng T, Enejosa J, Fisniku O, Othman AA. Characterization of the Effect of Upadacitinib on the Pharmacokinetics of Bupropion, a Sensitive Cytochrome P450 2B6 Probe Substrate. Clinical pharmacology in drug development. 2021 Mar:10(3):299-306. doi: 10.1002/cpdd.844. Epub 2020 Jul 9 [PubMed PMID: 32648334]
. Upadacitinib. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012:(): [PubMed PMID: 34633781]
. Upadacitinib. Drugs and Lactation Database (LactMed®). 2006:(): [PubMed PMID: 32816423]
Genovese MC, Fleischmann R, Combe B, Hall S, Rubbert-Roth A, Zhang Y, Zhou Y, Mohamed MF, Meerwein S, Pangan AL. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet (London, England). 2018 Jun 23:391(10139):2513-2524. doi: 10.1016/S0140-6736(18)31116-4. Epub 2018 Jun 18 [PubMed PMID: 29908670]
Level 1 (high-level) evidencePaller AS, Ladizinski B, Mendes-Bastos P, Siegfried E, Soong W, Prajapati VH, Lio P, Thyssen JP, Simpson EL, Platt AM, Raymundo EM, Liu J, Calimlim BM, Huang X, Gu Y, Hu X, Yang Y, Su JC, Zheng M, Yamamoto-Hanada K, Teixeira HD, Irvine AD. Efficacy and Safety of Upadacitinib Treatment in Adolescents With Moderate-to-Severe Atopic Dermatitis: Analysis of the Measure Up 1, Measure Up 2, and AD Up Randomized Clinical Trials. JAMA dermatology. 2023 May 1:159(5):526-535. doi: 10.1001/jamadermatol.2023.0391. Epub [PubMed PMID: 37043227]
Level 1 (high-level) evidenceWu Y, Wei M, Zhang J. A real-world pharmacovigilance analysis of FDA adverse event reporting system database for upadacitinib. Frontiers in pharmacology. 2023:14():1200254. doi: 10.3389/fphar.2023.1200254. Epub 2023 Aug 17 [PubMed PMID: 37663269]
Benjamin O, Goyal A, Lappin SL. Disease-Modifying Antirheumatic Drugs (DMARD). StatPearls. 2024 Jan:(): [PubMed PMID: 29939640]
Sandborn WJ, Ghosh S, Panes J, Schreiber S, D'Haens G, Tanida S, Siffledeen J, Enejosa J, Zhou W, Othman AA, Huang B, Higgins PDR. Efficacy of Upadacitinib in a Randomized Trial of Patients With Active Ulcerative Colitis. Gastroenterology. 2020 Jun:158(8):2139-2149.e14. doi: 10.1053/j.gastro.2020.02.030. Epub 2020 Feb 22 [PubMed PMID: 32092309]
Level 1 (high-level) evidenceCharles-Schoeman C, Choy E, McInnes IB, Mysler E, Nash P, Yamaoka K, Lippe R, Khan N, Shmagel AK, Palac H, Suboticki J, Curtis JR. MACE and VTE across upadacitinib clinical trial programmes in rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. RMD open. 2023 Nov:9(4):. doi: 10.1136/rmdopen-2023-003392. Epub [PubMed PMID: 37945286]
Wolfenbarger B, Britt E. A Case of Pulmonary Cryptococcosis Caused by Capsule-Deficient Cryptococcus neoformans. Cureus. 2023 Nov:15(11):e48196. doi: 10.7759/cureus.48196. Epub 2023 Nov 3 [PubMed PMID: 38054144]
Level 3 (low-level) evidenceValor-Méndez L, Manger B, Wacker J, Kleyer A, Schett G. Lymph node and pulmonary tuberculosis during upadacitinib treatment in a psoriatic arthritis patient. Rheumatology advances in practice. 2022:6(2):rkac032. doi: 10.1093/rap/rkac032. Epub 2022 May 13 [PubMed PMID: 35601270]
Level 3 (low-level) evidenceTrueman S, Mohamed MF, Feng T, Lacerda AP, Marbury T, Othman AA. Characterization of the Effect of Hepatic Impairment on Upadacitinib Pharmacokinetics. Journal of clinical pharmacology. 2019 Sep:59(9):1188-1194. doi: 10.1002/jcph.1414. Epub 2019 Apr 11 [PubMed PMID: 30973649]
Loftus EV Jr, Panés J, Lacerda AP, Peyrin-Biroulet L, D'Haens G, Panaccione R, Reinisch W, Louis E, Chen M, Nakase H, Begun J, Boland BS, Phillips C, Mohamed MF, Liu J, Geng Z, Feng T, Dubcenco E, Colombel JF. Upadacitinib Induction and Maintenance Therapy for Crohn's Disease. The New England journal of medicine. 2023 May 25:388(21):1966-1980. doi: 10.1056/NEJMoa2212728. Epub [PubMed PMID: 37224198]