Introduction
Pyogenic or suppurative flexor tenosynovitis (PFT) is a severe bacterial infection within the closed space of the digital flexor tendon sheaths.[1][2] PFT accounts for 2.5% to 9.5% of hand infections and can cause necrosis of the tendons and devitalization of fingers.[3] This infection alters the gliding mechanism and creates adhesions within the flexor tendon sheath, resulting in marked loss of finger movements. PFT can result from bloodstream infection but is generally caused by penetrating finger injuries involving the flexor tendon sheath. In 1912, Allen B. Kanavel described 3 cardinal signs of pyogenic flexor tenosynovitis: flexor sheath tenderness, flexed position of the affected digit, and painful passive digital extension. Later, a fourth sign, fusiform swelling of the digit, was also added to become the 4 cardinal signs.[4]
The detection of the 4 Kanavel signs on physical assessment has high sensitivity (91.4%-7.1%) to diagnose pyogenic flexor tenosynovitis.[5] A timely diagnosis and prompt treatment are paramount to limit the severe complications associated with this condition.[4]
Anatomy of Flexor Tendon Sheaths
The knowledge of the anatomy of hand flexor tendon sheaths is crucial for a better understanding and management of PFT. It has 2 parts: the inner synovial and the outer fibrous. Each flexor tendon sheath comprises 2 layers: an inner visceral and an outer parietal. The visceral layer closely covers the flexor tendon, forming the epitenon.[6] The outer parietal layer is conjoined with 5 annular and 3 cruciform pulleys. Space filled with synovium between the flexor sheath parietal and visceral layers provides nutrition to the tendons within the sheath. Flexor tendons receive their vascular supply from the surrounding digital arteries via the vincular system.[7] However, the vascular supply to the tendon sheaths is precarious, making the closed space an ideal breeding ground for infectious organisms.
As the PFT develops, pus accumulates within the flexor sheath synovial space, causing high pressures of up to 30 mm Hg within the flexor sheath closed space.[8] This high pressure further interferes with vascular supply to the flexor tendon, resulting in scarring and rupture.
The flexor tendon sheaths for the index, middle, ring, and little fingers terminate at the level of the flexor digitorum profundus tendon insertion into the distal phalanx. The flexor tendon sheath ends at the flexor pollicis longus tendon insertion level in the thumb. Proximally, the flexor sheaths of the index, middle, and ring fingers extend to the A1 pulley at the level of the neck of the metacarpal bone. The sheath of the flexor pollicis longus tendon communicates with the radial bursa proximally. The little finger flexor sheath communicates with the ulnar bursa in about 80% of the population.[9] The radial and ulnar bursa are connected in 80% of people in the space of Parona, a potential space between the digital flexor tendons and the pronator quadratus in the volar distal forearm. This anatomic space allows a little finger or thumb infection to cause a horseshoe abscess.[10][11]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Route of Infection
Most patients present with a finger penetrating injury involving the flexor tendon sheath, causing direct bacterial inoculation.[12] However, direct spread from a nearby felon, infected joint, or deep space infection is also possible.[12][13] PFT may result from a human or animal bite casing penetrative flexor sheath injury.[14] PFT caused by bloodstream infection is rare and raises the suspicion of disseminated gonococcal infection.[15]
Causative Organism
Staphylococcus aureus is the most common bacteria causing PFT, isolated in about 75% of cases.[16] Of the reported PFTs, 29% are caused by methicillin-resistant Staphyloccocus aureus (MRSA).[17] Other possible causative organisms of PFT include Staphylococcus aureus epidermidis, β-hemolytic streptococcus, and gram-negative Pseudomonas aeruginosa. PFT in immunocompromised patients can be due to infection by multiple organisms and gram-negative rods. Eikenella corrodens may cause PFT through penetrating human bites. PFT following animal bites could be a result of Pasteurella multocida infection.[14][18]
Epidemiology
PFT is found in about 2.5% to 9.5% of all hand infections, and a penetrating hand injury usually precedes this condition.[2][12] No difference in the PFT incidence rate among the different age groups or sexes is found.[5] In modern practice, the administration of intravenous antibiotics along with early surgical treatment in managing PFT have reduced the morbidity of this condition. The infection severity and the disease course are directly linked to the patient's immunity response. Patients who experience medical conditions causing reduced immunity (eg, malnutrition, chronic steroid use, autoimmune disease, peripheral vascular disease, and diabetes mellitus) are at higher risk of complications and poor outcomes after PFT.[19]
History and Physical
Clinicians should obtain a detailed history to cover the following:
- Identify any penetrating injury to the volar aspects of the hand and digits. The mechanism of injury may guide the identification of the causative organism of the infection. Penetrating hand injury usually precedes PFT presentation by 2 to 5 days.[20] However, this duration can be longer in immunocompromised and diabetic patients. Sometimes, penetrating injury can be trivial and overlooked. Often, the patients do not give any history of preceding trauma.
- Precisely identify the duration between symptom onset and the patient presentation. The duration and severity of the PFT symptoms can guide treatment options—a delayed patient presentation and treatment after PFT is associated with poor outcomes and increased finger amputation risk.
- The social history obtained should cover the patient's occupation and the dominant hand, as this information will guide the rehabilitation requirements and postoperative support.
- Medical comorbidities should be recognized and optimized before the surgical intervention as appropriate. Also, it has been reported that patients who develop PFT and have a medical history of diabetes mellitus, renal failure, or peripheral vascular disease are at higher risk of poor outcomes.[21][11]
Evaluation
Clinical Assessment
Physical findings and clinical suspicion are the basis of diagnosing PFT. The detection of the 4 Kanavel signs on physical assessment has high sensitivity (91.4%-97.1%) when diagnosing PFT.[5] The 4 cardinal Kanavel signs of PFT include
- Symmetrical swelling of the affected finger
- The affected finger is held in a flexed position
- Pain on any attempt at passive finger extension
- Tenderness along the course of flexor tendon sheath from distal phalanx to the level of A1 pulley
However, the Kanavel cardinal signs can be less evident on presentation in patients with compromised immune systems, diabetes, or intravenous drug use.[20]
A painful passive extension is the earliest sign of PFT, while flexor sheath tenderness is the last sign to be detected. Fever is present in only 17% of the affected patients. Examine the affected hand to identify any penetrating wound. The presence of erythema, swelling, and tenderness over the thenar eminence in the palm raises the suspicion of the proximal spread of infection to the radial bursa. Patients with an infected ulnar bursa present with pain, swelling, and erythema over the hypothenar eminence.[22] Results reported from a retrospective study of 33 patients with digit infections showed tenderness at the A1 pulley, which could be a useful modification of the flexor sheath tenderness cardinal sign in diagnosing PFT.[23]
According to Kanavel, tenderness over the tendon sheath was the most important cardinal sign.[4] In contrast, Pang, et al, reported a series of 75 patients (97%) with PFT where swelling of the digits was the most common sign. Tenderness over the tendon sheath was a late sign, signifying proximal extension of the infection. Due to this, PFT should not be excluded based on the lack of this sign.[2] Neviaser reported that pain on passive digit extension was the most reliable early Kanavel's sign and also described the inability of the digit to touch the palm as an additional sign to detect PFT.[4] In a retrospective case series of 41 patients with PFT, Dailiana, et al, found that only around half of the patients displayed all 4 of Kanavel's cardinal signs, bringing the sensitivity of the signs for detecting PFT into question.[16]
Diagnostic Imaging
- The anteroposterior, lateral, and oblique view x-rays of the hand should be obtained to rule out any retained foreign body. The presence of signs of osteomyelitis in the obtained x-rays suggests chronic infection. In a retrospective study of adult finger infections, Yi, et al, found that uniform finger swelling does not differentiate PFT from other hand infections. They also reported that PFT is characterized by differential radiographic soft tissue thickness on the volar aspect as compared to the dorsal aspect at the level of the proximal phalanx.[24]
- Hand ultrasonography is an inexpensive and noninvasive method to confirm the diagnosis of PFT. The ultrasound can visualize the flexor tendon and detect the presence of fluid collection within the flexor sheath. Ultrasound has good negative predictive value and specificity for diagnosing PFT and complements the clinical examination in detecting finger infection.[25] However, the ultrasound is operator-dependent and cannot differentiate pus from blood.
- Magnetic Resonance Imaging (MRI) is rarely required to diagnose PFT. MRI can identify the extent of the infection but cannot differentiate PFT from other inflammatory conditions.[26]
- Contrast-enhanced computed tomography of the hand has also been reported as helpful in detecting PFT, as the ratio of tendon sheath to tendon width in coronal and sagittal planes was found to improve objectivity.[27]
Laboratory Tests
- Inflammatory markers, including white blood cell count, C-reactive protein level, and erythrocyte sedimentation rate level, are usually elevated in patients with PFT. However, these tests are non-specific to diagnose PFT but can be helpful to monitor infection and response to the given treatment.
- Pus and necrotic tissue cultures are very beneficial in identifying the causative organism and directing the antibiotic therapy according to the sensitivity.
- Obtain a blood culture if there are signs of sepsis or hematogenous spread of the infection to isolate the causative organism.[28]
Treatment / Management
Non-operative Treatment of PFT
PFT could be treated nonoperatively if the patient presents within 48 hours of suffering from the hand penetrating injury with less dramatic Kanavel signs. Non-operative treatment measurements for PFT include intravenous antibiotics, high arm elevation, and splinting. Broad-spectrum antibiotics should be prescribed to cover the suspected organisms until the culture results are available. Non-operative PFT management includes close infection monitoring by repeated hand examination and laboratory inflammatory markers. If no clinical improvement is noticed or there is a worsening of the Kanavel signs, an urgent surgical washout should be performed. A retrospective review of 40 patients with PFT reported patients with a shorter duration of symptoms and fewer positive Kanavel's cardinal signs were successfully treated with antibiotics alone and did not require surgical management.[29]
Operative Treatment of PFT
Almost all cases of PFT require surgical management. Various surgical approaches and techniques have been reported to treat PFT. The flexor tendon sheath can be washed out with closed irrigation via two volar incisions. However, severe PFT with delayed presentation and tissue necrosis may require more invasive surgical treatment with debridement and open irrigation.[11]
Closed Flexor Sheath Catheter Irrigation
Flexor sheath irrigation can be performed through 2 small volar incisions. The proximal incision is made over the level of the A1 pulley and the distal proximal to the distal interphalangeal crease.[30] The tissues should be carefully dissected to identify the flexor tendon sheath without damaging the digital neurovascular bundle on each side. A small incision is made through the A1 pulley without damaging the underneath flexor tendon. Samples from the fluid inside the flexor sheath are collected and sent for microbiology examination and cultures. A second incision is made in the sheath at the A5 pulley, followed by passing a 16- or 18-gauge catheter or cannula through one of the two incisions. Sterile normal saline is injected from the catheter to irrigate the sheath thoroughly. Published evidence suggested that closed flexor sheath catheter irrigation can have the same effect as open flexor sheath irrigation and debridement.[31][32](B2)
Open Flexor Sheath Irrigation and Debridement
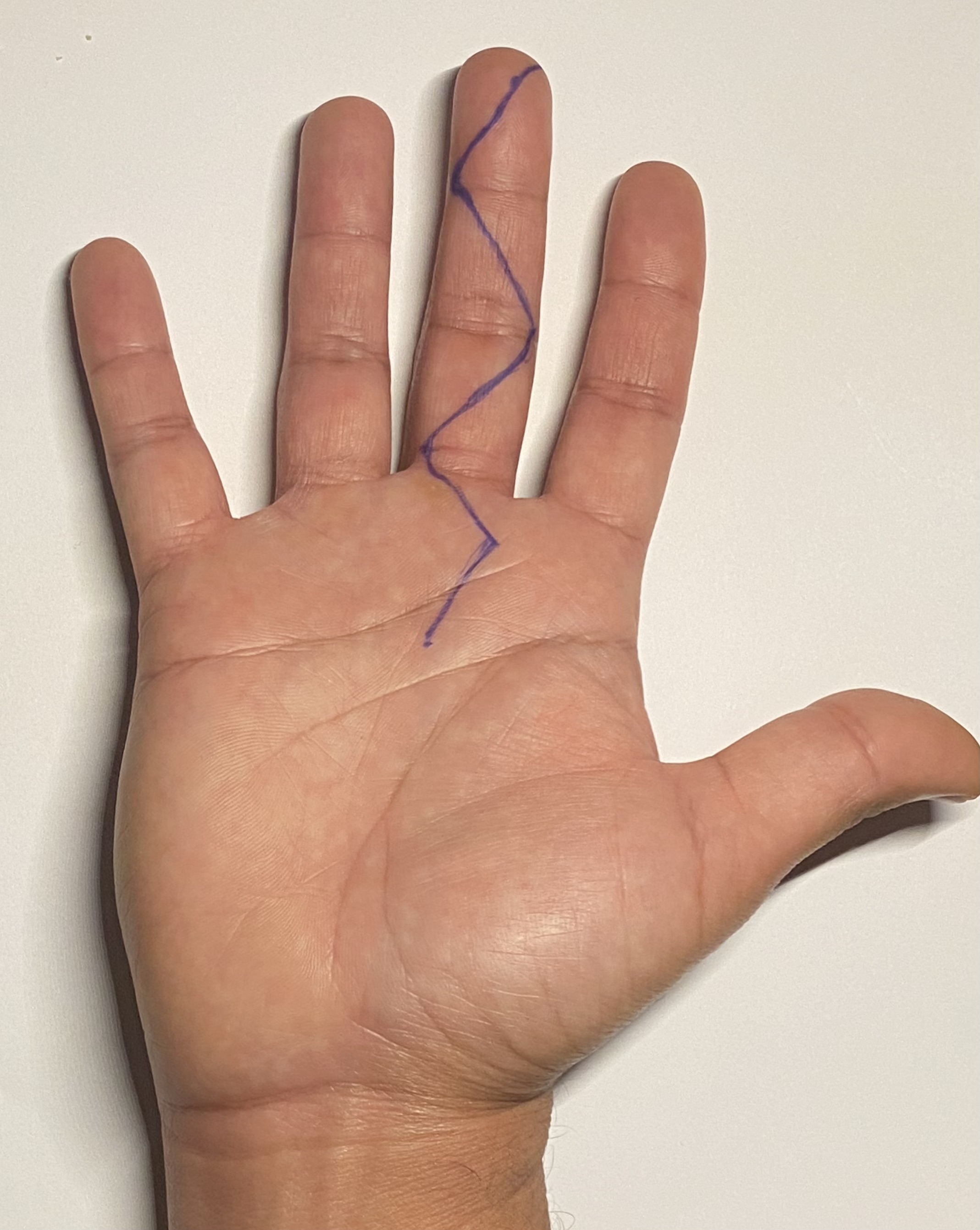
Open flexor sheath washout can be performed via a single extended volar incision to expose the entire flexor sheath. Two types of incisions have been described for open flexor sheath washout: a straight mid-axial incision and a zigzag incision, also known as a Bruner incision (see Figure 1. Bruner Incision Marking).[33] The involved digit's radial and ulnar neurovascular bundle should be identified and protected. After the entire exposure, the flexor sheath A1 and A5 pulleys are incised. The sheath is debrided and washed thoroughly with normal saline. Pulleys from A2 to A4 should be protected to avoid postoperative flexor tendon bowstringing. The Bruner incision aims to avoid vertical scarring at the flexor creases to minimize the risk of flexion contractures. A Bruner incision may increase the risk of wound breakdown after the surgery. However, this risk can be minimized by avoiding wound closure under tension.[34][20] Open flexor sheath irrigation and debridement are associated with a higher risk of postoperative finger stiffness and tendon adhesions.(B3)
Previously, intravenous antibiotics were routinely given for prolonged duration after the surgical management as per the culture and sensitivity reports. However, a retrospective review of 113 patients with PFT reported that even oral antibiotics for 7 to 14 days are effective after the surgery, thus allowing outpatient management for the patient.[35]
Differential Diagnosis
Felon (Distal Pulp Space Infection)
The felon is a closed space infection of the distal finger pulp that commonly affects the thumb or the index finger. Felon or pulp space infection is usually caused by Staphylococcus aureus infection due to penetrating injury to the fingertip pulp. However, this infection may result from diverse organisms or gram-negative bacteria in patients with immunosuppression.[36] The fibrous septa extend from the distal phalanx periosteum to the skin from a closed space; when infected, it causes severe throbbing pain. A felon presents with a painful throbbing abscess over the distal finger phalanx and requires surgical incisional drainage.[37]
Interphalangeal Joint or Metacarpophalangeal Joint Septic Arthritis
These 2 arthritic conditions usually present with signs of infection localized to the affected joint. The range of motion of the affected joint is generally restricted and painful due to joint effusion and capsular distention. The other cardinal Kanavel signs of PFT, including finger fusiform swelling and flexor sheath tenderness, are usually absent.
Herpetic Whitlow
This is a rare viral infection of the fingertips caused by the herpes simplex virus. Herpetic whitlow presents with painful blisters filled with clear fluid that may combine to form bullae. Herpetic whitlow requires treatment by antiviral medications such as acyclovir and does not require surgical intervention.[38]
Hand Cellulitis
Hand cellulitis is a diffuse hand inflammation without underlying pus collection.[39] Hand cellulitis presents diffuse swelling and erythema without abscess formation. Hand cellulitis can be treated by high arm elevation and intravenous antibiotics according to the causative organism.
Staging
Michon classified PFT into 3 different stages based on the severity of the infection:
- Stage 1: Serous exudate starts to accumulate within the flexor sheath
- Stage 2: The flexor sheath is filled with purulent fluid
- Stage 3: The flexor tendon, flexor sheath, and the pulley system show signs of necrosis [39]
Prognosis
PFT may result in poor outcomes with finger amputation and spread infection if appropriate treatment has not commenced early. Between 10% to 25% of the affected patients may not gain the full active range of movements.[40][13][41] Patients who suffer from diabetes mellitus, peripheral vascular disease, and renal failure are at a greater risk of finger amputation following PFT.[21]
Complications
Fingers Stiffness and Restricted Range of Motion
Stiffness after PFT can result from the infection process or secondary to the washout surgery. The inflammatory process in PFT may cause adhesions of the flexor tendons, thickened joint capsules, and damaged pulleys. Recommend early active exercises for the affected finger to minimize the risk of stiffness. Flexor tenolysis of the affected digit may be required to restore the range of movement after the complete subsidence of the inflammatory process.[20]
Flexor Tendon Scarring and Subsequent Rupture
The high pressure within the flexor sheath interferes with the flexor tendon blood flow and nutrition. Unhealthy, scarred flexor tendons lose elasticity and may rupture with further activity.
Soft Tissue Necrosis and Finger Ischemia
Critical ischemia of the affected finger may result from the interruption of the blood flow due to the high pressure in the finger or secondary to arterial thrombosis induced by the inflammatory process.
Hand Horseshoe Abscess
Horseshoe abscess formation may result from the spread of the infection from PFT of the thumb or little finger in patients who have communicated ulnar and radial bursae.[9]
Finger Amputation
Finger amputation at various levels can be inevitable because of the deep infection, significant soft tissue, or functionless stiff finger.[20]
Deterrence and Patient Education
Patients should be counseled and educated about PFT and the prognosis. Clinicians should use precise and nonmedical terminology to explain the necessity of the treatment and potential complications as a part of the informed consent. Clearly stating this condition is considered an emergency and necessitates urgent treatment to preserve the involved finger and hand viability and function is essential. Warn the patient about the possible complications resulting from the infective process, mainly restricted motion and the risk of amputation. Patients with an infection in the dominant hand will need extra support during rehabilitation and may require a more prolonged recovery.
Enhancing Healthcare Team Outcomes
Pyogenic flexor tenosynovitis is an emergent condition that requires timely diagnosis and prompt treatment. Effectively managing this potentially devastating infection requires an integrated approach from the healthcare team. The available diagnostic tools, including laboratory tests and imaging modalities, especially hand sonography, can be beneficial in confirming the diagnosis and monitoring the infection severity. However, clinicians should make this diagnosis based on the cardinal Kanavel signs. Early communication with an orthopedic or plastic surgeon is necessary to formulate the best management plan. The role of nurses in perioperative care and infection monitoring for PFT is crucial and must be supported. Postoperative care demands an integrated approach from doctors, nurses, hand therapists, and social workers. Rehabilitation after PFT requires early involvement of the hand therapist and early onset of active range of movement exercises.
Media
(Click Image to Enlarge)
References
Chapman T, Ilyas AM. Pyogenic Flexor Tenosynovitis: Evaluation and Treatment Strategies. Journal of hand and microsurgery. 2019 Dec:11(3):121-126. doi: 10.1055/s-0039-1700370. Epub 2019 Nov 2 [PubMed PMID: 31814662]
Pang HN, Teoh LC, Yam AK, Lee JY, Puhaindran ME, Tan AB. Factors affecting the prognosis of pyogenic flexor tenosynovitis. The Journal of bone and joint surgery. American volume. 2007 Aug:89(8):1742-8 [PubMed PMID: 17671013]
Stern PJ, Staneck JL, McDonough JJ, Neale HW, Tyler G. Established hand infections: a controlled, prospective study. The Journal of hand surgery. 1983 Sep:8(5 Pt 1):553-9 [PubMed PMID: 6355263]
Level 1 (high-level) evidenceKennedy CD, Huang JI, Hanel DP. In Brief: Kanavel's Signs and Pyogenic Flexor Tenosynovitis. Clinical orthopaedics and related research. 2016 Jan:474(1):280-4. doi: 10.1007/s11999-015-4367-x. Epub 2015 May 29 [PubMed PMID: 26022113]
Kennedy CD, Lauder AS, Pribaz JR, Kennedy SA. Differentiation Between Pyogenic Flexor Tenosynovitis and Other Finger Infections. Hand (New York, N.Y.). 2017 Nov:12(6):585-590. doi: 10.1177/1558944717692089. Epub 2017 Feb 1 [PubMed PMID: 28720000]
Patel DB, Emmanuel NB, Stevanovic MV, Matcuk GR Jr, Gottsegen CJ, Forrester DM, White EA. Hand infections: anatomy, types and spread of infection, imaging findings, and treatment options. Radiographics : a review publication of the Radiological Society of North America, Inc. 2014 Nov-Dec:34(7):1968-86. doi: 10.1148/rg.347130101. Epub [PubMed PMID: 25384296]
Ochiai N, Matsui T, Miyaji N, Merklin RJ, Hunter JM. Vascular anatomy of flexor tendons. I. Vincular system and blood supply of the profundus tendon in the digital sheath. The Journal of hand surgery. 1979 Jul:4(4):321-30 [PubMed PMID: 469207]
Schnall SB, Vu-Rose T, Holtom PD, Doyle B, Stevanovic M. Tissue pressures in pyogenic flexor tenosynovitis of the finger. Compartment syndrome and its management. The Journal of bone and joint surgery. British volume. 1996 Sep:78(5):793-5 [PubMed PMID: 8836073]
Aguiar RO, Gasparetto EL, Escuissato DL, Marchiori E, Trudell DJ, Haghighi P, Resnick D. Radial and ulnar bursae of the wrist: cadaveric investigation of regional anatomy with ultrasonographic-guided tenography and MR imaging. Skeletal radiology. 2006 Nov:35(11):828-32 [PubMed PMID: 16688447]
SCHELDRUP EW. Tendon sheath patterns in the hand; an anatomical study based on 367 hand dissections. Surgery, gynecology & obstetrics. 1951 Jul:93(1):16-22 [PubMed PMID: 14855245]
Goyal K, Speeckaert AL. Pyogenic Flexor Tenosynovitis: Evaluation and Management. Hand clinics. 2020 Aug:36(3):323-329. doi: 10.1016/j.hcl.2020.03.005. Epub [PubMed PMID: 32586458]
Abrams RA, Botte MJ. Hand Infections: Treatment Recommendations for Specific Types. The Journal of the American Academy of Orthopaedic Surgeons. 1996 Jul:4(4):219-230 [PubMed PMID: 10795057]
Harris PA, Nanchahal J. Closed continuous irrigation in the treatment of hand infections. Journal of hand surgery (Edinburgh, Scotland). 1999 Jun:24(3):328-33 [PubMed PMID: 10433448]
Small LN, Ross JJ. Suppurative tenosynovitis and septic bursitis. Infectious disease clinics of North America. 2005 Dec:19(4):991-1005, xi [PubMed PMID: 16297744]
Schaefer RA, Enzenauer RJ, Pruitt A, Corpe RS. Acute gonococcal flexor tenosynovitis in an adolescent male with pharyngitis. A case report and literature review. Clinical orthopaedics and related research. 1992 Aug:(281):212-5 [PubMed PMID: 1499214]
Level 3 (low-level) evidenceDailiana ZH, Rigopoulos N, Varitimidis S, Hantes M, Bargiotas K, Malizos KN. Purulent flexor tenosynovitis: factors influencing the functional outcome. The Journal of hand surgery, European volume. 2008 Jun:33(3):280-5. doi: 10.1177/1753193408087071. Epub [PubMed PMID: 18562357]
Katsoulis E, Bissell I, Hargreaves DG. MRSA pyogenic flexor tenosynovitis leading to digital ischaemic necrosis and amputation. Journal of hand surgery (Edinburgh, Scotland). 2006 Jun:31(3):350-2 [PubMed PMID: 16616976]
Level 3 (low-level) evidenceLanger MF, Grünert JG, Unglaub F, Ueberberg J, Glasbrenner J, Oeckenpöhler S. [Pyogenic Flexor Tenosynovitis]. Handchirurgie, Mikrochirurgie, plastische Chirurgie : Organ der Deutschsprachigen Arbeitsgemeinschaft fur Handchirurgie : Organ der Deutschsprachigen Arbeitsgemeinschaft fur Mikrochirurgie der Peripheren Nerven und Gefasse : Organ der V.... 2021 Jun:53(3):267-275. doi: 10.1055/a-1472-1689. Epub 2021 Jun 16 [PubMed PMID: 34134159]
Hyatt BT, Bagg MR. Flexor Tenosynovitis. The Orthopedic clinics of North America. 2017 Apr:48(2):217-227. doi: 10.1016/j.ocl.2016.12.010. Epub [PubMed PMID: 28336044]
Draeger RW, Bynum DK Jr. Flexor tendon sheath infections of the hand. The Journal of the American Academy of Orthopaedic Surgeons. 2012 Jun:20(6):373-82. doi: 10.5435/JAAOS-20-06-373. Epub [PubMed PMID: 22661567]
Level 3 (low-level) evidenceGiladi AM, Malay S, Chung KC. A systematic review of the management of acute pyogenic flexor tenosynovitis. The Journal of hand surgery, European volume. 2015 Sep:40(7):720-8. doi: 10.1177/1753193415570248. Epub 2015 Feb 10 [PubMed PMID: 25670687]
Level 1 (high-level) evidencePaniagua CT, Bean AS. Pyogenic flexor tenosynovitis: assessment and management in the emergency department setting. Advanced emergency nursing journal. 2014 Jan-Mar:36(1):36-43. doi: 10.1097/TME.0000000000000002. Epub [PubMed PMID: 24487262]
Level 3 (low-level) evidenceSiska RC, Davidson AL, Driscoll CR, Browne DT, Maus JC, Prabhu SS, Rudolph MA, Schneider MA, Runyan CM, Reynolds M. A1 Pulley Tenderness as a Modification to Tenderness along the Flexor Sheath in Diagnosing Pyogenic Flexor Tenosynovitis. Plastic and reconstructive surgery. Global open. 2022 Mar:10(3):e4165. doi: 10.1097/GOX.0000000000004165. Epub 2022 Mar 2 [PubMed PMID: 35261842]
Yi A, Kennedy C, Chia B, Kennedy SA. Radiographic Soft Tissue Thickness Differentiating Pyogenic Flexor Tenosynovitis From Other Finger Infections. The Journal of hand surgery. 2019 May:44(5):394-399. doi: 10.1016/j.jhsa.2019.01.013. Epub 2019 Feb 21 [PubMed PMID: 30797654]
Jardin E, Delord M, Aubry S, Loisel F, Obert L. Usefulness of ultrasound for the diagnosis of pyogenic flexor tenosynovitis: A prospective single-center study of 57 cases. Hand surgery & rehabilitation. 2018 Apr:37(2):95-98. doi: 10.1016/j.hansur.2017.12.004. Epub 2018 Feb 1 [PubMed PMID: 29396150]
Level 3 (low-level) evidenceChaudhary S, Maji S, Garg V, Singh V. Isolated multidrug-resistant tubercular tenosynovitis of the flexor tendon of the little finger. BMJ case reports. 2021 Feb 19:14(2):. doi: 10.1136/bcr-2020-238339. Epub 2021 Feb 19 [PubMed PMID: 33608337]
Level 3 (low-level) evidenceMyers DM, Goubeaux C, Skura B, Warmoth PJ, Taylor BC. Contrast Enhanced Computed Tomography in the Diagnosis of Acute Pyogenic Flexor Tenosynovitis. Hand (New York, N.Y.). 2023 Nov:18(8):1323-1329. doi: 10.1177/15589447221092058. Epub 2022 May 24 [PubMed PMID: 35611491]
Millerioux S, Rousset M, Canavese F. Pyogenic tenosynovitis of the flexor hallucis longus in a healthy 11-year-old boy: a case report and review of the literature. European journal of orthopaedic surgery & traumatology : orthopedie traumatologie. 2013 Nov:23 Suppl 2():S311-5. doi: 10.1007/s00590-012-1147-0. Epub 2012 Dec 8 [PubMed PMID: 23412272]
Level 3 (low-level) evidenceLatario L, Abeler J, Clegg S, Thurber L, Igiesuorobo O, Jones M. Antibiotics Versus Surgery in Treatment of Early Flexor Tenosynovitis. Hand (New York, N.Y.). 2023 Jul:18(5):804-810. doi: 10.1177/15589447211043187. Epub 2022 Jan 26 [PubMed PMID: 35081807]
Carter SJ, Burman SO, Mersheimer WL. Treatment of digital tenosynovitis by irrigation with peroxide and oxytetracycline: review of nine cases. Annals of surgery. 1966 Apr:163(4):645-50 [PubMed PMID: 5934641]
Level 3 (low-level) evidenceBorn TR, Wagner ER, Kakar S. Comparison of Open Drainage Versus Closed Catheter Irrigation for Treatment of Suppurative Flexor Tenosynovitis. Hand (New York, N.Y.). 2017 Nov:12(6):579-584. doi: 10.1177/1558944716675131. Epub 2016 Oct 25 [PubMed PMID: 29091483]
Gutowski KA, Ochoa O, Adams WP Jr. Closed-catheter irrigation is as effective as open drainage for treatment of pyogenic flexor tenosynovitis. Annals of plastic surgery. 2002 Oct:49(4):350-4 [PubMed PMID: 12370638]
Level 2 (mid-level) evidenceBruner JM. The zig-zag volar-digital incision for flexor-tendon surgery. Plastic and reconstructive surgery. 1967 Dec:40(6):571-4 [PubMed PMID: 6077719]
Stern PJ. Selected acute infections. Instructional course lectures. 1990:39():539-46 [PubMed PMID: 2186147]
Dujeux C, Cottebrune T, Malherbe M, Michon J, Fournier A, Hulet C. Use of antibiotics in pyogenic flexor tenosynovitis. Hand surgery & rehabilitation. 2022 Oct:41(5):624-630. doi: 10.1016/j.hansur.2022.07.006. Epub 2022 Aug 3 [PubMed PMID: 35933026]
McDonald LS, Bavaro MF, Hofmeister EP, Kroonen LT. Hand infections. The Journal of hand surgery. 2011 Aug:36(8):1403-12. doi: 10.1016/j.jhsa.2011.05.035. Epub [PubMed PMID: 21816297]
Nardi NM, McDonald EJ, Schaefer TJ. Felon. StatPearls. 2023 Jan:(): [PubMed PMID: 28613683]
Wu IB, Schwartz RA. Herpetic whitlow. Cutis. 2007 Mar:79(3):193-6 [PubMed PMID: 17674583]
Koshy JC, Bell B. Hand Infections. The Journal of hand surgery. 2019 Jan:44(1):46-54. doi: 10.1016/j.jhsa.2018.05.027. Epub 2018 Jul 14 [PubMed PMID: 30017648]
Lille S, Hayakawa T, Neumeister MW, Brown RE, Zook EG, Murray K. Continuous postoperative catheter irrigation is not necessary for the treatment of suppurative flexor tenosynovitis. Journal of hand surgery (Edinburgh, Scotland). 2000 Jun:25(3):304-7 [PubMed PMID: 10961561]
Level 2 (mid-level) evidenceGaston RG, Greenberg JA. Use of continuous marcaine irrigation in the management of suppurative flexor tenosynovitis. Techniques in hand & upper extremity surgery. 2009 Dec:13(4):182-6. doi: 10.1097/BTH.0b013e3181bef5a3. Epub [PubMed PMID: 19956043]
Level 2 (mid-level) evidence