Introduction
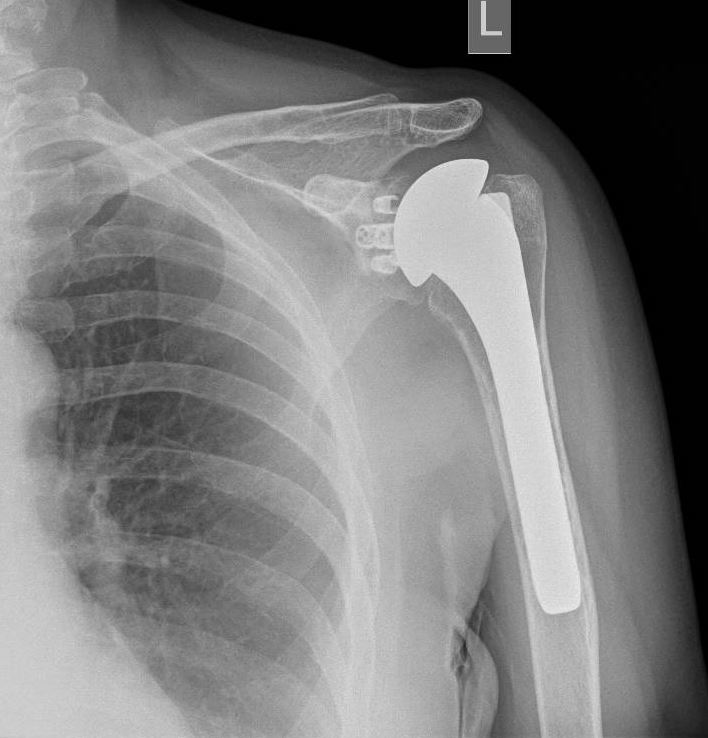
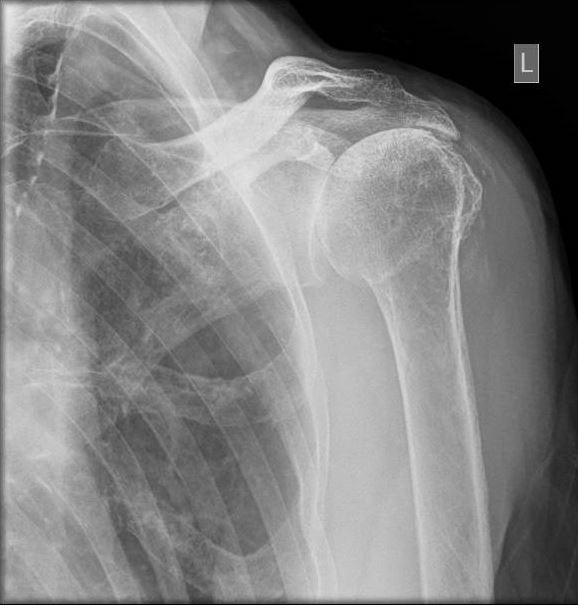
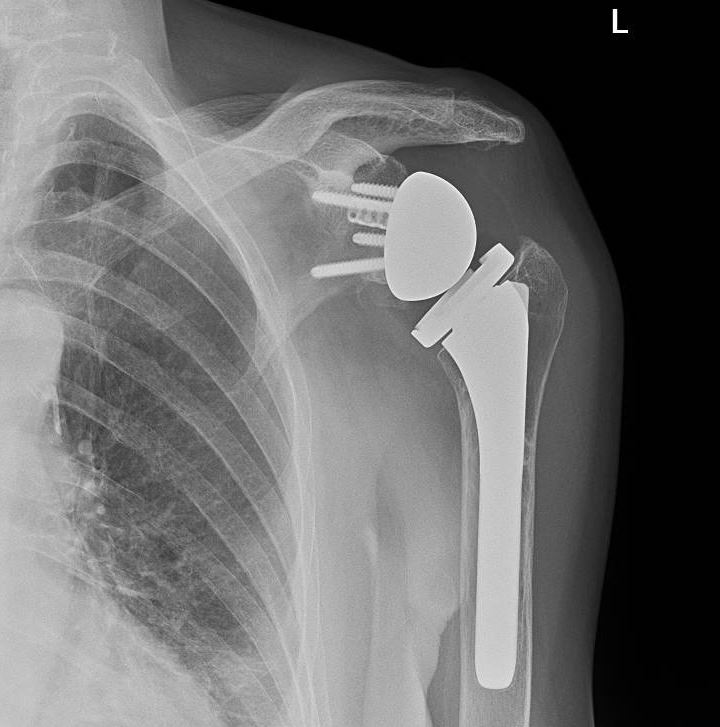
Reverse total shoulder arthroplasty (RTSA) is increasingly gaining popularity worldwide in treating various traumatic and degenerative glenohumeral diseases and irreparable rotator cuff arthropathies (see Image. Reverse Total Shoulder Arthroplasty). The number of performed RTSA in the United States of America has increased from 22,835 in 2011 to 62,705 in 2017.[1] RTSA was first described by Beddow and Alloy in 1970. However, they did not publish their surgical outcomes.[2] In 1987, Grammont et al reported the first 8 cases of rotator cuff arthropathy treated by an RTSA prototype called the “Trompette” (Medinov).[3] RTSA was introduced in the United States of America in 1998 after being used in Europe for several years.[4] The RTSA is a semi-constrained prosthesis with components different from the anatomical total shoulder arthroplasty prosthesis. The anatomical shoulder arthroplasty consists of a concave glenoid socket and proximal humeral ball prosthesis (see Image. Anatomical Shoulder Arthroplasty). In the reverse shoulder arthroplasty, the anatomy is reversed to a glenoid ball (glenosphere) and concave proximal humerus component.[5]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Understanding the shoulder joint anatomy and biomechanics is essential for selecting the suitable prosthesis for shoulder arthroplasty and successful outcomes. The Shoulder joint complex includes 4 combined articulations: the acromioclavicular joint, the glenohumeral joint (GH), the sternoclavicular joint, and the scapulothoracic joint.
The Glenohumeral (GH) Joint
GH joint is a synovial multiaxial ball and socket articulation between the humeral head and the bony glenoid that permits a wide range of motion.[6] Dynamic and static stabilizers achieve GH stability. Dynamic GH stabilizers include the long head of the biceps and the rotator cuff muscles. The rotor cuff muscles are subscapularis, supraspinatus, infraspinatus, and teres minor. During shoulder movements, the dynamic GH stabilizers keep the humeral head in a central position in the glenoid fossa. Static GH stabilizers include the glenoid bony configuration, glenoid labrum, shoulder joint capsule, and GH ligaments (superior, middle, and inferior GH ligaments).[7] The humeral head is retroverted about 20 degrees to the distal humerus inter epicondylar axis. The humeral neck-shaft angle is about 130-140 degrees.[8] The glenoid is titled upward about 5 degrees and retroverted about 5 degrees from the scapular body axis.
Reverse Total Arthroplasty Biomechanics
In the RTSA, the shoulder center of rotation is displaced inferiorly and medially. This modification in the center of rotation allows the deltoid muscle to work on a longer lever arm. This mechanical advantage allows more deltoid muscle fibers to act on shoulder abduction. Also, the downward humerus displacement increases the deltoid muscle tension.[9] The RTSA transforms the shear forces around the shoulder into compressive forces, creating a rotational moment that allows the deltoid muscle to start arm abduction.[4] The larger glenohumeral surface area in RTSA increases the shoulder stability and the potential range of movement. Also, the deltoid neutralization in RTSA minimizes the upward humeral translation associated with rotator cuff arthropathy, which enhances shoulder stability.[10]
Indications
RTSA has been used to manage and treat the following conditions:
- Rotator cuff arthropathy is a degenerative shoulder disease caused by a massive rotator cuff tear causing arthritic glenohumeral joint and proximal humerus migration (see Image. Advanced Glenohumeral Arthritic Changes).[11][12][13]
- Pseudo-shoulder paralysis due to an irreparable massive rotator cuff tear[14]
- Acute 3 or 4 parts proximal humerus fractures[15]
- Post-traumatic glenohumeral arthritis
- Chronic irreducible shoulder dislocation[16]
- Revision surgery for failed anatomical total shoulder arthroplasty[17]
- Revision surgery for failed proximal humerus hemiarthroplasty[18]
- Inflammatory joint conditions, eg, rheumatoid arthritis[19]
Contraindications
RTSA is contraindicated in patients with the following:
- Axillary nerve palsy
- Deficient deltoid muscle[20]
- Active infection
- Significant glenoid deficient bony stock
- Skeletal immaturity
- A neuromuscular disorder that may increase the risk of prosthesis dislocation
Equipment
The constrained RTSA prosthesis's initial designs were unsuccessful and had to be withdrawn because of the high failure rate.[21] In 1981, Grammont et al introduced the concept of medialization of the humeral head center of rotation to enhance the deltoid muscle lever arm. In 1991, a second-generation RTSA prosthesis was developed, and it has been used without change for about 15 years.[22] The newer prosthesis involved 2 main modifications: a large hemispherical glenoid component and increased humeral component inclination to the shaft to 155 degrees.[23]
The large size of the glenoid hemisphere causes medialization of the center of rotation. The bigger humeral neck-shaft ankle lowers the center of rotation and the proximal humerus, which increases the deltoid tension. The common problems associated with the newer prosthesis design were scapular notching, bony impingement, and polyethylene wear.[23] Over the last 20 years, more modifications have been introduced to overcome second-generation prosthesis problems. Prothesis designs with smaller neck-shaft angles (135 or 145 degrees) have shown a lower incidence of bony impingement and scapular notching.[24] Currently, various models of RTSA are available on the market, and their designs vary.[25] The RTSA usually involves the following components:
- A cementless glenoid base plate; is seated on the prepared glenoid and fixed by multidirectional screws.
- The modular geosphere is usually half a sphere and is secured at the glenoid base plate.
- The humeral stem is inserted into the prepared humerus. Two types of stems are available: cemented and cementless press-fit stems.
- The humeral cup is fitted on top of the humeral stem. It is designed to secure the concave insert on top of it.
- A concave polyethylene insert is fitted on top of the humeral cup. It is concave and articulates with the geosphere.
Personnel
To perform a reversed shoulder arthroplasty safely, the minimum operative team required to include:
- Anesthetist
- Anesthetist assistant
- Scrub nurse
- Operative room runner
- Shoulder surgeon
- Surgeon assistant
- Recovery nurse
- Prothesis company representative (optional): If the team is unfamiliar with the surgical instrument kit and the various prosthesis parts, sometimes the company representative's presence is required to identify them.
Preparation
The RTSA preoperative preparation includes obtaining a complete history, performing a full clinical assessment, arranging the appropriate imaging modalities, and medically optimizing the surgery.
Patient History
Detailed history, including personal, medical, and social history, is mandatory to identify all medical comorbidities, social circumstances, and the patient’s functional baseline. For example, patients who live on their own or usually use a mobility aid need extra support postoperatively.
Clinical Assessment
Full preoperative clinical assessment should be performed, including:
- The current shoulder's active and passive range of movement
- Rotator cuff examination,
- Axillary nerve function,
- Deltoid muscle function,
- Skin condition over the involved shoulder,
- The entire upper limb neurovascular status.
IMAGING MODALITIES
Shoulder X-rays
Anteroposterior, Y-scapular, and axillary views are usually obtained before RTSA. X-rays are useful for preparative templating, identifying bony lesions, and assessing bone quality.
Computed Tomography (CT) Scan
CT scan is beneficial in assessing the humerus and glenoid bony stock and glenoid version for preoperative planning. A preoperative 3-dimensional (3D) CT scan can determine the proximal humeral retroversion.[26] The 3-dimensional glenoid assessment using 3D-CT scan as a part of preoperative planning has been shown to guide surgeons in achieving more accurate glenoid component positioning.[27][28]
Magnetic Resonance Imaging (MRI) Scan
MRI could be used to assess the integrity of the rotator cuff before the surgery.
Medical Optimization
Patients with medical comorbidities, eg, diabetes, anemia, cardiac or renal diseases, require medical assessment, medication review, and optimization before the surgery.
Technique or Treatment
Anesthesia
General anesthesia associated with a regional nerve block, eg, interscalene brachial plexus block, is usually used for RTSA.[29] Prophylactic intravenous antibiotics and tranexamic acid are given during the induction.
Patient Position
For RTSA, the patient is usually positioned in a beach chair with the chest tilted to 60 degrees. The shoulder should be on the edge to allow full arm extension and adequate exposure of the humerus during the procedure. The anesthetic team should be on the other side of the operative table to give the surgeons enough access to the surgical field.
Examination Under Anesthesia
The shoulder's passive range of motion could be masked by pain, and it is challenging to determine the exact range while the patient is awake. After patient positioning, examining the shoulder for passive range movement and soft tissue tension is useful to allow proper planning for soft tissue contracture release as required.
Surgical Approach
RTSA is commonly performed through the deltopectoral approach or the anterior superior approach.[30] The deltopectoral approach allows better visualization of the lower glenoid, which is important for correct inferior sitting of the base plate. Other advantages of the deltopectoral approach include axillary nerve identification and protection, a lower incidence of axillary nerve palsy, and better access to the humerus shaft, mainly in revision procedures.[31] On the other hand, postoperative instability could be higher after the deltopectoral approach compared to the anterior superior approach.[32] The selection of the operative approach should be based on the surgeon's experience and patient suitability.
Deltopectoral Approach
The bony landmarks for the deltopectoral approach are the coracoid process, the acromion, and the proximal humerus. The inter-nervous plane for the deltopectoral approach is between the axillary nerve, which innervates the deltoid muscle, and medial and lateral pectoral nerves, which innervate the pectoralis major muscle. A 10 to 15-cm-long skin incision is required along the line between the coracoid process and the proximal humeral shaft over the deltopectoral groove. Careful dissection is required to identify and protect the cephalic vein. The cephalic vein is usually mobilized laterally toward the deltoid muscle. The conjoint tendon (the short head of the biceps and coracobrachialis) is then identified and retracted medially without much traction to protect the musculocutaneous nerve. The clavipectoral fascia should be incised lateral to the conjoint tendon to expose the subscapularis muscle. The subscapularis muscle can be divided lateral to the musculotendinous junction to reveal the shoulder joint capsule.
Anterior Superior Approach
Bony landmarks are the acromion, acromioclavicular joint, and anterior border of the clavicle. Skin incision starts 1 cm medial to and on the anterior half of the acromioclavicular joint. The incision extends in the line of the anterior clavicle border to the point 3 cm lateral to the acromion. The anterior deltoid muscle fibers are identified and detached from the acromion. The subacromial bursa is excised, and the rotator cuff muscles are explored.[30]
PROCEDURE STEPS
Humeral Head Dislocation
Regardless of the surgical approach, the humeral head is dislocated by adducting and externally rotating the arm while pushing the elbow upward and forward.[29] Anterior and inferior osteophytes are removed from the proximal humerus to identify the level of humeral resection and the anatomical neck.
Humeral Preparation
The humeral head is resected slightly below the greater tuberosity tip. Intramedullary or extramedullary guides can guide the humeral head resection depending on the surgeon's preference. Resection guides are specific for each prosthesis type and have different resection angles. The humeral head is usually resected at about 30 degrees of retroversion. The forearm axis with the elbow flexed at 90 degrees is used as a reference axis for retroversion.[29] The humeral canal is reamed using different reamer sizes and then prepared by humeral stem broaches. A version rod could be attached to the broach handle to monitor the version during humerus preparation. Two types of humeral stems are available, whether press-fit cementless or cemented. The size of the press-fit cementless stem is equal to the size of the final humeral broach size. The cemented stem size is smaller by 2 mm compared to the final broach size, allowing space for the cement around the stem.
Glenoid Exposure
The subscapularis muscle is released and retracted medially by a glenoid retractor to expose the glenoid. The labarum is excised from all around the glenoid for full glenoid exposure. The Diathermy tip is used to identify the anterior bony margin of the glenoid (position at 5 o’clock on the right shoulder and 7 o’clock on the left shoulder). The glenoid's bony margins are adequately exposed and examined to address any bony deficit in the glenoid before seating the base plate. Eccentric reaming and bone graft can manage glenoid bony defects under 25 degrees. For example, suppose there is bone loss from the superior glenoid. In that case, the upward tilt of the glenoid component is avoided by reaming inferiorly and superior bone graft insertion to maintain the desired inferior tilt of 10 degrees. Glenoid bony defect larger than 25 degrees requires a patient-specific graft prepared using preoperative 3D planning software.
Glenoid Preparation
Identifying the lower glenoid rim for proper base plate seating is important. Inferiorly placed base plate with 10 degrees inferior tilt was shown to reduce the base plate failure.[33] A central pilot wire is inserted to guide the glenoid reamer. The glenoid reamer is applied along the guidewire to prepare the glenoid surface and expose the subchondral bone for adequate seating of the base plate. Glenoid reaming could be performed at 0 or 10 degrees of inferior tilt. An inferiorly tilted position for the glenoid component increases the deltoid muscle tension due to inferior displacement of the humerus and reduces early glenoid failure. Still, it does not reduce the incidence of scapular notching.[33][34] The pilot wire is then removed from the glenoid. The base plate holder is used to apply for the glenoid plate, and it is flushed to the bone. The base is compressed to the exposed glenoid subchondral bone by the central screw. Superior and inferior screws aiming for dense bone are inserted to secure the base plate and reduce the micromotion at the plate-bone interface.
Glenosphere Trial and Offset Selection
Lateralized and eccentric glenosphere in modern prosthesis designs reduces the scapular notching and allows a better range of motion without bony impingement.[35] Depending on the prosthesis type, different geosphere sizes (32 to 42 mm) are available. A glenosphere trial determines the suitable diameter size, offset, and eccentricity direction. The suitable glenosphere should allow enough separation from the scapular pillar anteriorly, posteriorly, and inferiorly and free humeral component movement without superior impingement. The 36 mm glenosphere size is commonly used for female and 40 mm for male patients. Trials with different lateral offsets and eccentricities can be carried out to find adequate soft tissue tension. Proper soft tissue tension is necessary for prosthesis stability and deltoid muscle function after RTSA.[36]
Humeral Tray and Polyethylene Insert Trial
The humeral tray and polyethylene insert trials are mounted onto the stem trial, and the prosthesis trial is reduced and checked. Different tray and insert heights can be tried to achieve the optimal range of motion, soft tissue tension, and stability. The deltoid muscle, conjoint tendon, and triceps muscle tension are tested to ensure proper soft tissue tension. Joint stability in all directions without impingement is checked at this stage. The proximal humerus can be resected into a lower level if excessive soft tissue tension or the prosthesis trial cannot be reduced.
Definitive Prosthesis
Once all components are confirmed and checked, trial components are removed, and the definitive components are prepared for use. The glenosphere is implanted first while there is more space, followed by implanting the humeral stem (cemented or cementless). If a cemented stem is to be used, antibiotic-loaded cement is recommended. A cement restrictor is inserted, and the humeral canal is washed and dried before cementing. Once the stem is secured, the humeral cup is mounted onto the humeral stem, followed by the polyethylene insert on top. The prosthesis is reduced, and a final check is carried out for stability and soft tissue tension. The wound is then washed out with normal saline.
Subscapularis Muscles Repair and Wound Closure
It is still controversial whether to repair the subscapularis muscle during RTSA. Some published evidence indicates increased shoulder internal rotation after subscapularis muscle repair.[16][37] However, several studies showed no difference in shoulder instability in subscapularis muscle repair.[38][39][40] If the subscapularis muscle is of good soft tissue quality, the surgeon may decide whether to repair it.
Post-operative Care
The operated shoulder is immobilized in a board arm sling after the surgery for 2 weeks. The external rotation beyond the neutral level of the arm is avoided for the first 4 weeks. Pendulum shoulder exercises and elbow movement can start early. Post-operative X-rays are obtained to check the prosthesis components, anchoring screws, and the bone of the humerus and glenoid. Regular follow-up should be organized, starting 2 weeks after the surgery, to check the surgical wound and exclude any complications.
ADVANCES of RTSA
Stemless Humeral Component
The stemless humeral component is also known as the canal-sparing humeral component. The canal-sparing component is especially useful for patients who have a humeral deformity. The stemless humeral component preserves the bony stock of the proximal humerus if it is needed for future secondary revision procedures.[41] Humeral stemless components have been reported to reduce operative time and blood loss in anatomical total shoulder arthroplasty.[42] Midterm follow-up results for RTSA with the stemless humeral components are promising, with no reported component loosening.[41][43]
Preoperative 3D Planning Software
Digital software is a new development to allow virtual RTSA preoperative planning. This software creates a 3D virtual shoulder model from the shoulder 3D CT scan. It enables the surgeon to navigate the shoulder, identify bony loss, and plan for different component sizes, glenoid component tilt, and humeral component versions.[44] 3D preoperative planning is particularly beneficial for preparing patient-specific grafts for complex glenoid bone defects.
Complications
Prothesis Infection
The infection rate post-RTSA ranges from 1 % to 10 %.[16][45][46] Rheumatoid arthritis was found to increase the risk of postoperative infection in patients having RTSA.[19] Also, the infection rate is higher in revision RTSA compared to primary RTSA.[4] Acute infection (within 3 weeks) is manageable by debridement, antibiotics, polyethylene insert exchange, and retention of the prosthesis components. The presentation of infection after 3 weeks usually requires 1 or 2 stages of revision surgery.
Prothesis Instability and Dislocation
The reported prosthesis dislocation rate after RTSA is about 3.6%.[32] Factors that cause prosthesis instability after RTSA include inadequate soft tissue tension, mechanical impingement, deltoid dysfunction, axillary nerve palsy, deficient bony glenoid, malpositioned components, small glenosphere, and too medialised glenoid base plate.[47][48] Instability after RTSA is usually anterior, with the arm extended, adducted, and internally rotated.[49] The deltopectoral approach has been associated with a higher dislocation rate than the anterosuperior trans deltoid approach.[30]
Axillary Nerve Palsy
Transient axillary nerve neuropraxia may result from nerve traction caused by the arm position during glenoid exposure.[50] Humeral lengthening in some RTSA designs can cause brachial plexus traction and axillary nerve palsy.[4]
Scapular Notching
Mechanical impingement of the humeral prosthesis against the scapular neck may result in scapular notching.[4] There is a high incidence of scapular notching associated with the Grommet-style RTSA (51 to 96%).[51][52] The risk of inferior scapula notching could be reduced by placing the glenosphere inferiorly in the Grammot-style RTSA.
Sirveaux classified the scapular RTSA notching based on the extent of scapular bony defects in X-rays into 4 grades:
- Grade 1: scapular bony defects are confined to the pillar
- Grade 2; scapular bony defects extend to the glenoid baseplate lower screw
- Grade 3; scapular bony defects extend beyond the glenoid baseplate lower screw.
- Grade 4; loose glenoid base plate[53]
Base Plate Failure
The glenoid baseplate failure can result from increased motion at the baseplate–bone interface and inadequate bone growth into the plate. Several advancements in prosthesis design have been introduced to overcome this mode of prosthesis failure. The use of variable angle locking screws to fix the base plate has been suggested to engage the dense bone in the coracoid base scapular spine.[54] A central compressive locking screw has been introduced to compress the base plate into the glenoid and minimize plate micromotion.[55] The combination of base plate inferior tilt and locking screws has been proven to minimize the shearing forces at the baseplate-bone interface and minimize the failure rate.[56] To treat this mode of failure, a revision arthroplasty surgery is required to change the base plate. Bone grafts and locking screws are usually needed.[57]
Other Less Common Complications After RTSA
- Iatrogenic intraoperative Periprosthetic fracture
- Vascular injury
- Heterotopic ossification
- Surgical scar complications
Clinical Significance
RTSA is gaining popularity around the world to treat different shoulder pathological conditions in adult populations. More than 60,000 RTSA are performed yearly in the USA with satisfactory results. RTSA is mainly used to treat patients who suffer from deficient rotator cuff function with or without glenohumeral articulation arthritis. The alteration of the shoulder's rotation allows the deltoid muscle more mechanical advantage to mobilize the arm.
Enhancing Healthcare Team Outcomes
The healthcare practitioners managing patients with shoulder disease need to be familiar with the RTSA procedures. Understanding the indications and biomechanics for RTSA is necessary to select suitable patients for this type of treatment. Arthroplasty nurses and physicians should provide clear postoperative counseling about arm usage after the surgery. Patient education about safe arm use after RTSA reduces the risk of prosthesis dislocation. Post-operative proper pain management and physiotherapy lead rehabilitation are essential to achieve enhanced recovery and reduce the length of hospital stay. Regular follow-up consultations after the surgery with serial postoperative X-rays and clinical assessment are required to identify any postoperative complications.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Best MJ, Aziz KT, Wilckens JH, McFarland EG, Srikumaran U. Increasing incidence of primary reverse and anatomic total shoulder arthroplasty in the United States. Journal of shoulder and elbow surgery. 2021 May:30(5):1159-1166. doi: 10.1016/j.jse.2020.08.010. Epub 2020 Aug 26 [PubMed PMID: 32858194]
Reeves B, Jobbins B, Flowers F, Dowson D, Wright V. Some problems in the development of a total shoulder endo-prosthesis. Annals of the rheumatic diseases. 1972 Sep:31(5):425-6 [PubMed PMID: 5071643]
Baulot E, Sirveaux F, Boileau P. Grammont's idea: The story of Paul Grammont's functional surgery concept and the development of the reverse principle. Clinical orthopaedics and related research. 2011 Sep:469(9):2425-31. doi: 10.1007/s11999-010-1757-y. Epub [PubMed PMID: 21210311]
Level 3 (low-level) evidenceCheung E, Willis M, Walker M, Clark R, Frankle MA. Complications in reverse total shoulder arthroplasty. The Journal of the American Academy of Orthopaedic Surgeons. 2011 Jul:19(7):439-49 [PubMed PMID: 21724923]
Flatow EL, Harrison AK. A history of reverse total shoulder arthroplasty. Clinical orthopaedics and related research. 2011 Sep:469(9):2432-9. doi: 10.1007/s11999-010-1733-6. Epub [PubMed PMID: 21213090]
Chang LR, Anand P, Varacallo M. Anatomy, Shoulder and Upper Limb, Glenohumeral Joint. StatPearls. 2024 Jan:(): [PubMed PMID: 30725703]
Lugo R, Kung P, Ma CB. Shoulder biomechanics. European journal of radiology. 2008 Oct:68(1):16-24. doi: 10.1016/j.ejrad.2008.02.051. Epub 2008 Jun 3 [PubMed PMID: 18511227]
Jeong J, Bryan J, Iannotti JP. Effect of a variable prosthetic neck-shaft angle and the surgical technique on replication of normal humeral anatomy. The Journal of bone and joint surgery. American volume. 2009 Aug:91(8):1932-41. doi: 10.2106/JBJS.H.00729. Epub [PubMed PMID: 19651952]
Grammont PM, Baulot E. The classic: Delta shoulder prosthesis for rotator cuff rupture. 1993. Clinical orthopaedics and related research. 2011 Sep:469(9):2424. doi: 10.1007/s11999-011-1960-5. Epub [PubMed PMID: 21732025]
Boileau P, Watkinson DJ, Hatzidakis AM, Balg F. Grammont reverse prosthesis: design, rationale, and biomechanics. Journal of shoulder and elbow surgery. 2005 Jan-Feb:14(1 Suppl S):147S-161S [PubMed PMID: 15726075]
Drake GN, O'Connor DP, Edwards TB. Indications for reverse total shoulder arthroplasty in rotator cuff disease. Clinical orthopaedics and related research. 2010 Jun:468(6):1526-33. doi: 10.1007/s11999-009-1188-9. Epub [PubMed PMID: 20049573]
Boileau P, Sinnerton RJ, Chuinard C, Walch G. Arthroplasty of the shoulder. The Journal of bone and joint surgery. British volume. 2006 May:88(5):562-75 [PubMed PMID: 16645099]
Neer CS 2nd, Craig EV, Fukuda H. Cuff-tear arthropathy. The Journal of bone and joint surgery. American volume. 1983 Dec:65(9):1232-44 [PubMed PMID: 6654936]
Petrillo S, Longo UG, Papalia R, Denaro V. Reverse shoulder arthroplasty for massive irreparable rotator cuff tears and cuff tear arthropathy: a systematic review. Musculoskeletal surgery. 2017 Aug:101(2):105-112. doi: 10.1007/s12306-017-0474-z. Epub 2017 Apr 25 [PubMed PMID: 28444541]
Level 1 (high-level) evidenceBufquin T, Hersan A, Hubert L, Massin P. Reverse shoulder arthroplasty for the treatment of three- and four-part fractures of the proximal humerus in the elderly: a prospective review of 43 cases with a short-term follow-up. The Journal of bone and joint surgery. British volume. 2007 Apr:89(4):516-20 [PubMed PMID: 17463122]
Level 3 (low-level) evidenceWall B, Nové-Josserand L, O'Connor DP, Edwards TB, Walch G. Reverse total shoulder arthroplasty: a review of results according to etiology. The Journal of bone and joint surgery. American volume. 2007 Jul:89(7):1476-85 [PubMed PMID: 17606786]
Walker M, Willis MP, Brooks JP, Pupello D, Mulieri PJ, Frankle MA. The use of the reverse shoulder arthroplasty for treatment of failed total shoulder arthroplasty. Journal of shoulder and elbow surgery. 2012 Apr:21(4):514-22. doi: 10.1016/j.jse.2011.03.006. Epub 2011 Jun 8 [PubMed PMID: 21641825]
Level 2 (mid-level) evidenceLevy J, Frankle M, Mighell M, Pupello D. The use of the reverse shoulder prosthesis for the treatment of failed hemiarthroplasty for proximal humeral fracture. The Journal of bone and joint surgery. American volume. 2007 Feb:89(2):292-300 [PubMed PMID: 17272443]
Holcomb JO, Hebert DJ, Mighell MA, Dunning PE, Pupello DR, Pliner MD, Frankle MA. Reverse shoulder arthroplasty in patients with rheumatoid arthritis. Journal of shoulder and elbow surgery. 2010 Oct:19(7):1076-84. doi: 10.1016/j.jse.2009.11.049. Epub 2010 Apr 2 [PubMed PMID: 20363159]
Feeley BT, Gallo RA, Craig EV. Cuff tear arthropathy: current trends in diagnosis and surgical management. Journal of shoulder and elbow surgery. 2009 May-Jun:18(3):484-94. doi: 10.1016/j.jse.2008.11.003. Epub 2009 Feb 8 [PubMed PMID: 19208484]
Naveed MA, Kitson J, Bunker TD. The Delta III reverse shoulder replacement for cuff tear arthropathy: a single-centre study of 50 consecutive procedures. The Journal of bone and joint surgery. British volume. 2011 Jan:93(1):57-61. doi: 10.1302/0301-620X.93B1.24218. Epub [PubMed PMID: 21196544]
Grammont PM, Baulot E. Delta shoulder prosthesis for rotator cuff rupture. Orthopedics. 1993 Jan:16(1):65-8 [PubMed PMID: 8421661]
Level 2 (mid-level) evidenceGrassi FA, Murena L, Valli F, Alberio R. Six-year experience with the Delta III reverse shoulder prosthesis. Journal of orthopaedic surgery (Hong Kong). 2009 Aug:17(2):151-6 [PubMed PMID: 19721141]
Werner BS, Chaoui J, Walch G. The influence of humeral neck shaft angle and glenoid lateralization on range of motion in reverse shoulder arthroplasty. Journal of shoulder and elbow surgery. 2017 Oct:26(10):1726-1731. doi: 10.1016/j.jse.2017.03.032. Epub 2017 May 17 [PubMed PMID: 28528016]
Middernacht B, Van Tongel A, De Wilde L. A Critical Review on Prosthetic Features Available for Reversed Total Shoulder Arthroplasty. BioMed research international. 2016:2016():3256931. doi: 10.1155/2016/3256931. Epub 2016 Dec 25 [PubMed PMID: 28105417]
Bicknell RT, Patterson SD, King GJ, Chess DG, Johnson JA. Glenoid vault endosteal dimensions: an anthropometric study with special interest in implant design. Journal of shoulder and elbow surgery. 2007 May-Jun:16(3 Suppl):S96-101 [PubMed PMID: 17097310]
Iannotti J, Baker J, Rodriguez E, Brems J, Ricchetti E, Mesiha M, Bryan J. Three-dimensional preoperative planning software and a novel information transfer technology improve glenoid component positioning. The Journal of bone and joint surgery. American volume. 2014 May 7:96(9):e71. doi: 10.2106/JBJS.L.01346. Epub [PubMed PMID: 24806017]
Iannotti JP, Weiner S, Rodriguez E, Subhas N, Patterson TE, Jun BJ, Ricchetti ET. Three-dimensional imaging and templating improve glenoid implant positioning. The Journal of bone and joint surgery. American volume. 2015 Apr 15:97(8):651-8. doi: 10.2106/JBJS.N.00493. Epub [PubMed PMID: 25878309]
Nerot C, Ohl X. Primary shoulder reverse arthroplasty: surgical technique. Orthopaedics & traumatology, surgery & research : OTSR. 2014 Feb:100(1 Suppl):S181-90. doi: 10.1016/j.otsr.2013.06.011. Epub 2014 Jan 21 [PubMed PMID: 24461235]
Molé D, Wein F, Dézaly C, Valenti P, Sirveaux F. Surgical technique: the anterosuperior approach for reverse shoulder arthroplasty. Clinical orthopaedics and related research. 2011 Sep:469(9):2461-8. doi: 10.1007/s11999-011-1861-7. Epub [PubMed PMID: 21448776]
Gerber C, Pennington SD, Nyffeler RW. Reverse total shoulder arthroplasty. The Journal of the American Academy of Orthopaedic Surgeons. 2009 May:17(5):284-95 [PubMed PMID: 19411640]
Molé D, Favard L. [Excentered scapulohumeral osteoarthritis]. Revue de chirurgie orthopedique et reparatrice de l'appareil moteur. 2007 Oct:93(6 Suppl):37-94 [PubMed PMID: 18033091]
Tashjian RZ, Martin BI, Ricketts CA, Henninger HB, Granger EK, Chalmers PN. Superior Baseplate Inclination Is Associated With Instability After Reverse Total Shoulder Arthroplasty. Clinical orthopaedics and related research. 2018 Aug:476(8):1622-1629. doi: 10.1097/CORR.0000000000000340. Epub [PubMed PMID: 29781910]
Edwards TB, Trappey GJ, Riley C, O'Connor DP, Elkousy HA, Gartsman GM. Inferior tilt of the glenoid component does not decrease scapular notching in reverse shoulder arthroplasty: results of a prospective randomized study. Journal of shoulder and elbow surgery. 2012 May:21(5):641-6. doi: 10.1016/j.jse.2011.08.057. Epub 2011 Nov 12 [PubMed PMID: 22079769]
Level 1 (high-level) evidenceCuff D, Clark R, Pupello D, Frankle M. Reverse shoulder arthroplasty for the treatment of rotator cuff deficiency: a concise follow-up, at a minimum of five years, of a previous report. The Journal of bone and joint surgery. American volume. 2012 Nov 7:94(21):1996-2000. doi: 10.2106/JBJS.K.01206. Epub [PubMed PMID: 23138240]
Virani NA, Cabezas A, Gutiérrez S, Santoni BG, Otto R, Frankle M. Reverse shoulder arthroplasty components and surgical techniques that restore glenohumeral motion. Journal of shoulder and elbow surgery. 2013 Feb:22(2):179-87. doi: 10.1016/j.jse.2012.02.004. Epub 2012 May 22 [PubMed PMID: 22621793]
Friedman RJ, Flurin PH, Wright TW, Zuckerman JD, Roche CP. Comparison of reverse total shoulder arthroplasty outcomes with and without subscapularis repair. Journal of shoulder and elbow surgery. 2017 Apr:26(4):662-668. doi: 10.1016/j.jse.2016.09.027. Epub 2016 Oct 27 [PubMed PMID: 28277259]
Grassi FA, Zorzolo I. Reverse shoulder arthroplasty without subscapularis repair for the treatment of proximal humeral fractures in the elderly. Musculoskeletal surgery. 2014 Apr:98 Suppl 1():5-13. doi: 10.1007/s12306-014-0321-4. Epub 2014 Mar 23 [PubMed PMID: 24659198]
Level 2 (mid-level) evidenceClark JC, Ritchie J, Song FS, Kissenberth MJ, Tolan SJ, Hart ND, Hawkins RJ. Complication rates, dislocation, pain, and postoperative range of motion after reverse shoulder arthroplasty in patients with and without repair of the subscapularis. Journal of shoulder and elbow surgery. 2012 Jan:21(1):36-41. doi: 10.1016/j.jse.2011.04.009. Epub 2011 Jul 31 [PubMed PMID: 21803609]
Level 2 (mid-level) evidenceWerner BC, Wong AC, Mahony GT, Craig EV, Dines DM, Warren RF, Gulotta LV. Clinical Outcomes After Reverse Shoulder Arthroplasty With and Without Subscapularis Repair: The Importance of Considering Glenosphere Lateralization. The Journal of the American Academy of Orthopaedic Surgeons. 2018 Mar 1:26(5):e114-e119. doi: 10.5435/JAAOS-D-16-00781. Epub [PubMed PMID: 29419724]
Level 2 (mid-level) evidenceTeissier P, Teissier J, Kouyoumdjian P, Asencio G. The TESS reverse shoulder arthroplasty without a stem in the treatment of cuff-deficient shoulder conditions: clinical and radiographic results. Journal of shoulder and elbow surgery. 2015 Jan:24(1):45-51. doi: 10.1016/j.jse.2014.04.005. Epub 2014 Jul 11 [PubMed PMID: 25027480]
Berth A, Pap G. Stemless shoulder prosthesis versus conventional anatomic shoulder prosthesis in patients with osteoarthritis: a comparison of the functional outcome after a minimum of two years follow-up. Journal of orthopaedics and traumatology : official journal of the Italian Society of Orthopaedics and Traumatology. 2013 Mar:14(1):31-7. doi: 10.1007/s10195-012-0216-9. Epub 2012 Nov 9 [PubMed PMID: 23138538]
Moroder P, Ernstbrunner L, Zweiger C, Schatz M, Seitlinger G, Skursky R, Becker J, Resch H, Krifter RM. Short to mid-term results of stemless reverse shoulder arthroplasty in a selected patient population compared to a matched control group with stem. International orthopaedics. 2016 Oct:40(10):2115-2120 [PubMed PMID: 27438011]
Wylie JD, Tashjian RZ. Planning software and patient-specific instruments in shoulder arthroplasty. Current reviews in musculoskeletal medicine. 2016 Mar:9(1):1-9. doi: 10.1007/s12178-016-9312-4. Epub [PubMed PMID: 26809956]
Werner CM, Steinmann PA, Gilbart M, Gerber C. Treatment of painful pseudoparesis due to irreparable rotator cuff dysfunction with the Delta III reverse-ball-and-socket total shoulder prosthesis. The Journal of bone and joint surgery. American volume. 2005 Jul:87(7):1476-86 [PubMed PMID: 15995114]
Cho CH, Song KS, Koo TW. Clinical Outcomes and Complications during the Learning Curve for Reverse Total Shoulder Arthroplasty: An Analysis of the First 40 Cases. Clinics in orthopedic surgery. 2017 Jun:9(2):213-217. doi: 10.4055/cios.2017.9.2.213. Epub 2017 May 8 [PubMed PMID: 28567225]
Level 2 (mid-level) evidenceChae J, Siljander M, Wiater JM. Instability in Reverse Total Shoulder Arthroplasty. The Journal of the American Academy of Orthopaedic Surgeons. 2018 Sep 1:26(17):587-596. doi: 10.5435/JAAOS-D-16-00408. Epub [PubMed PMID: 30074512]
Boileau P. Complications and revision of reverse total shoulder arthroplasty. Orthopaedics & traumatology, surgery & research : OTSR. 2016 Feb:102(1 Suppl):S33-43. doi: 10.1016/j.otsr.2015.06.031. Epub 2016 Feb 12 [PubMed PMID: 26879334]
Affonso J, Nicholson GP, Frankle MA, Walch G, Gerber C, Garzon-Muvdi J, McFarland EG. Complications of the reverse prosthesis: prevention and treatment. Instructional course lectures. 2012:61():157-68 [PubMed PMID: 22301230]
Lynch NM, Cofield RH, Silbert PL, Hermann RC. Neurologic complications after total shoulder arthroplasty. Journal of shoulder and elbow surgery. 1996 Jan-Feb:5(1):53-61 [PubMed PMID: 8919443]
Simovitch RW, Zumstein MA, Lohri E, Helmy N, Gerber C. Predictors of scapular notching in patients managed with the Delta III reverse total shoulder replacement. The Journal of bone and joint surgery. American volume. 2007 Mar:89(3):588-600 [PubMed PMID: 17332108]
Lévigne C, Boileau P, Favard L, Garaud P, Molé D, Sirveaux F, Walch G. Scapular notching in reverse shoulder arthroplasty. Journal of shoulder and elbow surgery. 2008 Nov-Dec:17(6):925-35. doi: 10.1016/j.jse.2008.02.010. Epub 2008 Jun 16 [PubMed PMID: 18558499]
Sirveaux F, Favard L, Oudet D, Huquet D, Walch G, Molé D. Grammont inverted total shoulder arthroplasty in the treatment of glenohumeral osteoarthritis with massive rupture of the cuff. Results of a multicentre study of 80 shoulders. The Journal of bone and joint surgery. British volume. 2004 Apr:86(3):388-95 [PubMed PMID: 15125127]
Codsi MJ, Iannotti JP. The effect of screw position on the initial fixation of a reverse total shoulder prosthesis in a glenoid with a cavitary bone defect. Journal of shoulder and elbow surgery. 2008 May-Jun:17(3):479-86. doi: 10.1016/j.jse.2007.09.002. Epub 2008 Feb 20 [PubMed PMID: 18282725]
Harman M, Frankle M, Vasey M, Banks S. Initial glenoid component fixation in "reverse" total shoulder arthroplasty: a biomechanical evaluation. Journal of shoulder and elbow surgery. 2005 Jan-Feb:14(1 Suppl S):162S-167S [PubMed PMID: 15726076]
Gutiérrez S, Keller TS, Levy JC, Lee WE 3rd, Luo ZP. Hierarchy of stability factors in reverse shoulder arthroplasty. Clinical orthopaedics and related research. 2008 Mar:466(3):670-6. doi: 10.1007/s11999-007-0096-0. Epub 2008 Feb 10 [PubMed PMID: 18264855]
Holcomb JO, Cuff D, Petersen SA, Pupello DR, Frankle MA. Revision reverse shoulder arthroplasty for glenoid baseplate failure after primary reverse shoulder arthroplasty. Journal of shoulder and elbow surgery. 2009 Sep-Oct:18(5):717-23. doi: 10.1016/j.jse.2008.11.017. Epub 2009 Mar 17 [PubMed PMID: 19278872]
Level 2 (mid-level) evidence