Introduction
Pseudoexfoliation syndrome (PEX) is a systemic disorder that leads to the accumulation of extracellular material in various ocular tissues, [1] which presents primarily via its characteristic ocular manifestations.[2] PEX is considered an age-related microfibillopathy that affects different systemic organs and is characterized by a progressive chronic deposition and accumulation of extracellular greyish-white material in several organs.[3] PEX can lead to secondary glaucoma, known as pseudoexfoliative glaucoma (PEG), which is a major cause of blindness worldwide.
This systemic disorder is typically clinically diagnosed during routine ophthalmic examination with slit-lamp visualization of white, flaky fibrillar (pseudoexfoliative) material on the pupillary margin of the iris and the anterior lens capsule.[4] This condition affects the eyes, particularly the lens and the trabecular meshwork, which are responsible for regulating the flow of fluid in the eye.[5] Small flakes of material, resembling dandruff, tend to accumulate on the surface of the lens, the iris, and the ciliary body. This accumulation can cause increased intraocular pressure (IOP) inside the eye, leading to glaucoma and other vision problems. PEX is more common in individuals over the age of 60 and is often associated with a higher risk of cataract development. While there is currently no cure for PEX, early diagnosis, and treatment can help prevent or slow the progression of vision loss.
The history of PEX dates back to the early 20th century in 1917 when John G. Lindberg first described the characteristic flaky material found in the eyes of patients with glaucoma.[6] However, it wasn't until the 1950s that the term "Pseudoexfoliation" was used to describe the condition.[7] Initially, PEX was thought to be a benign age-related change in the eye, but as more research was conducted, it became clear that PEX was associated with an increased risk of glaucoma and other vision problems. Since then, PEX has been extensively studied, and numerous researchers have contributed to our understanding of the condition's pathogenesis, genetics, and clinical implications. Despite ongoing research, the exact cause of PEX remains unclear, and there is currently no cure for the condition.
PEX may be present unilaterally or bilaterally. PEX is strongly associated with raised intraocular pressures (IOP) in up to 44% of patients and subsequent development of pseudoexfoliation glaucoma (PEG), making it the most commonly identifiable cause of secondary open-angle glaucoma.[8] PEX is also associated with technically challenging cataract surgery: PEX eyes dilate poorly and have unstable lens zonules, which may lead to a higher risk of complications such as capsular bag rupture, zonular dialysis, and loss of vitreous.[9]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
PEX is characterized by the formation of white, flaky deposits on the lens and iris, which can cause several ocular complications, including cataracts, glaucoma, and corneal endothelial dysfunction. The exact etiology of PEX is not known, however, genetic susceptibility is supported by numerous genetic studies in population studies worldwide.[10] Variants of certain genes have been found to be more common in individuals with PEX and PEG, suggesting that they may play a role in the development of the condition.
A genetic predisposition has been shown for the following genes:
- Lysyl oxidase-like 1 (LOXL1) enzyme is part of a family of copper-dependent monoamine oxidases secreted by fibroblasts and smooth muscle cells. These enzymes are involved in cross-linking collagen and elastin fibers in the extracellular matrix. Multiple single nucleotide polymorphisms (SNPs) in this gene are associated with PEX, and various high-risk alleles have been identified in different populations, with important associations between LOLX1 and PEX in individuals from Scandinavia, Europe, Asia, Africa, Australia, and North America.[11]
- Chromosome 8p21, Clusterin (CLU) gene: Clusterin is a multifunctional glycoprotein that has been proposed to inhibit stress-induced aggregation of misfolded proteins. Clusterin downregulation has been shown to occur in all anterior segment tissues in PEX eyes, suggesting that its deficiency may be responsible for the chronic accumulation of abnormal extracellular fibrillar material in this condition.[12]
- Calcium Voltage-Gated Channel Subunit Alpha 1 (CACNA1A): Gene variants could possibly influence calcium levels that may lead to PEX depositions.[13]
- Chromosome 1p13.3, Glutathione transferase (GST) gene: GST has a role in protecting cells from oxidative damage. Polymorphisms of GST have been detected in Pakistani patients with PEX. However, no significant association was found in other patient populations.[14]
- Fibulin-5 (FBLN5) gene: Studies have shown that downregulation of two variants of this gene that encode for this extracellular matrix protein have been associated with risks of developing PEG.[15]
PEX deposits are composed of different extracellular, molecular, and membrane proteins and enzymes that include tropoelastin, fibrillin-1, elastin, LOL1, amyloid, fibulin, vitronectin, clusterin, etc.[5] Genetic variants or mutations that cause dysregulation and production of these components can potentially be of importance in the genetic susceptibility for PEX and PEG.[16] Other genetic associations have also been discovered, including polymorphisms of the CNTNAP2 gene,[17] vimentin, [18] tumor necrosis factor-alpha, tumor growth factor beta 1, matrix metalloproteinase-1, and 3, proteasome maturation protein (POMP), transmembrane protein 136, semaphorin 6A (SEMA6A), etc.[10][19] Current studies have reported the presence of altered microRNA molecules, which regulate post-transcriptional gene expression, in the aqueous humor of individuals with PEX and PEG.[20]
Environmental factors that have been proposed to play a role in the development of PEX include exposure to sunlight and ultraviolet light,[21] long-term alcohol consumption,[22] excessive caffeine,[23] higher temperatures, climatic and geographical factors, diet, viral infection, and trauma or surgery involving the anterior segment.[11][24] Current studies have shown that factors associated with increased oxidative stress, production of free radicals, disruption of the blood-aqueous barrier, and limited antioxidant defense mechanisms can play important roles in the etiopathogenesis of PEX and PEG.[25][10]
The insoluble extracellular aggregates in the anterior chamber can decrease aqueous humor outflow and congestion in the trabecular meshwork, causing large fluctuations and increases in IOP, thus leading to PEG in some individuals with PEX. PEG is a type of secondary glaucoma as a result of PEX, which is a progressive disease that can lead to blindness if left untreated. Signs of this type of secondary glaucoma include irreversible optic nerve head and retinal nerve fiber layer damage and/or visual field defects,[26] which tend to be more severe and progress at faster rates when compared to primary open-angle glaucoma (POAG).[27][26]
Epidemiology
The prevalence of PEX increases markedly with age. It is estimated that up to 20% of the over-60 population may be affected.[4] PEX is found in all geographic populations with a significant variation in prevalence, which range from 0% to 38%.[28] In populations of Scandinavian, Northern European, and Mediterranean descent, the prevalence of PEX is estimated to be between 5% and 20%. Nordic and Eastern Mediterranean countries are most affected, and East Asian and Inuit populations have the lowest reported prevalence. Studies have reported that the prevalence of PEX can be as low as 0% in Greenland Inuits[29] and as high as 38 % in Navajo Nation Indians.[30]The variation in prevalence has been proposed to be due to epigenetics, the attitude of inhabitants, UV exposure, climatic conditions, proximity to the equator, dietary factors, oxidative stress mechanisms, genetic predisposition, etc.[31][32]
Risk Factors include:
- Age (strongest risk factor; PEX rarely occurs below the age of 50, with an incidence increasing with age).[26][33]
- Race (Nordic and Eastern Mediterranean populations have increased risk).[34]
- high altitude and/or solar/cosmic radiation.[35][36]
- Female sex (possible risk factor with more recent studies showing equal prevalence in males and females). Studies reporting on association with gender have produced conflicting results, and so the link remains unclear.[37]
- Low consumption of dietary anti-oxidants, smoking, caffeine, alcohol, and factors that favor oxidative stress[10][25]
The diagnosis of secondary glaucoma or PEG is reached when PEX is associated with elevated and fluctuating IOP levels, in addition to functional alterations in computerized perimetry and/or defects in the optic nerve and retinal nerve fiber layer. PEG accounts for 25 % of individuals with open-angle glaucoma worldwide, in which PEX shows a cumulative probability to develop in PEG in 15% of cases within 10 years.[38] There is progressive damage and loss of retinal ganglion cells that cause irreversible peripheral vision loss in PEG.
Pathophysiology
PEX material is most readily found in the structures of the eye bathed by the aqueous. PEX is a systemic disorder that causes the accumulation of extracellular material in various other organs and body tissues including blood vessels, lungs, heart, liver, gallbladder, kidneys, meninges, and skin.[1][39]
The exact pathophysiological processes that underline PEX remain unclear. Still, it is now well known that PEX is a fibrillopathy. PEX material arises from the abnormal accumulation of elastic microfibrils composed of fibrillin-1, fibulin-2, vitronectin, the enzyme lysyl oxidase, and clusterin, amongst other proteins.[16] Fibrillin molecules aggregate to form microfibrils which are then crosslinked to form PEX fibrils.
Transforming growth factor-beta 1 (TGF-B1) is considered a key mediator of abnormal accumulation of PEX material. It has been shown to promote PEX material formation in vitro and, increased concentrations have been found in the aqueous humor of PEX eyes. There is evidence that oxidative stress plays a role in developing PEX.[40] Decreased levels of ascorbic acid and increased levels of oxidative stress markers have been found in aqueous humor. Iris hypoperfusion and anterior chamber hypoxia are also associated with PEX.
The vasoconstrictor endothelin-1 is found in increased concentrations in PEX, and nitric oxide (a vasodilator) is reduced. It has been suggested that this may further exacerbate cellular stress and lead to the processes behind the development of PEX. Accumulation of abnormal PEX fibrils may be promoted via a deficiency of clusterin, a molecular chaperone that inhibits aggregation of misfolded proteins. Moreover, the dysfunction of matrix metalloproteinases (MMPs) prevents the breakdown and clearance of this abnormal material.[4]
Histopathology
Light microscopy of anterior segment structures demonstrates deposition of PEX material on the iris pigment epithelium, anterior lens capsule, and corneal endothelium (See image of PEX).[41][42] Electron microscopy and immunohistochemical studies have demonstrated the production of PEX material by the non-pigmented epithelial cells of the ciliary body, iris pigment epithelium, pre-equatorial lens epithelium, and corneal endothelium.[43] PEX material seen on the anterior lens capsule and the zonular fibers is thought to be transmitted from the cellular tissues via the aqueous.[44]
Zonular weakness is a characteristic feature of PEX, and loosening of the zonular attachments to the basement membrane of the ciliary body is considered to be primarily responsible for this. This is aggravated by further weakness along the free zonular fibers and their attachment to the basement membrane of the lens capsule. Electron microscopies of conjunctival biopsies taken from fellow eyes in unilateral cases demonstrate the presence of subclinical pseudoexfoliative material, confirming that PEX is a bilateral condition with marked clinical asymmetry.[45][46]
History and Physical
PEX and PEG are typically diagnosed during a comprehensive eye exam. The presence of flaky deposits on the lens and iris is a hallmark of PEX, while an increase in IOP is a characteristic of PEG. Other tests that may be performed include visual field testing, optic nerve imaging, and gonioscopy. Patients are often asymptomatic but may present with peripheral visual field loss from secondary glaucoma or PEG. The diagnosis is made clinically via characteristic slit-lamp biomicroscopy and gonioscopic findings:
Slit Lamp Examination
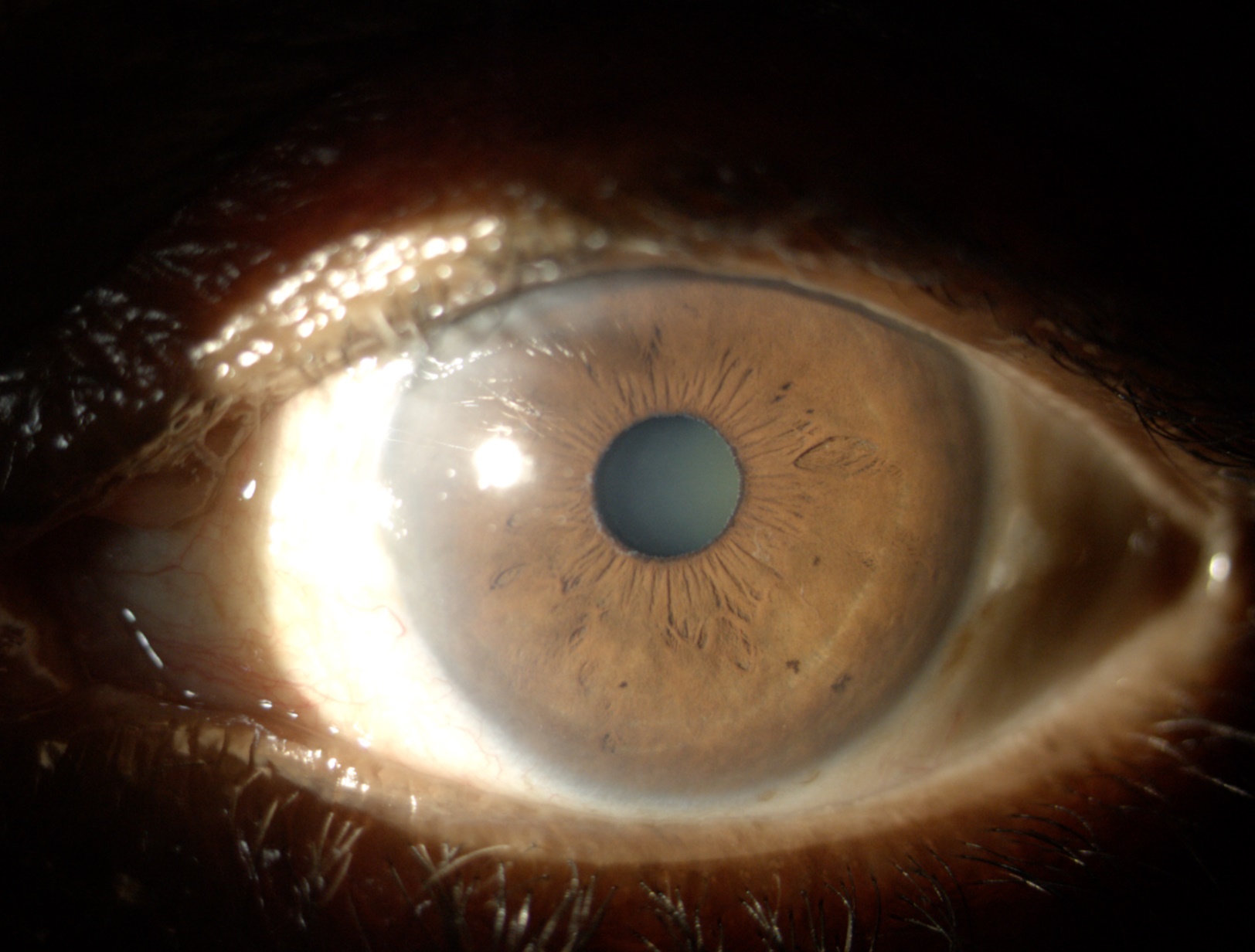
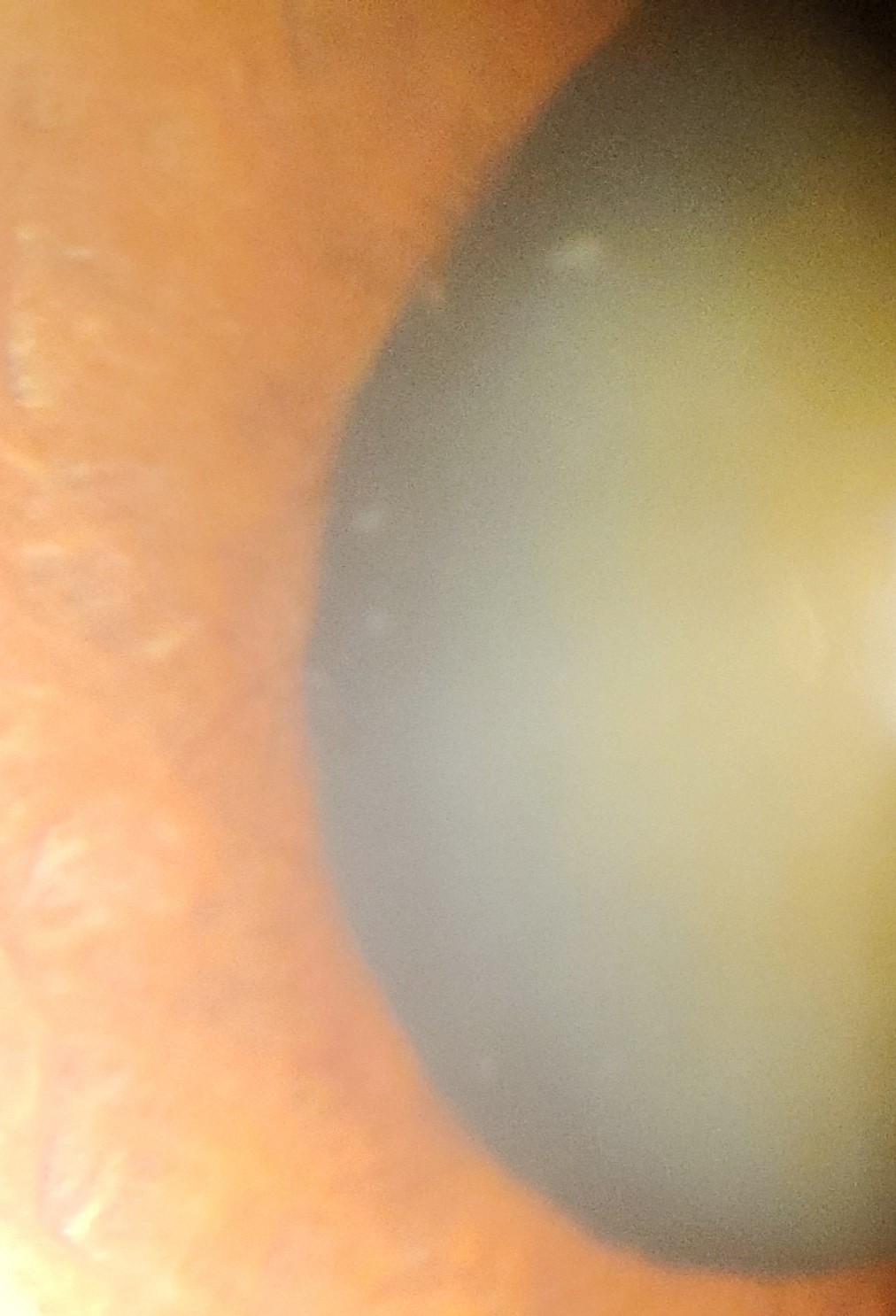
The characteristic finding in PEX is the visualization of white, flaky, dandruff-like PEX material along the pupillary margin and anterior lens capsule (See image of PEX deposits on anterior structures). The anterior lens capsule shows a central disc and peripheral ring of PEX material with a clear intermediate zone maintained by pupillary abrasion. The whitish double concentric ring that is seen on the anterior portion of the lens is probably due to the movement of the iris over the lens. The central disc may be absent in up to 20% of cases, and the peripheral zone may not be completely visualized without the aid of pharmacological dilation.[47]
A thorough, systematic examination may reveal additional signs. The corneal endothelium may show deposition of PEX material that may be erroneously interpreted as keratic precipitates or inflammatory debris. Fine scattered pigment deposits are also present, which may form a vertical line known as a Krukenberg spindle, similar to what may be seen in pigment dispersion syndrome.[48] Studies have shown lower corneal endothelial cell counts and guttae in eyes with PEX.[49]
The aqueous humor may demonstrate PEX particles and mild flare from an impaired blood-aqueous barrier. The iris may demonstrate poor mydriasis secondary to atrophy of the dilator muscle and loss of elasticity due to PEX material accumulation within the iris stroma.[41] There may be a loss of pupillary ruff due to rubbing against the lens, and transillumination defects may be seen along the pupillary margin.[50] This is in contrast to the more mid-peripheral iris transillumination seen in pigment dispersion syndrome.
There may be phacodonesis or lens subluxation/dislocation secondary to zonular weakness. The fragility is thought to be due to the deposition of extracellular material on the zonules and ciliary processes and/or histological fiber alteration.[44] Studies have reported an increased incidence of nuclear sclerotic and subcapsular cataracts in PEX eyes compared to non-PEX eyes, although the pathophysiology is not yet understood.[51][52]
Gonioscopy
Gonioscopy is fundamental and should be performed in all patients with PEX during slit-lamp examination. PEX deposits may be visualized over angle structures. Patchy hyperpigmentation over the trabecular meshwork and Schwalbe line can be observed.[53] This hyperpigmentation may coalesce to form a band of hyperpigmentation on the Schwalbe line known as the Sampaolesi line.[54] In unilateral cases where no PEX material is visible in the fellow eye, trabecular meshwork pigmentation may be an early sign of PEX development. Up to 20% of PEX eyes may have occludable angles predisposing them to acute angle-closure glaucoma.[4][9]
Evaluation
All patients suspected of PEX should undergo a dilated slit-lamp examination and gonioscopy. Baseline IOP must be measured at the time of diagnosis and periodically due to the high risk of developing ocular hypertension and PEG.[55] Genetic testing of eyes is not routinely performed, considering that PEX is a clinical diagnosis.
The evaluation of patients suspected of PEG is identical to those suspected of primary open-angle glaucoma (POAG).[56]
To summarise, the clinical evaluation involves:
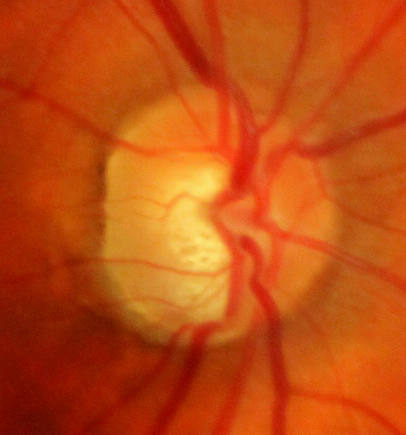
- Visual examination of optic nerve head: This is evaluated using a slit lamp and condensing lens of sufficient magnification. The typical signs associated with glaucomatous damage include an increased cup-to-disc ratio (CDR) greater than 0.5, optic nerve cupping and notching (See image of glaucomatous optic nerve cupping), asymmetry of CDR between both eyes and disc hemorrhages.
- IOP measurement: Glaucomatous damage in pseudoexfoliation is associated with raised IOP and shows marked variation in diurnal IOP levels. IOP should therefore be measured at multiple different times of the day. Goldmann applanation tonometry is the gold standard for assessment,[57] although clinicians should consider the effect of central corneal thickness on the measured value.[58]
- Visual Field Analysis: Static computer automated perimetry is useful for a baseline investigation of glaucoma suspects and monitoring progression in those with confirmed glaucoma. Characteristic field defects are seen as with POAG, but those seen with PEG are typically more severe and tend to progress at a faster rate.[59]
- Optical coherence tomography (OCT): Peripapillary retinal nerve fiber layer (RNFL) thinning is associated with glaucomatous damage. Inferotemporal thinning tends to be associated with early glaucomatous changes, whereas superotemporal thinning may be used to differentiate severe glaucoma from healthy controls.[60]
Treatment / Management
There is currently no treatment available to halt the deposition of PEX material in affected eyes. Management of this condition is primarily aimed at regular (at least annual) eye examinations for early detection and treatment of glaucoma.
Local eye drops: The first-line treatment of PEG is medical. IOP-lowering topical medications that are effective in PEG include prostaglandin analogs, beta-blockers, carbonic anhydrase inhibitors, or a combination of these. Pilocarpine is not recommended due to the risk of worsening angle-closure glaucoma and posterior synechiae formation. PEG is generally more resistant to medical therapy than POAG. Considering the aggressive nature and the risk of faster progression in PEX, the target IOP should be lower than those used to manage POAG.[59]
Selective laser trabeculoplasty (SLT): Laser treatment may be used as a first-line treatment or to avoid more invasive surgery. It is very successful in PEG eyes, reducing IOP by 30%. The increased effectiveness of SLT may be due to the increased pigmentation of the trabecular meshwork, which leads to increased absorption of laser energy. SLT, however, is not permanent, and a significant proportion of patients may require further surgery in the long term. Argon laser trabeculoplasty (ALT), which induces a greater histopathological effect on the trabecular meshwork due to the stronger energy laser applied for a longer time, has also been reported to be useful as a temporary regulation treatment of IOP in PEG.[61] SLT has shown to be less destructive and can be repeatable, thus a viable alternative to traditional ALT.[62](A1)
Surgery: Similar to POAG, surgical management of PEG may be considered following the failure of maximal medical therapy and/or SLT.[63] Trabeculectomy in PEG patients has shown similar outcomes as with POAG patients, without an increased risk of complications.[64] Glaucoma drainage devices and alternative methods like canaloplasty and viscocanalostomy may also be considered, although target pressures after surgery do not tend to be low, thus local eyedrops may still be needed in some patients.[65] If there is a component of angle closure secondary to cataract or anterior lens movement secondary to zonular laxity, laser iridoplasty or cataract extraction may be beneficial.[66](A1)
Cataract surgery may be challenging in PEX eyes due to poor mydriasis, corneal endotheliopathy, zonular instability, and lens subluxation.[67] Zonular laxity may predispose to zonular dialysis, compromised blood-aqueous barrier, capsular bag rupture, and subsequent loss of vitreous.[68] There is also an increased risk of postoperative complications, which include increased inflammation, iris vascular leaks, spikes in IOP, corneal edema, capsular opacification, capsular phimosis, and late intra-ocular lens (IOL) decentration.[69] PEX patients often need a more aggressive and longer duration of treatment with postoperative steroids. Therefore, it is essential to evaluate all eyes undergoing cataract extraction for the presence of pseudoexfoliation to aid surgical planning, prognostication, and follow-up.[67]
Current studies in the literature have shown that new treatments for PEG are constantly being studied. These alternatives to traditional treatment are still being performed in preclinical and animal models, and include magnetic phage display,[70] microRNAs,[71]gene therapies,[72] stem cell therapy,[73] nanotechnology,[74] immunotherapy,[75] and photobiomodulation.[76]
Differential Diagnosis
- Pigment dispersion syndrome: Corneal endothelial changes are similar to those of PEX (Krukenberg spindle).[77] Iris transillumination demonstrates mid-peripheral atrophy as compared to the pupillary border defects seen in PEX. Gonioscopy shows posterior bowing of the iris and more homogenous trabecular meshwork pigmentation than the patchy pigmentation seen in PEX.[78]
- True exfoliation of the lens capsule: This is a rare disorder where the superficial and deeper layers of the anterior lens capsule separate in association with exposure to infrared radiation. Slit-lamp examination shows a thin white membrane emanating from the lens into the anterior chamber.[79]
- Amyloidosis: Amyloid can appear similar to PEX material and deposit within the anterior segment structures.[80]
- Primary open-angle glaucoma: There are no signs of white, flaky deposits on the anterior segment of the eye.[81]
- Primary angle-closure glaucoma: Elevated IOP is due to angle closure without the presence of PEX material.[82]
Prognosis
PEX is significantly associated with the development of glaucoma. In one study, 44% of patients diagnosed with PEX developed ocular hypertension requiring treatment or glaucoma within 15 years of follow-up. In patients diagnosed with unilateral PEX, the risk of the fellow eye developing clinically evident PEX was 29% by 15 years.[8] When compared to POAG, PEX eyes have higher IOPs with an increased diurnal variation. There is a rapid progression of nerve damage, and subsequent field loss may be worse than POAG.
Complications
PEX eyes may be complicated by progression to ocular hypertension or glaucoma (PEG). Cataract surgery may be challenging in these eyes because of corneal endotheliopathy, poor mydriasis, lens subluxation, and zonular instability.[67] Cataract surgical complications include corneal edema, compromised blood-aqueous barrier, capsular opacification, spikes in IOP, capsular phimosis, increased inflammation, zonular dialysis, capsular bag rupture, iris vascular leaks, and late intra-ocular lens (IOL) decentration.[68] PEX and PEG patients tend to require more frequent follow-ups and aggressive therapies in the presence of elevated IOP and signs of glaucomatous functional and anatomical progression.
Deterrence and Patient Education
Patients should be advised to seek annual eye screening to develop ocular hypertension and/or glaucoma. Patients on medical therapy must be advised on the importance of treatment adherence in preventing the progression of glaucomatous damage. Those undergoing cataract surgery must be counseled regarding the increased risks associated with surgery and the possible need for further surgery if there are associated complications. They should be advised of the risk of IOL decentration years following the surgery. PEX is significantly associated with cardiovascular and cerebrovascular disease, and patients may be advised to seek optimization of vascular risk factors with their primary care physician.[83]
Enhancing Healthcare Team Outcomes
Untreated glaucoma may lead to severe peripheral vision loss, followed by central visual loss. Patient outcomes can be improved through ophthalmologist collaboration with other healthcare professionals within the community setting. Community optometrists are well-equipped to perform routine eye examinations, monitor patients’ intraocular pressures, and perform perimetry testing. They are aiming to refer all glaucoma suspects to secondary care. Patients diagnosed with glaucoma need to be closely monitored and provided with ongoing education. The primary care physician can work with patients to treat co-morbidities that may impair the self-administration of eye drops such as arthritis of the hands and optimize vascular risk factors that may be elevated in this patient group. [Level 5]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Mastronikolis S, Pagkalou M, Baroutas G, Kyriakopoulou K, Makri ΟE, Georgakopoulos CD. Pseudoexfoliation syndrome: The critical role of the extracellular matrix in pathogenesis and treatment. IUBMB life. 2022 Oct:74(10):995-1002. doi: 10.1002/iub.2606. Epub 2022 Feb 24 [PubMed PMID: 35201654]
Padhy B, Alone DP. Is pseudoexfoliation glaucoma a neurodegenerative disorder? Journal of biosciences. 2021:46():. pii: 97. Epub [PubMed PMID: 34785624]
Pompoco CJ, Curtin K, Taylor S, Paulson C, Shumway C, Conley M, Barker DJ, Swiston C, Stagg B, Ritch R, Wirostko BM. Summary of Utah Project on Exfoliation Syndrome (UPEXS): using a large database to identify systemic comorbidities. BMJ open ophthalmology. 2021:6(1):e000803. doi: 10.1136/bmjophth-2021-000803. Epub 2021 Oct 27 [PubMed PMID: 34765740]
Schlötzer-Schrehardt U, Naumann GO. Ocular and systemic pseudoexfoliation syndrome. American journal of ophthalmology. 2006 May:141(5):921-937 [PubMed PMID: 16678509]
Challa P, Johnson WM. Composition of Exfoliation Material. Journal of glaucoma. 2018 Jul:27 Suppl 1():S29-S31. doi: 10.1097/IJG.0000000000000917. Epub [PubMed PMID: 29965899]
Grzybowski A, Kanclerz P, Ritch R. The History of Exfoliation Syndrome. Asia-Pacific journal of ophthalmology (Philadelphia, Pa.). 2019 Jan-Feb:8(1):55-61. doi: 10.22608/APO.2018226. Epub 2018 Nov 13 [PubMed PMID: 30421589]
Bansal R, Spivey BE, Honavar SG. PXF, the power of the X-factor - Georgiana Dvorak-Theobald. Indian journal of ophthalmology. 2022 Feb:70(2):359-360. doi: 10.4103/ijo.IJO_63_22. Epub [PubMed PMID: 35086196]
Jeng SM, Karger RA, Hodge DO, Burke JP, Johnson DH, Good MS. The risk of glaucoma in pseudoexfoliation syndrome. Journal of glaucoma. 2007 Jan:16(1):117-21 [PubMed PMID: 17224761]
Level 2 (mid-level) evidencePlateroti P, Plateroti AM, Abdolrahimzadeh S, Scuderi G. Pseudoexfoliation Syndrome and Pseudoexfoliation Glaucoma: A Review of the Literature with Updates on Surgical Management. Journal of ophthalmology. 2015:2015():370371. doi: 10.1155/2015/370371. Epub 2015 Oct 29 [PubMed PMID: 26605078]
Mastronikolis S, Pagkalou M, Plotas P, Kagkelaris K, Georgakopoulos CD. Emerging roles of oxidative stress in the pathogenesis of pseudoexfoliation syndrome (Review). Experimental and therapeutic medicine. 2022 Sep:24(3):602. doi: 10.3892/etm.2022.11539. Epub 2022 Jul 28 [PubMed PMID: 35949329]
Elhawy E, Kamthan G, Dong CQ, Danias J. Pseudoexfoliation syndrome, a systemic disorder with ocular manifestations. Human genomics. 2012 Oct 10:6(1):22. doi: 10.1186/1479-7364-6-22. Epub 2012 Oct 10 [PubMed PMID: 23157966]
Zenkel M, Kruse FE, Jünemann AG, Naumann GO, Schlötzer-Schrehardt U. Clusterin deficiency in eyes with pseudoexfoliation syndrome may be implicated in the aggregation and deposition of pseudoexfoliative material. Investigative ophthalmology & visual science. 2006 May:47(5):1982-90 [PubMed PMID: 16639006]
Aung T, Ozaki M, Mizoguchi T, Allingham RR, Li Z, Haripriya A, Nakano S, Uebe S, Harder JM, Chan AS, Lee MC, Burdon KP, Astakhov YS, Abu-Amero KK, Zenteno JC, Nilgün Y, Zarnowski T, Pakravan M, Safieh LA, Jia L, Wang YX, Williams S, Paoli D, Schlottmann PG, Huang L, Sim KS, Foo JN, Nakano M, Ikeda Y, Kumar RS, Ueno M, Manabe S, Hayashi K, Kazama S, Ideta R, Mori Y, Miyata K, Sugiyama K, Higashide T, Chihara E, Inoue K, Ishiko S, Yoshida A, Yanagi M, Kiuchi Y, Aihara M, Ohashi T, Sakurai T, Sugimoto T, Chuman H, Matsuda F, Yamashiro K, Gotoh N, Miyake M, Astakhov SY, Osman EA, Al-Obeidan SA, Owaidhah O, Al-Jasim L, Al Shahwan S, Fogarty RA, Leo P, Yetkin Y, Oğuz Ç, Kanavi MR, Beni AN, Yazdani S, Akopov EL, Toh KY, Howell GR, Orr AC, Goh Y, Meah WY, Peh SQ, Kosior-Jarecka E, Lukasik U, Krumbiegel M, Vithana EN, Wong TY, Liu Y, Koch AE, Challa P, Rautenbach RM, Mackey DA, Hewitt AW, Mitchell P, Wang JJ, Ziskind A, Carmichael T, Ramakrishnan R, Narendran K, Venkatesh R, Vijayan S, Zhao P, Chen X, Guadarrama-Vallejo D, Cheng CY, Perera SA, Husain R, Ho SL, Welge-Luessen UC, Mardin C, Schloetzer-Schrehardt U, Hillmer AM, Herms S, Moebus S, Nöthen MM, Weisschuh N, Shetty R, Ghosh A, Teo YY, Brown MA, Lischinsky I, Blue Mountains Eye Study GWAS Team, Wellcome Trust Case Control Consortium 2, Crowston JG, Coote M, Zhao B, Sang J, Zhang N, You Q, Vysochinskaya V, Founti P, Chatzikyriakidou A, Lambropoulos A, Anastasopoulos E, Coleman AL, Wilson MR, Rhee DJ, Kang JH, May-Bolchakova I, Heegaard S, Mori K, Alward WL, Jonas JB, Xu L, Liebmann JM, Chowbay B, Schaeffeler E, Schwab M, Lerner F, Wang N, Yang Z, Frezzotti P, Kinoshita S, Fingert JH, Inatani M, Tashiro K, Reis A, Edward DP, Pasquale LR, Kubota T, Wiggs JL, Pasutto F, Topouzis F, Dubina M, Craig JE, Yoshimura N, Sundaresan P, John SW, Ritch R, Hauser MA, Khor CC. A common variant mapping to CACNA1A is associated with susceptibility to exfoliation syndrome. Nature genetics. 2015 Apr:47(4):387-92. doi: 10.1038/ng.3226. Epub 2015 Feb 23 [PubMed PMID: 25706626]
Level 3 (low-level) evidenceYilmaz A, Tamer L, Ates NA, Yildirim O, Yildirim H, Atik U. Is GST gene polymorphism a risk factor in developing exfoliation syndrome? Current eye research. 2005 Jul:30(7):575-81 [PubMed PMID: 16020292]
Kapuganti RS, Bharati B, Mohanty PP, Alone DP. Genetic variants and haplotypes in fibulin-5 (FBLN5) are associated with pseudoexfoliation glaucoma but not with pseudoexfoliation syndrome. Bioscience reports. 2023 Mar 31:43(3):. doi: 10.1042/BSR20221622. Epub [PubMed PMID: 36794549]
Sharma S, Chataway T, Klebe S, Griggs K, Martin S, Chegeni N, Dave A, Zhou T, Ronci M, Voelcker NH, Mills RA, Craig JE. Novel protein constituents of pathological ocular pseudoexfoliation syndrome deposits identified with mass spectrometry. Molecular vision. 2018:24():801-817 [PubMed PMID: 30713420]
Karaca I, Yilmaz SG, Palamar M, Onay H, Akgun B, Aytacoglu B, Aykut A, Ozkinay FF. Evaluation of CNTNAP2 gene rs2107856 polymorphism in Turkish population with pseudoexfoliation syndrome. International ophthalmology. 2019 Jan:39(1):167-173. doi: 10.1007/s10792-017-0800-3. Epub 2017 Dec 19 [PubMed PMID: 29260496]
Kapuganti RS, Mohanty PP, Alone DP. Quantitative analysis of circulating levels of vimentin, clusterin and fibulin-5 in patients with pseudoexfoliation syndrome and glaucoma. Experimental eye research. 2022 Nov:224():109236. doi: 10.1016/j.exer.2022.109236. Epub 2022 Aug 31 [PubMed PMID: 36055390]
Level 2 (mid-level) evidenceAung T, Chan AS, Khor CC. Genetics of Exfoliation Syndrome. Journal of glaucoma. 2018 Jul:27 Suppl 1():S12-S14. doi: 10.1097/IJG.0000000000000928. Epub [PubMed PMID: 29965897]
Czop M, Gasińska K, Kosior-Jarecka E, Wróbel-Dudzińska D, Kocki J, Żarnowski T. Twenty Novel MicroRNAs in the Aqueous Humor of Pseudoexfoliation Glaucoma Patients. Cells. 2023 Feb 24:12(5):. doi: 10.3390/cells12050737. Epub 2023 Feb 24 [PubMed PMID: 36899874]
Jiwani AZ, Pasquale LR. Exfoliation Syndrome and Solar Exposure: New Epidemiological Insights Into the Pathophysiology of the Disease. International ophthalmology clinics. 2015 Fall:55(4):13-22. doi: 10.1097/IIO.0000000000000092. Epub [PubMed PMID: 26322422]
Level 2 (mid-level) evidenceHanyuda A, Rosner BA, Wiggs JL, Negishi K, Pasquale LR, Kang JH. Long-term Alcohol Consumption and Risk of Exfoliation Glaucoma or Glaucoma Suspect Status among United States Health Professionals. Ophthalmology. 2023 Feb:130(2):187-197. doi: 10.1016/j.ophtha.2022.08.023. Epub 2022 Aug 28 [PubMed PMID: 36041586]
Dewundara S, Pasquale LR. Exfoliation syndrome: a disease with an environmental component. Current opinion in ophthalmology. 2015 Mar:26(2):78-81. doi: 10.1097/ICU.0000000000000135. Epub [PubMed PMID: 25594763]
Level 3 (low-level) evidenceGhaffari Sharaf M, Damji KF, Unsworth LD. Recent advances in risk factors associated with ocular exfoliation syndrome. Acta ophthalmologica. 2020 Mar:98(2):113-120. doi: 10.1111/aos.14298. Epub 2019 Nov 17 [PubMed PMID: 31736276]
Level 3 (low-level) evidenceOzkan D, Altan C, Er MO, Gultekin F, Kuraş S, Artunay O. The Role of Oxidative Status in the Pathogenesis of Primary Open-Angle Glaucoma, Pseudoexfolyation Syndrome and Glaucoma. European journal of ophthalmology. 2023 Jan:33(1):352-360. doi: 10.1177/11206721221113199. Epub 2022 Jul 11 [PubMed PMID: 35818741]
Aboobakar IF, Johnson WM, Stamer WD, Hauser MA, Allingham RR. Major review: Exfoliation syndrome; advances in disease genetics, molecular biology, and epidemiology. Experimental eye research. 2017 Jan:154():88-103. doi: 10.1016/j.exer.2016.11.011. Epub 2016 Nov 11 [PubMed PMID: 27845061]
Level 3 (low-level) evidenceKim JH, Rabiolo A, Morales E, Yu F, Afifi AA, Nouri-Mahdavi K, Caprioli J. Risk Factors for Fast Visual Field Progression in Glaucoma. American journal of ophthalmology. 2019 Nov:207():268-278. doi: 10.1016/j.ajo.2019.06.019. Epub 2019 Jun 22 [PubMed PMID: 31238025]
Patil A, Swiston C, Wallace RT, Paulson C, Conley ME, McCoy L, Chaya C, Wirostko B. Exfoliation Syndrome and Exfoliation Glaucoma in the Navajo Nation. Vision (Basel, Switzerland). 2022 Oct 3:6(4):. doi: 10.3390/vision6040061. Epub 2022 Oct 3 [PubMed PMID: 36278673]
Forsius H. Prevalence of pseudoexfoliation of the lens in Finns, Lapps, Icelanders, Eskimos, and Russians. Transactions of the ophthalmological societies of the United Kingdom. 1979 Jul:99(2):296-8 [PubMed PMID: 298430]
Faulkner HW. Pseudo-exfoliation of the lens among the Navajo Indians. American journal of ophthalmology. 1971 Jul 30:72(1):206-7 [PubMed PMID: 5571208]
Pasquale LR, Kang JH, Wiggs JL. Prospects for gene-environment interactions in exfoliation syndrome. Journal of glaucoma. 2014 Oct-Nov:23(8 Suppl 1):S64-7. doi: 10.1097/IJG.0000000000000113. Epub [PubMed PMID: 25275911]
Thorleifsson G, Magnusson KP, Sulem P, Walters GB, Gudbjartsson DF, Stefansson H, Jonsson T, Jonasdottir A, Jonasdottir A, Stefansdottir G, Masson G, Hardarson GA, Petursson H, Arnarsson A, Motallebipour M, Wallerman O, Wadelius C, Gulcher JR, Thorsteinsdottir U, Kong A, Jonasson F, Stefansson K. Common sequence variants in the LOXL1 gene confer susceptibility to exfoliation glaucoma. Science (New York, N.Y.). 2007 Sep 7:317(5843):1397-400 [PubMed PMID: 17690259]
Level 2 (mid-level) evidenceMansour AM, Konstas AGP, Mansour HA, Charbaji AR, El Jawhari KM. A Case-Cohort Study of Exfoliation Risk Factors and Literature Review. Middle East African journal of ophthalmology. 2021 Jan-Mar:28(1):36-50. doi: 10.4103/meajo.MEAJO_358_20. Epub 2021 Apr 30 [PubMed PMID: 34321821]
Level 3 (low-level) evidenceYildirim N, Yasar E, Gursoy H, Colak E. Prevalence of pseudoexfoliation syndrome and its association with ocular and systemic diseases in Eskisehir, Turkey. International journal of ophthalmology. 2017:10(1):128-134. doi: 10.18240/ijo.2017.01.21. Epub 2017 Jan 18 [PubMed PMID: 28149789]
Chan TCW, Bala C, Siu A, Wan F, White A. Risk Factors for Rapid Glaucoma Disease Progression. American journal of ophthalmology. 2017 Aug:180():151-157. doi: 10.1016/j.ajo.2017.06.003. Epub 2017 Jun 15 [PubMed PMID: 28624324]
Pasquale LR, Jiwani AZ, Zehavi-Dorin T, Majd A, Rhee DJ, Chen T, Turalba A, Shen L, Brauner S, Grosskreutz C, Gardiner M, Chen S, Borboli-Gerogiannis S, Greenstein SH, Chang K, Ritch R, Loomis S, Kang JH, Wiggs JL, Levkovitch-Verbin H. Solar exposure and residential geographic history in relation to exfoliation syndrome in the United States and Israel. JAMA ophthalmology. 2014 Dec:132(12):1439-45. doi: 10.1001/jamaophthalmol.2014.3326. Epub [PubMed PMID: 25188364]
Level 2 (mid-level) evidenceArnarsson AM. Epidemiology of exfoliation syndrome in the Reykjavik Eye Study. Acta ophthalmologica. 2009 Dec:87 Thesis 3():1-17. doi: 10.1111/j.1755-3768.2009.01806.x. Epub [PubMed PMID: 20017735]
Level 2 (mid-level) evidenceRitch R, Schlötzer-Schrehardt U. Exfoliation syndrome. Survey of ophthalmology. 2001 Jan-Feb:45(4):265-315 [PubMed PMID: 11166342]
Level 3 (low-level) evidenceAriga M, Nivean M, Utkarsha P. Pseudoexfoliation Syndrome. Journal of current glaucoma practice. 2013 Sep-Dec:7(3):118-20. doi: 10.5005/jp-journals-10008-1148. Epub 2013 Sep 6 [PubMed PMID: 26997794]
Yüksel N, Karabaş VL, Arslan A, Demirci A, Cağlar Y. Ocular hemodynamics in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Ophthalmology. 2001 Jun:108(6):1043-9 [PubMed PMID: 11382627]
Asano N, Schlötzer-Schrehardt U, Naumann GO. A histopathologic study of iris changes in pseudoexfoliation syndrome. Ophthalmology. 1995 Sep:102(9):1279-90 [PubMed PMID: 9097764]
Schlötzer-Schrehardt UM, Dörfler S, Naumann GO. Corneal endothelial involvement in pseudoexfoliation syndrome. Archives of ophthalmology (Chicago, Ill. : 1960). 1993 May:111(5):666-74 [PubMed PMID: 8489451]
Level 3 (low-level) evidenceRitch R. Ocular and systemic manifestations of exfoliation syndrome. Journal of glaucoma. 2014 Oct-Nov:23(8 Suppl 1):S1-8. doi: 10.1097/IJG.0000000000000119. Epub [PubMed PMID: 25275896]
Schlötzer-Schrehardt U, Naumann GO. A histopathologic study of zonular instability in pseudoexfoliation syndrome. American journal of ophthalmology. 1994 Dec 15:118(6):730-43 [PubMed PMID: 7977599]
Hammer T, Schlötzer-Schrehardt U, Jünemann A. [Unilateral or asymmetric PEX syndrome? An electron microscopy study]. Klinische Monatsblatter fur Augenheilkunde. 2000 Aug:217(2):100-8 [PubMed PMID: 11022664]
Level 2 (mid-level) evidenceKivelä T, Hietanen J, Uusitalo M. Autopsy analysis of clinically unilateral exfoliation syndrome. Investigative ophthalmology & visual science. 1997 Sep:38(10):2008-15 [PubMed PMID: 9331264]
SUNDE OA. On the so-called senile exfoliation of the anterior lens capsule; a clinical and anatomical study. Acta ophthalmologica. Supplementum. 1956:(Suppl 45):1-85 [PubMed PMID: 13339292]
Zeppieri M. Pigment dispersion syndrome: A brief overview. Journal of clinical and translational research. 2022 Oct 31:8(5):344-350 [PubMed PMID: 36518550]
Level 3 (low-level) evidenceInoue K, Okugawa K, Oshika T, Amano S. Morphological study of corneal endothelium and corneal thickness in pseudoexfoliation syndrome. Japanese journal of ophthalmology. 2003 May-Jun:47(3):235-9 [PubMed PMID: 12782156]
Aasved H. Incidence of defects in the pigmented pupillary ruff in eyes with and without fibrillopathia epitheliocapsularis (so-called senile exfoliation or pseudoexfoliation of the anterior lens capsule). Acta ophthalmologica. 1973:51(5):710-5 [PubMed PMID: 4801166]
Puska P. Lens opacity in unilateral exfoliation syndrome with or without glaucoma. Acta ophthalmologica. 1994 Jun:72(3):290-6 [PubMed PMID: 7976257]
Rumelaitiene U, Speckauskas M, Tamosiunas A, Radisauskas R, Peto T, Larsen MB, Zaliūniene D. Exploring association between pseudoexfoliation syndrome and ocular aging. International ophthalmology. 2023 Mar:43(3):847-857. doi: 10.1007/s10792-022-02486-0. Epub 2022 Sep 21 [PubMed PMID: 36127504]
Sternfeld A, Luski M, Sella R, Zahavi A, Geffen N, Pereg A, Megiddo E, Gaton D. Diagnosis of Pseudoexfoliation Syndrome in Pseudophakic Patients. Ophthalmic research. 2021:64(1):28-33. doi: 10.1159/000508336. Epub 2020 Apr 30 [PubMed PMID: 32353850]
SAMPAOLESI R, AMALRIC P, BESSOU P. [On early diagnosis and heredity in capsular pseudoexfoliation of the crystalline lens]. Archivos de oftalmologia de Buenos Aires. 1961 Jul:36():159-64 [PubMed PMID: 14496647]
Level 3 (low-level) evidenceSalvetat ML, Zeppieri M, Tosoni C, Brusini P, Medscape. Baseline factors predicting the risk of conversion from ocular hypertension to primary open-angle glaucoma during a 10-year follow-up. Eye (London, England). 2016 Jun:30(6):784-95. doi: 10.1038/eye.2016.86. Epub 2016 May 13 [PubMed PMID: 27174381]
Mahabadi N, Foris LA, Tripathy K. Open Angle Glaucoma. StatPearls. 2023 Jan:(): [PubMed PMID: 28722917]
Brusini P, Salvetat ML, Zeppieri M. How to Measure Intraocular Pressure: An Updated Review of Various Tonometers. Journal of clinical medicine. 2021 Aug 27:10(17):. doi: 10.3390/jcm10173860. Epub 2021 Aug 27 [PubMed PMID: 34501306]
Zeppieri M, Brusini P, Miglior S. Corneal thickness and functional damage in patients with ocular hypertension. European journal of ophthalmology. 2005 Mar-Apr:15(2):196-201 [PubMed PMID: 15812759]
Ayala M. Risk factors for visual field progression in newly diagnosed exfoliation glaucoma patients in Sweden. Scientific reports. 2022 Jun 24:12(1):10763. doi: 10.1038/s41598-022-14962-9. Epub 2022 Jun 24 [PubMed PMID: 35750795]
Huo YJ, Thomas R, Li L, Cao K, Wang HZ, Wang NL. Comparison of Peripapillary Retinal Nerve Fiber Layer Thickness, Functional Subzones, and Macular Ganglion Cell-Inner Plexiform Layer in Differentiating Patients With Mild, Moderate, and Severe Open-angle Glaucoma. Journal of glaucoma. 2020 Sep:29(9):761-766. doi: 10.1097/IJG.0000000000001598. Epub [PubMed PMID: 32657819]
Todorović D, Šarenac Vulović T, Srećković S, Jovanović S, Petrović N. THE EFFECT OF PRIMARY ARGON LASER TRABECULOPLASTY ON INTRAOCULAR PRESSURE REDUCTION AND QUALITY OF LIFE IN PATIENTS WITH PSEUDOEXFOLIATION GLAUCOMA. Acta clinica Croatica. 2021 Jun:60(2):231-236. doi: 10.20471/acc.2021.60.02.08. Epub [PubMed PMID: 34744272]
Level 2 (mid-level) evidenceTran E, Sanvicente C, Hark LA, Myers JS, Zhang Q, Shiuey EJ, Tran J, Bonafede L, Hamershock RA, Withers C, Katz LJ. Educational intervention to adopt selective laser trabeculoplasty as first-line glaucoma treatment: Randomized controlled trial: Educational intervention on selective laser trabeculoplasty. European journal of ophthalmology. 2022 May:32(3):1538-1546. doi: 10.1177/11206721211018365. Epub 2021 May 27 [PubMed PMID: 34041935]
Level 1 (high-level) evidencePose-Bazarra S, López-Valladares MJ, López-de-Ullibarri I, Azuara-Blanco A. Surgical and laser interventions for pseudoexfoliation glaucoma systematic review of randomized controlled trials. Eye (London, England). 2021 Jun:35(6):1551-1561. doi: 10.1038/s41433-021-01424-1. Epub 2021 Feb 9 [PubMed PMID: 33564134]
Level 1 (high-level) evidenceTekcan H, Mangan MS, Imamoglu S. Uneventful phacoemulsification after trabeculectomy in pseudoexfoliation glaucoma versus primary open-angle glaucoma. Ophthalmic research. 2023 Feb 23:():. doi: 10.1159/000529642. Epub 2023 Feb 23 [PubMed PMID: 36822166]
Fea AM, Laffi GL, Martini E, Economou MA, Caselgrandi P, Sacchi M, Au L. Effectiveness of MicroShunt in Patients with Primary Open-Angle and Pseudoexfoliative Glaucoma: A Retrospective European Multicenter Study. Ophthalmology. Glaucoma. 2022 Mar-Apr:5(2):210-218. doi: 10.1016/j.ogla.2021.08.005. Epub 2021 Aug 31 [PubMed PMID: 34478904]
Level 2 (mid-level) evidenceRao A, Cruz RD. Cataract versus combined surgery in pseudoexfoliation glaucoma. Indian journal of ophthalmology. 2023 Mar:71(3):797-802. doi: 10.4103/ijo.IJO_1669_22. Epub [PubMed PMID: 36872681]
Desai MA, Lee RK. The medical and surgical management of pseudoexfoliation glaucoma. International ophthalmology clinics. 2008 Fall:48(4):95-113. doi: 10.1097/IIO.0b013e318187e902. Epub [PubMed PMID: 18936639]
Shingleton BJ, Crandall AS, Ahmed II. Pseudoexfoliation and the cataract surgeon: preoperative, intraoperative, and postoperative issues related to intraocular pressure, cataract, and intraocular lenses. Journal of cataract and refractive surgery. 2009 Jun:35(6):1101-20. doi: 10.1016/j.jcrs.2009.03.011. Epub [PubMed PMID: 19465298]
Joshi RS, Singanwad SV. Frequency and surgical difficulties associated with pseudoexfoliation syndrome among Indian rural population scheduled for cataract surgery: Hospital-based data. Indian journal of ophthalmology. 2019 Feb:67(2):221-226. doi: 10.4103/ijo.IJO_931_18. Epub [PubMed PMID: 30672474]
Ghaffari Sharaf M, Waduthanthri KD, Crichton A, Damji KF, Unsworth LD. Towards preventing exfoliation glaucoma by targeting and removing fibrillar aggregates associated with exfoliation syndrome. Journal of nanobiotechnology. 2022 Oct 27:20(1):459. doi: 10.1186/s12951-022-01665-6. Epub 2022 Oct 27 [PubMed PMID: 36303134]
Tomczyk-Socha M, Tomczak W, Turno-Kręcicka A. The Importance of MicroRNA Expression in Pseudoexfoliation Syndrome. International journal of molecular sciences. 2022 Oct 31:23(21):. doi: 10.3390/ijms232113234. Epub 2022 Oct 31 [PubMed PMID: 36362020]
Lanza M, Benincasa G, Costa D, Napoli C. Clinical Role of Epigenetics and Network Analysis in Eye Diseases: A Translational Science Review. Journal of ophthalmology. 2019:2019():2424956. doi: 10.1155/2019/2424956. Epub 2019 Dec 23 [PubMed PMID: 31976085]
Wang Y, Xie T. Extracellular, stem cells and regenerative ophthalmology. Journal of glaucoma. 2014 Oct-Nov:23(8 Suppl 1):S30-3. doi: 10.1097/IJG.0000000000000112. Epub [PubMed PMID: 25275901]
Cetinel S, Montemagno C. Nanotechnology Applications for Glaucoma. Asia-Pacific journal of ophthalmology (Philadelphia, Pa.). 2016 Jan-Feb:5(1):70-8. doi: 10.1097/APO.0000000000000171. Epub [PubMed PMID: 26693592]
Wallace DM, Clark AF, Lipson KE, Andrews D, Crean JK, O'Brien CJ. Anti-connective tissue growth factor antibody treatment reduces extracellular matrix production in trabecular meshwork and lamina cribrosa cells. Investigative ophthalmology & visual science. 2013 Dec 2:54(13):7836-48. doi: 10.1167/iovs.13-12494. Epub 2013 Dec 2 [PubMed PMID: 24204045]
Ahn SH, Suh JS, Lim GH, Kim TJ. The Potential Effects of Light Irradiance in Glaucoma and Photobiomodulation Therapy. Bioengineering (Basel, Switzerland). 2023 Feb 7:10(2):. doi: 10.3390/bioengineering10020223. Epub 2023 Feb 7 [PubMed PMID: 36829717]
Zeppieri M, Tripathy K. Pigment Dispersion Glaucoma. StatPearls. 2024 Jan:(): [PubMed PMID: 35593820]
Scuderi G, Contestabile MT, Scuderi L, Librando A, Fenicia V, Rahimi S. Pigment dispersion syndrome and pigmentary glaucoma: a review and update. International ophthalmology. 2019 Jul:39(7):1651-1662. doi: 10.1007/s10792-018-0938-7. Epub 2018 May 2 [PubMed PMID: 29721842]
Karp CL, Fazio JR, Culbertson WW, Green WR. True exfoliation of the lens capsule. Archives of ophthalmology (Chicago, Ill. : 1960). 1999 Aug:117(8):1078-80 [PubMed PMID: 10448754]
Level 3 (low-level) evidenceFuta R, Inada K, Nakashima H, Baba H, Kojima Y, Okamura R, Araki S. Familial amyloidotic polyneuropathy: ocular manifestations with clinicopathological observation. Japanese journal of ophthalmology. 1984:28(3):289-98 [PubMed PMID: 6098757]
Level 3 (low-level) evidenceJoshi P, Dangwal A, Guleria I, Kothari S, Singh P, Kalra JM, Jakhmola V. Glaucoma in Adults-diagnosis, Management, and Prediagnosis to End-stage, Categorizing Glaucoma's Stages: A Review. Journal of current glaucoma practice. 2022 Sep-Dec:16(3):170-178. doi: 10.5005/jp-journals-10078-1388. Epub [PubMed PMID: 36793264]
Ribeiro M, Barbosa-Breda J, Gonçalves F, Faria Pereira A, Falcão-Reis F, Alves F, E Silva S, B Melo A. [Evaluation of the Manchester Triage System in Patients with Acute Primary Angle Closure Attack: A Retrospective Study]. Acta medica portuguesa. 2023 Mar 17:():. doi: 10.20344/amp.19170. Epub 2023 Mar 17 [PubMed PMID: 36929920]
Level 2 (mid-level) evidenceChung H, Arora S, Damji KF, Weis E. Association of pseudoexfoliation syndrome with cardiovascular and cerebrovascular disease: a systematic review and meta-analysis. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2018 Aug:53(4):365-372. doi: 10.1016/j.jcjo.2017.10.039. Epub [PubMed PMID: 30119791]
Level 1 (high-level) evidence