Introduction
Cranial nerve (CN) testing is the physical functional assessment of the nerves arising from the brain and innervating the head, neck, and trunk. This testing is widely applicable to emergency and clinical situations and can be performed relatively quickly with equipment readily available in the hospital or ambulatory environment.[1]
Abnormalities discovered during the examination can be valuable harbingers of neurologic pathologies such as mass lesions or disease progression requiring urgent intervention (ie, enlarging intracranial aneurysm).
CN testing is particularly useful in the continuous evaluation of unconscious patients. Patients with sequelae from neurotrauma, strokes, or intracranial pathology require frequent monitoring for worsening function or condition. Higher-level tests such as brainstem auditory evoked potentials (BAEP) typically require a referral to a tertiary care center; they can be performed if abnormalities are revealed on initial CN testing.[2]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Cranial nerves innervate different structures in the head, neck, and trunk. The olfactory and optic nerves exit from the cerebrum, while the remaining cranial nerves exit from the brainstem.[3] Abnormalities in cranial nerve function help localize the lesion to a specific level of the brain or brainstem.[4][5]
Cranial nerves have motor, sensory, and autonomic functions. Generally, a singular cranial nerve deficit indicates a peripheral nerve lesion. A lesion in the brainstem may produce multiple cranial nerve deficits and motor and sensory abnormalities of the extremities.[6]
Cranial Nerve I
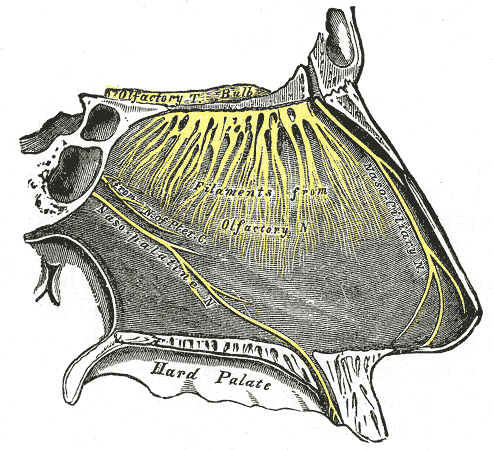
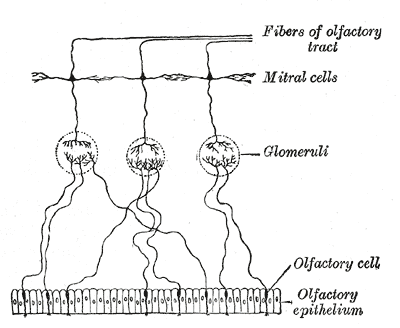
The olfactory bulb lies within the cranial vault on the inferior portion of the frontal lobes. The olfactory nerve (CN I) is composed of special visceral afferents, which provide the sense of smell and contribute to the flavor-discriminatory aspect of taste.[7] The sense of smell is 95% of chemo-sensation, with taste comprising only 5%. Chemosensory receptors in the olfactory mucosal lining bind to odorant molecules and conduct a signal through the nerves traveling through the cribriform plate of the ethmoid bone to synapse on the neurons of the olfactory bulb.[6]
The central processes of these olfactory bulb neurons project through the olfactory trigone medially to the septal area and the contralateral bulb via the anterior commissure. At the same time, other fibers travel laterally to the amygdala and piriform cortex, also known as the primary olfactory cortex, where conscious odorant sense is processed. These fibers travel to the secondary olfactory cortex. The secondary olfactory cortex comprises the insula and entorhinal cortex (hippocampus input area connected to the parahippocampal cortex).[8]
Cranial Nerve II
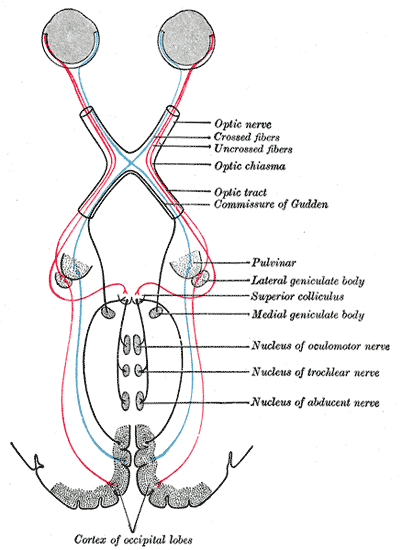
Optic nerve (CN II) fibers are formed from the projections of ganglion cells, whose cell bodies are located deep in the retina. Next, the optic nerve fibers traverse the optic canal and enter the cranium.[9] Fibers from the medial visual fields travel posteriorly without crossing at the optic chiasm, while fibers of the lateral visual fields cross within the chiasm. The regions of the visual field are retinotopically organized within the optic nerve and at the synapses in the lateral geniculate nucleus. Collaterals are also given off centrally to innervate the superior colliculus, responsible for the pupillary light reflex.[6]
Cranial Nerves III, IV, and VI
The oculomotor (CN III), trochlear (CN IV), and abducens (CN VI) nerves are grouped and tested together as they all function in the motion of the eye.[1] Each nerve originates from its like-named nucleus in the brainstem and travels through the superior orbital fissure. The oculomotor and abducens nerves then pass through the common tendinous ring, while the trochlear nerve remains outside the ring.[6] The oculomotor nerve innervates the superior, inferior, and medial recti muscles and the inferior oblique muscle. The trochlear nerve innervates the superior oblique muscle, and the abducens nerve innervates the lateral rectus muscle.
Cranial Nerve V
The trigeminal nerve (CN V) provides the general sensory function to the face through 3 divisions: the ophthalmic (V1), maxillary (V2), and mandibular (V3).[1] The trigeminal nerve also provides somatic efferents to the muscles of mastication, anterior belly of the digastric, mylohyoid, tensor veli palatini, and tensor tympani muscles.[10]
Cranial Nerve VII
The facial nerve (CN VII) has motor and autonomic fibers with minor somatosensory components. Special visceral efferent (SVE) motor innervation is to the muscles of facial expression and exits the skull through the stylomastoid foramen deep to the parotid gland. Damage to these fibers results in ipsilateral facial paralysis.[11]
General visceral efferents (GVE) and special visceral afferents (SVA) fibers initially exit the brainstem as the nervus intermedius. This separate nerve bundle joins with the other components of the facial nerve within the facial canal. The GVE components from the superior salivary nucleus are responsible for parasympathetic innervation of the glands and mucosae of the face, except for the parotid gland and the smaller buccal and labial glands.
Taste fibers from the anterior two-thirds of the tongue travel centrally as the chorda tympani nerve to their cell body of origin in the geniculate ganglion before synapsing centrally in the solitary nucleus. Depending on the location of the lesion, these visceral components may also be impacted in lesions of the facial nerve. General somatic afferents (GSA) provide sensory innervation from the auricle and a small external portion of the auditory canal.[6]
Cranial Nerve VIII
The vestibulocochlear nerve (CN VIII) is responsible for auditory sensation and the vestibular sense of orientation of the head. This nerve conveys special sensory afferents (SSA) from the inner ear to the cochlear nuclei and the vestibular nuclei in the caudal medulla oblongata. Hair cells within the cochlear duct, semicircular canals, utricle, and saccule are polarized sensory receptor cells with apical ciliary extensions that transduce an electrochemical signal upon mechanical deformation.[2]
Ganglionic neurons within the cochlea and the vestibular nerve receive this signal peripherally and transmit it centrally through the internal auditory meatus before entering the medulla. These nerves run through the internal acoustic meatus, a narrow canal of the temporal bone.[6]
Cranial Nerve IX
The glossopharyngeal nerve (CN IX) is responsible for motor (SVE) innervation of the stylopharyngeus and the pharyngeal constrictor muscles by the nucleus ambiguus. Inferior salivary nucleus fibers travel with cranial nerve IX to provide general visceral efferent (GVE) innervation to parotid, buccal and labial glands. In contrast, visceral afferents (GVA and SVA) receive sensory information from the carotid body and carotid sinus and taste from the posterior third of the tongue to synapse on the solitary nucleus.[1] Whereas the sensory afferents (GSA) receive information from the mucosa of the tongue, oropharynx, middle ear cavity, and auditory canal.[6]
Cranial Nerve X
The vagus nerve (CN X) conveys parasympathetic efferents (GVE) fibers from the dorsal vagal nucleus to the thoracic and abdominal viscera to the splenic flexure of the colon representing its major neural component. These fibers form a comprehensive plexus that travels along the esophageal serosa to the viscera. It also has a considerable motor output (SVE) from the nucleus ambiguous to the pharyngeal and soft palate muscles, as well as the intrinsic laryngeal muscles via the superior and recurrent laryngeal nerves.
Somatic afferents (GSA) supply the posterior cranial dura and a portion of the ear and external auditory canal epithelium. Visceral afferents (GVA) from the pharynx, larynx, aorta, thoracic and abdominal viscera, and taste buds from the root of the tongue and epiglottis (SVA) synapse on the solitary nucleus as well.[6]
Cranial Nerve XI
The spinal accessory nerve (CN XI) is responsible for the general somatic efferent (GSE) motor innervation of the trapezius and sternocleidomastoid muscles through the spinal nucleus of the accessory nerve.[6] The spinal nucleus of the accessory nerve is located within the cervical spinal cord from the levels of C1 through approximately C5/6. The fibers emerge as independent roots, separate from the anterior or dorsal spinal roots of the central spinal grey matter, and ascend through the foramen magnum to enter the cranial cavity. These fibers exit the cranium with cranial nerves IX and X via the jugular foramen.[12]
Central root or nuclear damage to the spinal accessory nerve results in ipsilateral flaccid paralysis of the sternocleidomastoid (with difficulty turning the head against force) and partial ipsilateral trapezius paralysis leading to shoulder drop. The trapezius is innervated by anterior horn grey matter from cervical spinal regions C3 through C4/5 in addition to the spinal accessory nerve. Thus, complete paralysis of the trapezius muscle will not occur following a simple focal lesion.
Cranial Nerve XII
The hypoglossal nerve (CN XII) is responsible for the general somatic efferent (GSE) innervation of the intrinsic and extrinsic muscles of the tongue, except the palatoglossus muscle, from the nerve’s synonymous nucleus.[1] This includes the genioglossus, geniohyoid, hyoglossus, and styloglossus muscles. Fibers from the hypoglossal nucleus exit the medulla from the sulcus between the pyramids and the olives as a collection of fibers that coalesce before entering the hypoglossal canal to exit the cranium.[6] Damage to the nucleus or nerve fibers results in tongue deviation toward the side of the lesion, as the ipsilateral genioglossus muscle becomes weak or flaccid, reducing its ability to protrude the tongue.
Indications
There are many indications for CN testing in prehospital, outpatient, and inpatient settings. These indications include but are not limited to the following clinical conditions:
- Neurologic symptoms, including headaches, seizures, and sensory or motor disturbances
- Traumatic brain injury
- Intracranial hemorrhage
- Cerebral aneurysm
- Intracranial masses
- Cerebrovascular accidents
- Evaluation for brain death
- Unconscious or comatose patient
Contraindications
Relative contraindications to CN testing include severe facial trauma or excessive swelling, which could preclude proper and complete testing. High cervical spine injury or significant concern for cervical spine injury is a particular contraindication to vestibulo-ocular reflex testing.[5]
Equipment
Specialized equipment needed for performing CN testing is minimal. Many of the supplies are readily available in the hospital or clinic setting. A 256-hertz or 512-hertz tuning fork is required to perform the Rinne and Weber tests.[1]
Ishihara or Hardy-Rand-Ritter plates are used to evaluate color perception, and a fundoscopy is required for optic nerve testing. The University of Pennsylvania Smell Identification Test or Sniffin’ Sticks are helpful in testing the olfactory nerve. If these cannot be obtained, items commonly found in the office or hospital, such as coffee, soap, or peanut butter, can be used.[7]
A pen light or flashlight, cotton wisps, safety pins or blunt tip needles, and a hand-held Snellen chart are portable testing instruments that can be carried in a white coat. Sweet, sour, salty, and bitter solutions assess taste on the anterior two-thirds of the tongue.
Technique or Treatment
Cranial Nerve I
The olfactory nerve is the least tested cranial nerve.[3] Nasal blockage can lead to diminished olfaction. The cold spatula test can be used to assess for nasal blockage. Place a cold metal spatula or metal tongue depressor under the nose of the patient. Instruct her to breathe normally and look for misting on the spatula. Move the spatula away from the patient's nose and inspect the misting pattern.[13] Decreased moisture or absent moisture on one side signifies nasal blockage.
CN I functional assessment is easily performed by having patients close their eyes and occlude one nostril. A familiar scent, such as coffee, soap, or cloves, is introduced to the open nostril, and the patient is instructed to sniff and identify the substance.[1] This is repeated with the other nostril, and differences between nostrils are noted. The substance should be less than 30 cm from the nostril, and care should be taken to avoid touching the patient's face or giving auditory clues that indicate when the substance has been introduced. A different, second scent is attempted if the patient cannot identify the odor.
Failure to identify either scent or distinguish between the 2 smells makes diagnosing anosmia highly probable. Standardized kits such as the University of Pennsylvania Smell Identification Test (UPSIT), the Connecticut Chemosensory Clinical Research Center (CCCRC) test, the Brief Smell Identification Test (B-SIT), and Sniffin' Sticks Test (SST) are frequently used, allowing for portable and consistent testing materials.[14] Irritants like alcohol, peppermint, or ammonia are only used when the clinician suspects malingering. These substances activate the nociceptive receptors of the trigeminal nerve.[7][7]
Other tests to measure olfaction include the odor discrimination test to distinguish between odorous and odorless substances and the odor identification test to identify the substance. This method was developed based on the T&T Olfactometer, a standardized olfactometer used in Japan.[14] The odor detection threshold test, or alcohol sniff test, can also be used. This testing requires a standard 70% isopropyl alcohol pad and a 30-cm metric ruler. The patient is instructed to close their eyes and occlude one nostril with their finger. The alcohol pad is introduced starting at the 30-cm mark, moving closer to the nose in 1-cm increments. The patient is diagnosed with anosmia if he cannot smell at a distance of 0 cm to 7 cm, hyposmia if he can detect the smell between 7 cm and 12 cm, and normosmia if he can detect the smell at greater than 12 cm from the unobstructed naris.[14]
Chemosensory event-related potentials (CSERPs) can be used to evaluate chemosensory function objectively. Psychophysical olfactory testing is a helpful technique for evaluating olfactory function in those who have lost their sense of smell. This testing may also enable us to gain a semi-objective and fundamental assessment of olfactory performances.[15] Patients with intact olfactory nerve fibers could be chosen using thallium-based imaging for long-term monitoring of olfactory impairment.[16]
Cranial Nerve II
Functional testing of the optic nerve requires multiple operational assessments to assess the integrity of the nerve. Testing includes evaluation of visual acuity, the visual fields, pupillary light reflexes, the accommodation reflex, and fundoscopy (ophthalmoscopy).
Visual acuity is tested using a Snellen eye chart placed 20 feet away from the patient. Have the patient read the smallest line he can see and record the corresponding visual acuity fraction listed beside the row on the chart. Each eye is assessed individually, while the examiner covers the other eye or the patient covers the eye using an occluder. Test the weaker eye first, and ensure the patient is wearing any glasses or corrective lenses they have for distance vision.[1]
If the patient cannot read the largest (top) line at 20 feet, have them move closer three feet at a time until they can read the top line. Adjust the top portion of the visual acuity fraction accordingly. If the patient cannot read the letters at three feet, have them count fingers at a distance of fewer than 3 feet. Testing light perception is the last resort if the patient cannot count fingers. Charts with pictures instead of letters can be used for patients who cannot read letters due to language or literacy.[17]
The same process is undertaken to assess near vision using a handheld Snellen chart held 30-40 cm away from the patient's face. Any glasses the patient has for near vision should be worn. To test accommodation, have the patient focus on a pen or the examiner's finger. Slowly bring the object closer to the patient and stop within 3 cm of the patient's eyes. Look at both eyes to observe convergence and pupillary constriction. There is a physiological decline of accommodation associated with aging and presbyopia.[18] Hardy-Reed-Ritter or Ishihara plates (cards with numbers in a field of specifically colored dots) can be used to evaluate color perception.[19]
Visual fields are tested by direct confrontation. Each eye is tested individually, with the other eye covered. The examiner stands 3 feet away from the patient, and the patient focuses on the examiner's eyes or nose. A pen or a finger is moved toward the center of the visual field in all 4 quadrants, and the patient will indicate when they can see the object. Alternatively, hold a select number of fingers up in a quadrant and have the patient identify how many fingers they see. Repeat this for all quadrants.
In comatose patients, blink to threat can be used to evaluate the visual fields. Quickly move a hand toward the patient's lateral eye and watch for blinking. Unilateral loss of the blink reflex is suggestive of hemianopia. Note any diminished vision and the location of the deficit (ie, inability to see the temporal fields bilaterally). Visual fields are described from the perspective of the patient. An ophthalmologist should further evaluate deficits in detail.[19]
The pupillary light reflex is assessed using the swinging flashlight test. In a dimly lit room, a flashlight or penlight is shone into the patient's pupil for 1 to 3 seconds.[9] Quickly move the light to the other eye for 1 to 3 seconds, then move the light to the first eye. Observe the pupil for constriction throughout the test, noting any asymmetry between pupils. The direct response is the constriction of the pupil exposed to the light, and the consensual response is the simultaneous constriction of the opposite pupil.[20] A normal result is an equal pupillary constriction in the direct and consensual responses. Damage to the pre-chiasmal portion of the optic nerve leads to greater constriction in the consensual response (in the damaged eye), so when the light is swung to the damaged eye, it appears to dilate. This is a relative afferent pupillary defect or Marcus Gunn pupil.[21][19]
The accommodation reflex is assessed by asking the patient to keep looking at the tip of the examiner's index finger as it is slowly brought from a distance toward the tip of the patient's nose. Observe for the convergence of the eyes and pupillary constriction.[18]
The fundoscopic exam requires significant practice. Typically, the examiner's eye will view the same eye as the patient (use the left eye to look into the patient's left eye with the ophthalmoscope). Dim the lights in the room. With the patient looking slightly upward and inward, slowly move closer from the temporal side with the ophthalmoscope and look through the pupil. View the ocular vessels and follow their course back to the papilla. Note any evidence of optic disc edema or optic atrophy.[19] Alternatives to fundoscopy are being developed. Ocular fundus photography and smartphone fundoscopy have been demonstrated to provide relevant clinical information and be cost-effective. This technology also has a learning curve, and phone-holding attachments can become expensive.[22][23]
Cranial Nerves III, IV, and VI
The oculomotor, trochlear, and abducens nerves are tested by holding a pen or finger 30 to 40 cm in front of the patient and moving in an H-shaped pattern pausing during vertical and lateral gaze.[1] The patient should follow the target with their eyes, carefully keeping their head still. Any eye deviation, abnormal head posture, or nystagmus should be noted. In oculomotor nerve palsy, the involved eye would deviate downward and laterally at rest with ptosis. When looking down, the eye would be mildly adducted and rotated. Oculomotor nerve control of pupillary constriction is tested during the evaluation of the pupillary light reflex.[24] An efferent defect would present as a pupil that sluggishly reacts or is nonreactive to direct and consensual light stimulation.
Patients with trochlear nerve palsy often tilt their heads away from the affected eye and may have strabismus.[25] Midface hypoplasia may also be present in instances of congenital trochlear nerve palsy. Intorsion, depression, and abduction of the eye will be impaired. The dysfunction is usually incomplete as the oculomotor and abducens nerves also depress and abduct the eye.
A head tilt may also be associated with abducens nerve palsy; however, the head will be tilted toward the affected eye. Patients may also exhibit a constant head-turning motion to try and lessen the diplopia. Eye abduction will be impaired or absent on examination.[26]
Cranial Nerve V
The trigeminal nerve has sensory and motor functions.
The sensory portions of the trigeminal nerve are evaluated by lightly touching a blunt tip needle and a cotton swab or ball to the patient's face in each of the divisions while their eyes are closed. Have the patient indicate whether the sensation is soft or sharp. The sensation of the angle of the mandible should be tested if the facial sensation is diminished, as the C2 spinal root innervates this area. Sparing of the angle of the jaw is indicative of trigeminal pathology.[1]
Evaluation of trigeminal nerve function should also include testing the corneal reflex. With the patient looking away, gently touch a cotton swab to the middle or lateral portion of the cornea to test the corneal reflex.[20] Approach the eye from the periphery and avoid placing the swab in the area of the pupil, as the patient may see the swab and blink. The direct response is the closure of the ipsilateral eye after stimulation, and the closure of the contralateral eye is the consensual response.
A patient with a lower motor neuron facial nerve palsy will have a stronger and more brisk consensual response when testing the eye on the affected side.[27][10] The reflex afferents are A-delta fibers that pass through long ciliary nerves and the ophthalmic division of the trigeminal nerve to reach the pons. The efferents are the motor fibers of the bilateral facial motor nuclei in the facial nerve that terminate in the orbicularis oculi muscles.[28] Another distinction between a weak blink in the corneal reflex due to facial nerve palsy versus a depressed corneal sensation is that a patient with facial nerve palsy will feel the cotton swab normally on both sides. The patient with dampened corneal sensation will not feel the cotton swab when it touches the cornea.[10]
Motor function of the trigeminal nerve is tested by having the patient open their mouth against resistance. If weakness is present, the jaw will deviate to the side with the weakened pterygoid muscle. With the patient's teeth clenched, palpate the masseter muscles and note any asymmetry.[1][10]
The masseter reflex, or jaw jerk, is tested by tapping the examiner's thumb kept over the patient's chin with a knee hammer with the patient's mouth partially opened. This causes reflex contraction of the masseter leading to the closure of the mouth. Afferent neurons located in the mesencephalic nucleus of the midbrain travel through the motor root of V3, and the efferent motor neurons are located in the pontine trigeminal motor nucleus and stimulate the ipsilateral masseter muscle.[28] Weakness will occur with lower motor neuron lesions. Paralysis from upper motor neuron lesions is rare as there is bilateral innervation. In the event of bilateral upper motor neuron lesions leading to paralysis, there will also be a hyperactive jaw jerk and possibly clonus.
Cranial Nerve VII
Facial nerve function is tested by assessing for asymmetric facial movements. This can be observed while obtaining the medical history, particularly during talking, blinking, and smiling.[1] A widened palpebral fissure, a flattened nasolabial fold, and delayed or incomplete blinking are signs of facial weakness. Have the patient smile, puff out their cheeks, raise eyebrows, and show their teeth. Try to open the patient's eyes while they hold them shut. Note any asymmetry in response between both sides of the face.[11]
Upper motor neuron (UMN) lesions are classified as damage to the corticobulbar tract from the motor cortex to the facial nerve nucleus. Unilateral damage will result in drooping of the mouth, flattening of the nasolabial fold, and paralysis of the contralateral lower face. Eye closure and forehead movement will be spared as bilateral UMN innervation exists in that area.[29] Lower motor neuron (LMN) lesions occur from within the facial nucleus along the path of the facial nerve. Injuries manifest partial or complete paralysis of the ipsilateral upper and lower face. Signs include incomplete eye closure due to orbicularis oculi dysfunction, hyperacusis, drooping of the corner of the mouth, flattened nasolabial fold, smoothing of the eyebrow, and diminished taste of the anterior tongue. Look for signs of incomplete eye closure, such as dry eye and corneal ulcers.[29]
To evaluate taste, ask the patient to stick out her tongue and close her eyes. Apply a small amount of salt (for salty), quinine hydrochloride or caffeine strips (for bitter), tartaric acid (for sour), or sugar (for sweet) to the lateral surface and side of the anterior tongue, then have the patient identify the substance. Rinse the mouth thoroughly with water and assess the opposite side with a different substance. Solution kits with varying concentrations are commercially available for quantitative testing, and premade taste strips have also been developed as an alternative to solutions.[30][31][32]
Cranial Nerve VIII
Initial hearing testing is conducted by rubbing the fingers by one ear while occluding the other, then repeating for the other ear. This can also be accomplished by whispering in one ear while occluding the other and repeating for the opposite side.[1] Note any asymmetry in response or hearing. Further evaluation using the Rinne and Weber tests is warranted if hearing loss is suspected; the Rinne and Weber tests help to distinguish conductive from sensorineural hearing loss.
The Rinne test is performed by placing a vibrating 512-hertz tuning fork on the mastoid process, and once the sound is no longer heard, moving the fork to just outside the ear. In a normal (positive) Rinne test, air conduction is greater than bone conduction. In an abnormal (negative) Rinne test, bone conduction is greater than air conduction in the affected ear. A patient with profound sensorineural hearing loss may not hear anything from the tuning fork placed on the mastoid process or near the external auditory canal. Sound will transmit through the skull to the opposite ear, and the patient may be unable to identify which ear heard the sound. In this situation, it would appear that bone conduction is greater than air conduction when in fact, the ear is completely nonfunctional. This is called a false negative Rinne test. A Weber test can delineate between a negative and a false negative test.[33]
The Weber test is performed by placing a vibrating 512-hertz tuning fork on the center of the forehead. Sound is louder in, or "lateralizes to," the ear experiencing conductive hearing loss or opposite the ear with sensorineural hearing loss. Sensorineural hearing loss can be further divided into sensory or neural based on brainstem auditory evoked responses (BSAERs), and the patient should be referred for further testing.[34]
To assess vestibular function, test for nystagmus and note the direction, duration, and trigger of the nystagmus. Further in-depth testing is used to distinguish central from peripheral sources, particularly if the patient is experiencing vertigo during the examination. Frenzel lenses or +30 diopter glasses can be used to prevent visual fixation, which can suppress nystagmus. Testing for acute vestibular syndrome involves the head thrust maneuver; the Dix-Hallpike maneuver tests for positional vertigo.
The head thrust maneuver is performed by holding the head of the sitting patient while they focus on an object, such as the examiner's nose, and quickly turning the patient's head 20 degrees to the right or left. This is a normal result if the eyes remain focused on the examiner's nose. Temporary deviation away from the object with a corrective saccade that brings the eyes back to the object indicates a peripheral source of nystagmus, such as vestibular neuronitis.[34]
For the Dix-Hallpike maneuver, the patient is quickly lowered to the supine position with the head extended 45 degrees below the table and turned 45 degrees to one side. Note the direction and duration of nystagmus and assess for vertigo. Raise the patient back to the upright position and perform the maneuver with the head turned to the other side. Nystagmus with a latency period of five to ten seconds that is vertical when the eyes are turned away from the affected ear and rotary when the eyes face the involved ear is pathognomonic for benign paroxysmal positional vertigo. This response also diminishes with repeated testing. Positional nystagmus caused by central nervous system dysfunction will not have a latency period and will not diminish with further testing.
Cranial Nerves IX and X
Testing of the glossopharyngeal and vagus nerves is performed simultaneously. Have the patient open their mouth and say, "Aah." Determine whether the palate elevates symmetrically and the uvula remains in the midline. If weakness is present, the uvula will lift away from the paretic side of the palate.[3]
To assess the pharyngeal gag reflex, lightly touch one side of the posterior pharynx with a tongue blade and watch for gagging, then the opposite side. Contraction of the pharyngeal musculature ipsilateral to the side of the stimulus is known as the direct gag reflex, and contraction of the musculature on the contralateral side is known as the consensual gag reflex. Unilateral damage to the glossopharyngeal nerve will result in the absence of a gag response when that side of the pharynx is stimulated. When the vagus nerve is damaged, the palate will elevate and deviate toward the affected side with stimulation of either side of the posterior pharynx. Unilateral injury to both the glossopharyngeal and vagus nerves leads to deviation of the palate to the intact side when the intact side is stimulated. When the damaged side is stimulated, there is no response. The bilateral absence of the gag reflex is common. Stimulating the soft palate with a tongue blade can also elicit the gag reflex; however, the trigeminal nerve provides the efferent portion of this reflex.[35]
In an intubated patient, suction the endotracheal tube and note the presence or absence of coughing.[20]
Note any hoarseness in the patient's voice, as the vocal cords will need inspection and visualization if hoarseness is present.[1] Hoarseness with intact gag reflexes and symmetric palate elevation suggests compression of the recurrent laryngeal nerve and warrants further evaluation for mass lesions.
Cranial Nerve XI
Evaluation of the spinal accessory nerve involves the sternocleidomastoid and trapezius muscles. To assess the left sternocleidomastoid, place one hand on the patient's right cheek and have the patient turn their head to the right while providing resistance. Have the patient turn their head to the left against resistance to test the left side. Weakness with turning the head to the left indicates right-sided pathology, and weakness when turning the head to the right indicates left-sided pathology. Further testing involves having the patient flex their head against resistance.[36][37]
For the trapezius, inspect the shoulder and upper back for any asymmetry, drooping of the shoulder, or winging of the scapula; winging is noted when the medial side of the scapular appears more prominently than the unaffected scapula. Note any atrophy of the trapezius or internal rotation of the humerus. There may also be hypertrophy or subluxation of the sternoclavicular joint due to increased strain on the joint from loss of trapezius muscle support. Have the patient shrug their shoulders while providing resistance by pressing down on the shoulders.[1][3] The examiner can also have the patient press against a wall with his arms extended to evaluate for lateral winging of the scapula.[37]
Ipsilateral upper motor neuron lesions present with ipsilateral sternocleidomastoid weakness and contralateral trapezius weakness. Pathology of the lower cervical cord, ventral brainstem, or lower spinal accessory nerve roots displays isolated trapezius muscle weakness.
Isolated sternocleidomastoid weakness indicates pathology within the brainstem tegmentum or upper cervical accessory root. Peripheral lesions can cause isolated weakness of either the sternocleidomastoid or the trapezius. Damage within the contralateral brainstem, ipsilateral high cervical cord, or an accessory nerve lesion peripherally before the nerve bifurcates to both muscles results in ipsilateral weakness of both the sternocleidomastoid and the trapezius.[36]
Cranial Nerve XII
To test the hypoglossal nerve, have the patient protrude her tongue. Assess for deviation of the tongue, atrophy, and fasciculations.[3] Atrophy, fasciculations, and deviation in the direction of the lesion are associated with lower motor neuron pathology. The tongue will deviate away from the lesion with upper motor neuron pathology, and there will not be atrophy or fasciculations. Have the patient press the tongue against each cheek while the examiner provides gentle resistance to the cheek.[1] Also, note the patient's ability to pronounce T and D words, which will be impaired with hypoglossal nerve palsy.
Clinical Significance
The cranial nerve examination is a key component of any neurological evaluation, but it is particularly crucial in comatose patients and brain death evaluation.[20][38] The cranial nerves that can be reliably tested in a comatose patient are the optic and oculomotor nerves via the pupillary light reflex, trigeminal nerve via the corneal reflex, vestibulocochlear nerve via the vestibulo-ocular or doll’s eye reflex, and the glossopharyngeal and vagus nerves via the gag reflex.[39][40]
A diminished or absent pupillary light reflex has significant prognostic value and is often an indicator of increased intracranial pressure, cerebral edema, secondary brain injury, hydrocephalus, or intracranial shift.[5]
Signs discovered during cranial nerve testing can aid in narrowing the differential diagnosis of neurologic disease and differentiate among various pathologies. For example, multiple sclerosis and neurosarcoidosis can both present with diplopia.[9] The latter frequently presents with other cranial nerves concomitantly involved, including the facial nerve and the oculomotor nerve, while the former presents with hyperreflexia, spasticity, and diminished sensation.[41]
Cranial Nerve I
Head trauma, particularly anterior skull base fractures, concussion, and whiplash injuries, lead to anosmia 5% to 17% of the time.[7] The olfactory dysfunction can be conductive due to sinonasal tract disruption from nasal fracture or swelling of nasal mucosa or neurosensory due to the shearing of the olfactory nerve fibers in the cribriform plate or olfactory cortex contusion.[42] Anosmia can also be a sign of congenital disorders such as Kallmann syndrome and has a strong association with neuropsychiatric conditions like Lewy body dementia and Parkinson disease. Rhinitis and viral upper respiratory infection commonly result in decreased olfaction. Unilateral anosmia combined with ipsilateral optic nerve atrophy and contralateral papilledema suggests a sphenoid ridge tumor. Schizophrenia and depression have also been linked with diminished olfaction.
No specific treatment has been shown to improve olfactory dysfunction's speed or odds of recovery. Recovery rates of post-traumatic anosmia range from 33% to 36%; improvement is seen predominantly within the first year following the injury.[42]
Cranial Nerve II
Damage to the optic nerve leads to visual field defects or vision loss. The location of the disruption to the optic nerve determines the visual field defect. Compromise of the optic nerve anterior to the optic chiasm causes ipsilateral monocular blindness.[21] Damage at the optic chiasm disrupts the nasal retinal fibers of both eyes, resulting in a loss of both temporal visual fields. This is termed bitemporal hemianopia and may result from a pituitary adenoma compressing the optic chiasm. Pathology occurring posterior to the chiasm involving the optic tracts or visual cortex results in contralateral homonymous hemianopia or quadrantanopia, respectively.[19]
For example, a lesion in the right optic tract results in a loss of the left visual fields of both eyes. Damage to the inferior retrochiasmatic pathway (Meyer loop in the temporal lobe) produces a superior quadrantanopia, while injuries to the superior retrochiasmatic pathway (Baum loop in the parietal cortex) lead to an inferior quadrantanopia.[19]
An altitudinal deficit is decreased vision in the inferior or superior visual field that does not cross the horizontal midline. This deficit is typically caused by occlusion of a branch of the retinal artery, retinal detachment, or ischemic optic neuropathy. Blind spot enlargement can be caused by papilledema and optic nerve drusen. Loss of the bilateral nasal or medial visual fields is termed a binasal hemianopia, which is rare and caused by glaucoma or retinitis pigmentosa. Glaucoma, retinitis pigmentosa, and bilateral occipital lobe infarction can also lead to loss of the peripheral visual fields. Central scotoma, or vision loss in the central visual field, is typically seen with macular disease and optic neuropathy.[43]
Damage to the optic nerve may also attenuate the pupillary light reflex resulting in an afferent pupillary defect (APD) or a relative afferent pupillary defect (RAPD), also known as a Marcus Gunn pupil. Optic neuropathies, such as glaucoma, optic neuritis, as seen in multiple sclerosis, and severe macular degeneration, may all cause an RAPD.[21]
Optic neuritis can be treated with steroids. Underlying disease processes such as tubercular or cryptococcal meningitis, cavernous sinus thrombosis, or compressive tumor should be treated. Vascular causes of acute ischemic optic neuropathy are treated with platelet aggregation inhibitors; however, the effects are modest. A diagnosis of giant cell arteritis warrants immediate steroid therapy to prevent severe vision loss.[44][19]
Cranial Nerve III
Complete oculomotor palsy involves loss of parasympathetic function and levator palpebrae superioris function.[19] As a result, the affected eye has ptosis with a dilated, nonreactive pupil. With incomplete palsy, ptosis is mild, and the pupil is reactive. The manifestations include diplopia, impaired adduction, elevation, accommodation, and depression in adduction. Microvascular etiologies like hypertension or diabetes typically spare parasympathetic function, while compressive pathologies such as tumors or aneurysms, especially those arising from the posterior communicating artery, cause significant parasympathetic dysfunction.[45] Oculomotor nerve compression can occur after head trauma, frequently due to an epidural hematoma with uncal herniation.
Conservative management of acquired oculomotor palsy leads to complete resolution of symptoms in 63% of patients.[46] Botulinum toxin can be administered in acute oculomotor nerve palsy with isolated medial rectus paralysis. Surgical intervention is indicated for patients with no improvement following six months of conservative treatment and those with compressive aneurysms.[45]
Cranial Nerve IV
Trochlear nerve palsy manifests as vertical binocular diplopia, a duplicate image appearing superiorly and to the side of the actual image.[47][48] This is most pronounced when looking down (ie, walking downstairs) or participating in near-vision activities such as reading.[19] Patients frequently tilt their heads towards the unaffected side to alleviate the symptoms. The most common etiology is idiopathic or congenital, accounting for 49% of all cases.[25] Other causes of trochlear nerve palsy include trauma (18% of cases), hypertension (18% of cases), hypertension and diabetes (5% of cases), or tumors (1% of cases). Another rare cause is cavernous sinus syndrome.
Trochlear nerve palsy is usually unilateral. Frontal head trauma from boxing or motor vehicle collisions and lesions of the dorsal midbrain can cause bilateral trochlear nerve palsy.[49]
Surgical interventions such as tucking the superior oblique tendon or inferior oblique weakening correct most cases of trochlear nerve palsy.[49] More conservative management methods include eye patches and prisms.[50]
Cranial Nerve V
Common trigeminal nerve pathologies include trigeminal neuralgia, cluster headache, and lateral medullary or Wallenberg syndrome.
Trigeminal neuralgia presents as neuropathic, shock-like facial pain within the trigeminal dermatomal distribution (V2 and V3 divisions).[51] This neuralgia is commonly due to trigeminal nerve compression by an aneurysm, arteriovenous malformation, tumor, or trauma.[52] Trigeminal neuralgia can be treated surgically via microvascular decompression or ablative treatments, or it can be treated medically using medications such as carbamazepine, lamotrigine, oxcarbazepine, phenytoin, gabapentin, pregabalin, or baclofen. Medical pharmaceutical therapy is generally attempted before surgical intervention, with carbamazepine or oxcarbazepine being the first-line treatment.[52][53] The corneal reflex is used as a practical test to evaluate the trigeminal nerve injury following the treatment of trigeminal neuralgia using percutaneous balloon compression.[54]
The presentation of cluster headaches is similar to that of trigeminal neuralgia, and the two diseases are often considered within the same differential. Cluster headaches are unilateral in the trigeminal dermatomal distribution, with nasal congestion, swelling, and lacrimation of the ipsilateral side. Prophylactic treatment of cluster headaches includes trigger avoidance and pharmacotherapy. Acute attacks are managed with oxygen supplementation and fast-acting triptans.
The lateral medullary or Wallenberg syndrome is caused by damage to the lateral medulla, commonly from a posterior circulation stroke.[55] Signs include ipsilateral sensory loss in the trigeminal territory and contralateral sensory loss in the rest of the body. Management of lateral medullary syndrome focuses on stroke management and prevention of secondary stroke.[55]
Cranial Nerve VI
The abducens nerve is the ocular motor nerve most frequently affected by pathology. Abducens palsy results in binocular diplopia or double vision in the horizontal plane when looking at objects side by side and with distance vision. Etiologies leading to abducens palsy can be classified based on the location of the nerve. Tumors and trauma such as skull base fractures or orbital trauma can injure the abducens nerve anywhere along the path of the nerve.[56]
In the pons, ischemic stroke, demyelinating disease, and metabolic diseases such as Wernicke encephalitis damage the abducens nucleus and fascicles. An aneurysm can damage the portion of the abducens nerve within the subarachnoid space; infections like syphilis, tuberculosis, and Lyme disease; inflammatory diseases such as neurosarcoidosis or lupus; and carcinomatous meningitis.[26] This is primarily due to increased intracranial pressure affecting the nerve. Complicated otitis media or mastoiditis extending to the petrous apex can also lead to abducens nerve palsy. Within the cavernous sinus, the abducens nerve is subject to injury from internal carotid artery aneurysm or dissection, cavernous sinus thrombosis, and cavernous sinus fistula. Infections, tumors, inflammation, or trauma involving the orbit may also lead to abducens nerve dysfunction.
Abducens nerve palsy is typically self-limiting in adults, and patients can be observed. For dysfunction caused by a significant underlying disease process, treatment of the disease is warranted.[26][56] The treatment of abducens nerve palsy in children includes prism therapy, alternate eye patching, strabismus surgery, or botulinum toxin injection.
Cranial Nerve VII
Facial nerve palsy is iatrogenic in 70% of cases, colloquially known as Bell palsy. Trauma accounts for 10% to 23% of facial nerve palsy cases, most commonly due to fractures of the petrous portion of the temporal bone or deep facial wounds that disrupt the nerve.[57]
Accompanying signs of facial nerve palsy are dictated by etiology and may include hemotympanum, battle sign, and nystagmus. Iatrogenic injury can occur by stretching, crushing, or lacerating the nerve during parotid gland surgery, vestibular schwannoma resection, or otologic procedures. Other etiologies include various infections such as varicella-zoster virus, acute otitis media, otitis externa, and COVID-19 with 4.5% to 7% incidence, and various neoplasms such as a parotid malignancy, vestibular schwannoma, meningioma, facial neuroma, or arachnoid cyst in another 2.2% to 5%.[58]
While bilateral facial nerve palsy has a rare occurrence of 0.3% to 2%, it is a crucial finding as it usually indicates systemic disease. Lyme disease is the cause in 35% of these cases. Parkinson disease, multiple sclerosis, and pseudobulbar palsy can also cause bilateral facial nerve palsy.
Various treatment modalities are utilized in the management of facial nerve palsy. Artificial tears, lubrication, and taping the eye closed at night prevent corneal ulceration.[59] Facial massage and exercises are other components of conservative management. Steroids and analgesics have been shown to increase recovery of motor function if a regimen is started within 72 hours of symptom onset. Acyclovir is added to the medical treatment in cases of Ramsay Hunt syndrome due to varicella-zoster infection, although there is no concrete evidence of its efficacy.[57]
Over 70% of patients with Bell palsy have complete motor recovery without treatment. Appropriate antibiotic therapy is used for cases caused by otitis media, otitis externa, or Lyme disease. Surgical intervention is indicated in cases of Bell palsy with over 90% degeneration on electroneuronography or temporal bone fractures with immediate and complete facial paralysis. Myringotomy or mastoidectomy can be undertaken in patients with suppurative otitis media or mastoiditis.
Cranial Nerve VIII
There are multiple etiologies of vestibulocochlear nerve dysfunction, and pathology can occur anywhere along the auditory pathway. Presenting symptoms include vertigo, nystagmus, tinnitus, and sensorineural hearing loss.[60]
The most common mechanisms of injury are infarcts and demyelination diseases such as multiple sclerosis. Patients can present with unilateral or bilateral sensorineural loss, depending on the location of the injury. Additionally, traumatic injury to the temporal bone can present with unilateral sensorineural loss due to an internal auditory canal or bony labyrinth fracture. There can also be a concussive injury to the labyrinth. Imaging studies for such injuries are typically negative unless magnetic resonance imaging (MRI) shows an intralabyrinthine hemorrhage.[61]
Notable congenital malformations of CN VIII can be classified as aplasia or hypoplasia. These malformations are subdivided into three subtypes: 1, 2A, and 2B. Type 1 refers to an aplastic CN VIII with a normal labyrinth. Type 2A is an aplastic or hypoplastic cochlear branch with an accompanying labyrinth malformation. Type 2B, the most common subtype, refers to an aplastic or hypoplastic cochlear branch with a normal labyrinth.[62]
Vestibular schwannomas arise from Schwann cells. These are the most common cerebellopontine angle tumors, followed by meningioma.[63] The usual presentation is progressive, unilateral sensorineural hearing loss; commonly associated symptoms are tinnitus and vertigo. Bilateral vestibular schwannomas are a hallmark sign of neurofibromatosis type 2 (NF-2), an autosomal dominant genetic syndrome. Notably, facial nerve palsy rarely occurs with vestibular schwannomas because the facial nerve is resistant to chronic pressure. MRI is the diagnostic imaging of choice.[62][64]
Acute vestibular syndrome (AVS) is characterized by the acute onset over minutes to hours of vertigo, nausea, vomiting, and gait disturbances associated with nystagmus and head-motion intolerance over the following days to weeks. The etiology of AVS can either be peripheral, most commonly vestibular neuritis, or central, such as brainstem or cerebellar stroke. Vestibular neuritis is usually viral in origin and self-limiting, while a central stroke can have permanent effects. Therefore, it is critical to distinguish between these two etiologies to ensure proper patient care. This can be done at the bedside using the “Head-Impulse—Nystagmus—Test-of-Skew” (HINTS) exam.[60]
The horizontal head-impulse test, which assesses the vestibulo-ocular reflex, would be normal with central etiologies and abnormal with peripheral etiologies. Nystagmus would be vertical or bidirectional with central etiologies and unidirectional with peripheral etiologies, regardless of gaze direction. A skew deviation would be present with central etiologies and absent with peripheral etiologies.[65]
The mainstays of treatment for vestibulocochlear nerve dysfunction focus on managing the underlying cause. The Epley maneuver is recommended for benign positional vertigo, and other conservative measures, such as physiotherapy or gait training, can be used for multiple types of vestibular dysfunction.[66]
Cranial Nerve IX
Etiologies of glossopharyngeal nerve palsy or dysfunction include trauma (styloid fracture), iatrogenic injury (carotid endarterectomy, laryngeal mask airway placement), Eagle syndrome, glossopharyngeal neuralgia, stroke, and tumor (tonsillar carcinoma).[67] The most common jugular foramen syndromes associated with CN IX dysfunction are Vernet syndrome, Collet-Sicard syndrome, and Villaret syndrome.
Presentation of glossopharyngeal nerve palsy involves dysphagia, dysphonia, impaired taste over the posterior third of the tongue and palate, absent gag reflex, loss of carotid sinus reflex, decreased parotid gland secretions, and diminished sensation of the posterior third of the tongue, palate, and pharynx.[68]
Glossopharyngeal neuralgia consists of episodic, unilateral sharp pain in the posterior throat, tonsils, base of the tongue, and inferior to the angle of the mandible that can last from seconds to minutes.[69] It is triggered by mandibular actions such as chewing, swallowing, coughing, and yawning.[68] Some patients with glossopharyngeal neuralgia also experience excessive vagal stimulation during attacks, with symptoms such as bradycardia, hypotension, syncope, seizures, or cardiac arrest.[70]
Treatment of glossopharyngeal neuralgia includes carbamazepine, gabapentin, and pregabalin medications as first-line treatment. Microvascular decompression is indicated in patients with refractory symptoms.[68]
Cranial Nerve X
The recurrent laryngeal branch of the vagus nerve can suffer injury in head or neck surgeries such as carotid endarterectomy.[71] Central vagus nerve lesions can cause dysphagia, dysarthria, hoarseness, uvula deviation towards the side opposite the lesion, and transient parasympathetic effects. The vagus nerve can also become damaged due to lateral medullary syndrome.[72]
Management of dysphagia requires dietary and postural modifications. Placement of percutaneous endoscopic gastrostomy (PEG) may be necessary for instances of severe prolonged dysphagia. Active oral exercises help strengthen swallowing musculature. Botulinum toxin type-A injections have been used to treat severe dysphagia associated with trismus.[73][74]
The vagus nerve also has clinical implications as a minimally invasive treatment modality for central neurologic structures. Vagus nerve stimulation has been approved for the treatment of epilepsy and depression.[75] With the wide distribution of the vagus nerve throughout the body, stimulation is being explored for other purposes, such as treating obesity.[76]
Conventional vagus nerve stimulation involves the placement of a device under the skin in the chest, with electrical wires connected to the left vagus nerve (the left is used more frequently than the right, as the right vagus nerve is more likely to have branches to the heart).[77] Stimulation of the larynx provides reflexes, including cough and apnea, and effects on the cardiovascular system, such as bradycardia and hypotension.
Cranial Nerve XI
Due to the superficial course in the posterior triangle of the neck, the spinal accessory nerve is subject to injury secondary to blunt or direct trauma. Blunt trauma can cause injury during contact sports such as football or hockey. Penetrating trauma from a gunshot wound or a deep laceration from sharp objects or weapons can also compromise the spinal accessory nerve.[78]
Iatrogenic injuries are most common and may occur during lymph node biopsies or tumor resection within the posterior triangle of the neck, carotid or internal jugular vein surgeries, neck dissections of any complexity, or cosmetic surgery. Palsy usually stems from the mechanical stress exerted on the neck due to positioning throughout the procedure.[37] Damage results in trapezius paralysis, leading to winging of the scapula and drooping of the affected shoulder.[79]
Neurologic causes of spinal accessory nerve palsy occur from nerve compression, such as that seen with a tumor at the jugular foramen.[37] Vernet, Collet-Sicard, and Villaret syndromes involve the spinal accessory nerve. Other causes of CN XI injury are syringomyelia, brachial neuritis, poliomyelitis, motor neuron disease, and traction palsies to the brachial plexus and thoracic outlet.[80]
The approach to managing accessory nerve injury and resulting trapezius muscle deformity is an interprofessional approach that revolves around conservative care, physical therapy, and surgical treatment. Treatment and management depend on the severity and the cause of the spinal accessory nerve injury. Immediate therapy should be considered for severe presentations, such as penetrating traumas and iatrogenic injuries.[37]
Surgical interventions include re-anastomosis and nerve grafting. Nonsteroidal anti-inflammatory drugs, nerve stimulation, local or regional nerve blocking, and physical therapy with rehabilitation are non-surgical treatment options.[78]
Cranial Nerve XII
The hypoglossal nerve can be damaged at the hypoglossal nucleus (nuclear), above the hypoglossal nucleus (supranuclear), or interrupted at the motor axons (infranuclear).[81] Such damage causes paralysis, fasciculations, and eventual atrophy of the tongue muscles. Common etiologies include tumors, infection, or trauma such as injury from carotid endarterectomy or cervical spine surgery.[82]
Upper motor neuron (supranuclear) lesions occur in the cerebral cortex, the corticobulbar tract of the internal capsule, cerebral peduncles, or the pons. Supranuclear lesions do not tend to cause atrophy but can lead to an uncoordinated tongue with slow, spastic tongue movements. Supranuclear lesions frequently result from strokes but can also be caused by pseudobulbar palsy or amyotrophic lateral sclerosis.[83] Lower motor neuron (infranuclear and nuclear) lesions cause tongue weakness and ipsilateral atrophy. Lower motor neuron disease can also cause fasciculation.[81]
Unilateral lesions portend a favorable prognosis for patients as deficits are partially compensated for by the remaining hypoglossal nerve. Bilateral lesions can be caused by radiation therapy and cause impairment of speech and swallowing as the patient cannot protrude the tongue for these necessary functions.[81]
In obstructive sleep apnea (OSA), the decrease in muscle tone of the genioglossus muscle causes the tongue to retract and impede airflow into the trachea. The hypoglossal nerve stimulator is one possible treatment if the patient is refractory to continuous positive airway pressure (CPAP), oral devices, or surgery. Mild hypoglossal nerve stimulation causes the nerve to pull the tongue forward, enabling better airflow.[84][85]
Enhancing Healthcare Team Outcomes
The cranial nerve examination is a detailed procedure that requires practice and vigilance to master. Deficits found during the cranial nerve examination may require further evaluation and involvement of otolaryngology, radiology, and neurophysiologic testing professionals. Instances such as intracranial hemorrhage, coma, or stroke necessitate serial testing as part of the overall neurologic examination to evaluate for worsening or progression of pathology.[5]
Assistance from the nursing and intensive care unit staff is vital in discovering changes in conditions that require further evaluation or urgent intervention. An interprofessional approach is suggested to lower morbidity, reduce unnecessary imaging, and improve outcomes.[4] [Level 5]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
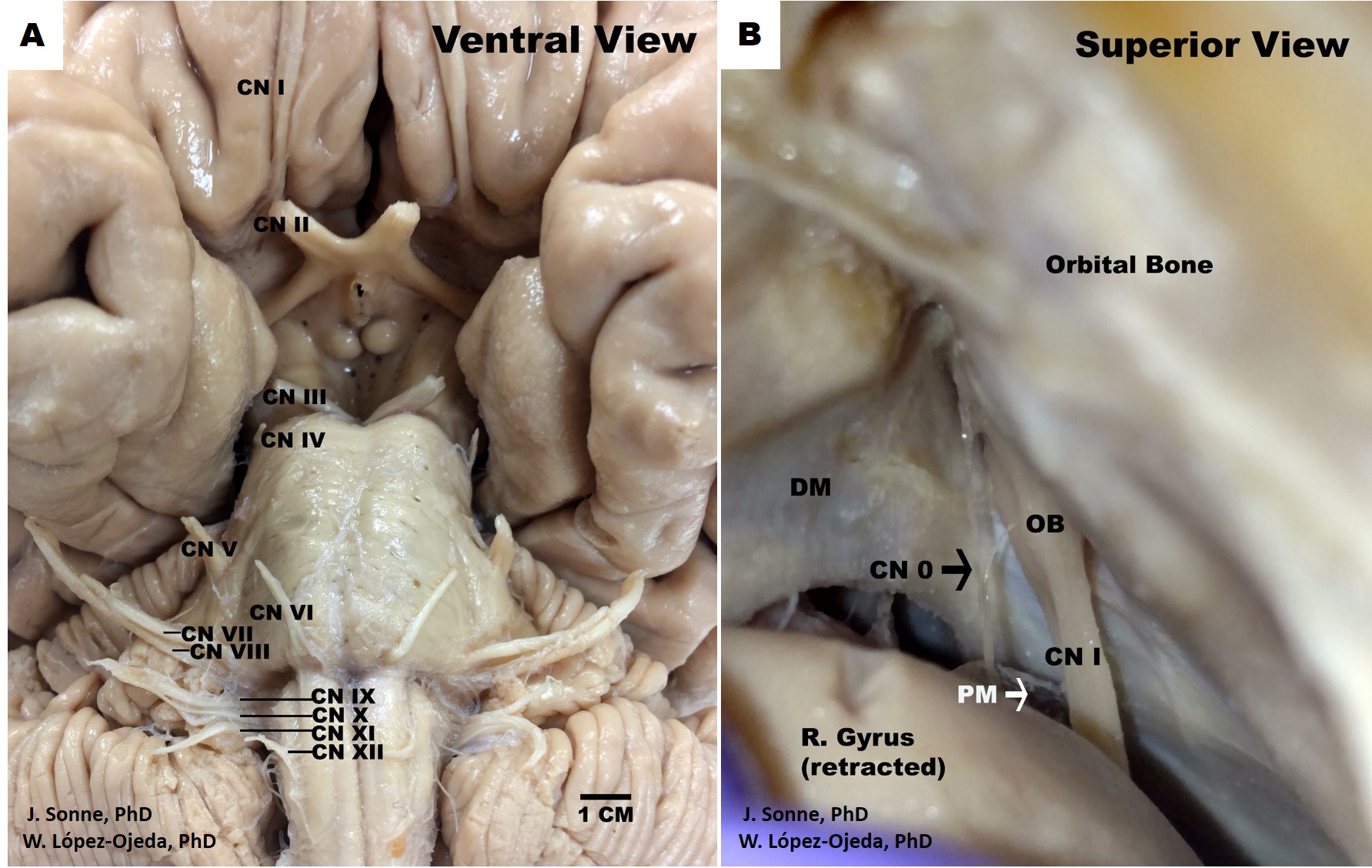
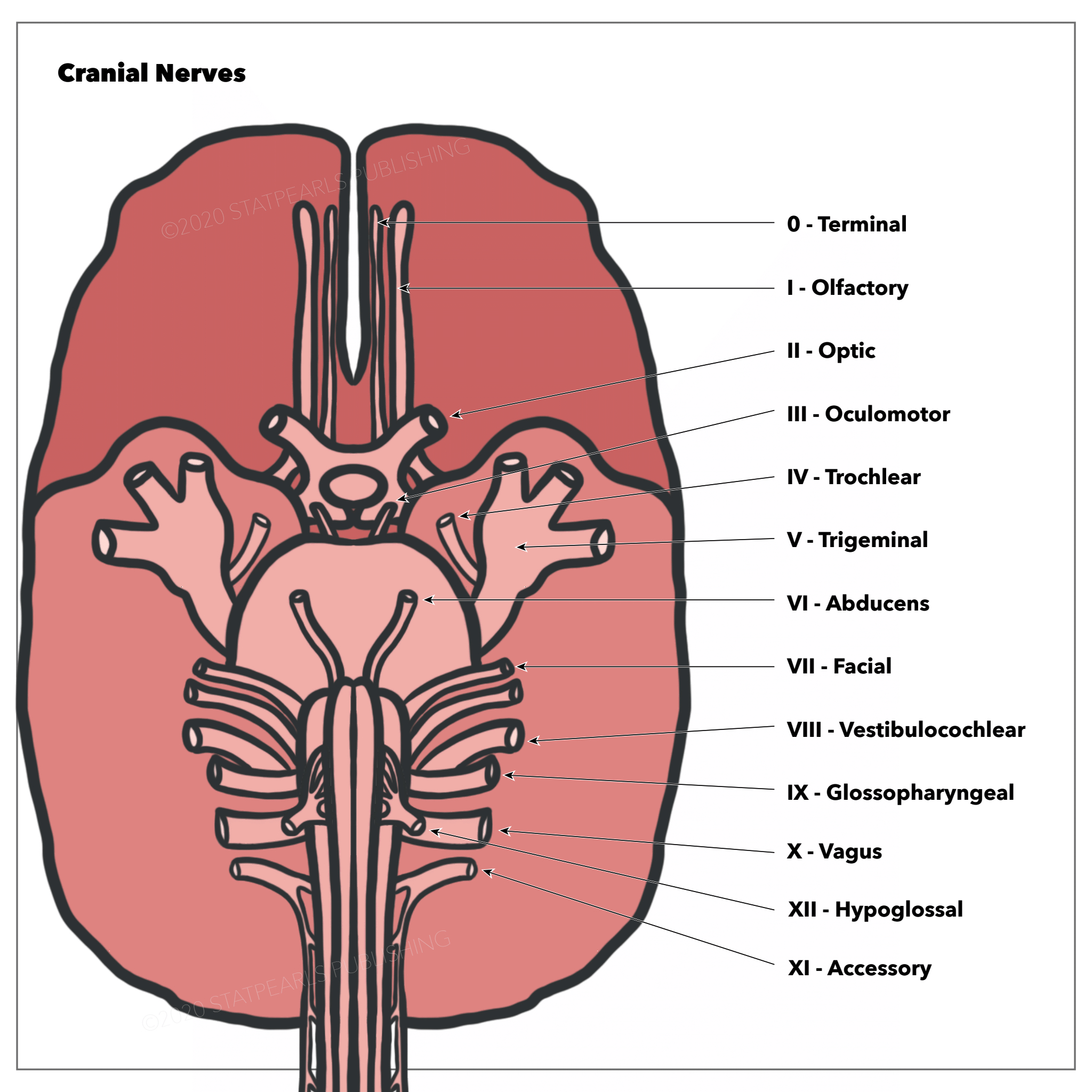
Human Brain Cranial Nerves. Ventral view of the human brain depicting the canonical organization of the 12 pairs of cranial nerves (CN) as described in the classical medical nomenclature (A). Roman numerals (I-XII) are used in progressive order to identify the rostrocaudal organization of the 12 pairs of cranial nerves, (B). Superior view of the human skull and frontal brain lobe following a special dissection procedure to reveal the intact cranial nerve zero (CN 0) (black arrow). DM= dura matter, OB= olfactory bulb, PM= pia matter.
Contributed by J Sonne, PhD, and W López-Ojeda, PhD
(Click Image to Enlarge)
References
Taylor A, Mourad F, Kerry R, Hutting N. A guide to cranial nerve testing for musculoskeletal clinicians. The Journal of manual & manipulative therapy. 2021 Dec:29(6):376-389. doi: 10.1080/10669817.2021.1937813. Epub 2021 Jun 29 [PubMed PMID: 34182898]
Møller MB. Vascular compression of the eighth cranial nerve as a cause of vertigo. The Keio journal of medicine. 1991 Sep:40(3):146-50 [PubMed PMID: 1753557]
Shahrokhi M, Asuncion RMD. Neurologic Exam. StatPearls. 2023 Jan:(): [PubMed PMID: 32491521]
Mandavia DP, Villagomez J. The importance of serial neurologic examination and repeat cranial tomography in acute evolving epidural hematoma. Pediatric emergency care. 2001 Jun:17(3):193-5 [PubMed PMID: 11437146]
Level 3 (low-level) evidenceMusick S, Alberico A. Neurologic Assessment of the Neurocritical Care Patient. Frontiers in neurology. 2021:12():588989. doi: 10.3389/fneur.2021.588989. Epub 2021 Mar 22 [PubMed PMID: 33828517]
Sonne J, Lopez-Ojeda W. Neuroanatomy, Cranial Nerve. StatPearls. 2023 Jan:(): [PubMed PMID: 29261885]
Sanders RD, Gillig PM. Cranial nerve I: olfaction. Psychiatry (Edgmont (Pa. : Township)). 2009 Jul:6(7):30-5 [PubMed PMID: 19724767]
Soudry Y, Lemogne C, Malinvaud D, Consoli SM, Bonfils P. Olfactory system and emotion: common substrates. European annals of otorhinolaryngology, head and neck diseases. 2011 Jan:128(1):18-23. doi: 10.1016/j.anorl.2010.09.007. Epub 2011 Jan 11 [PubMed PMID: 21227767]
Baughman RP, Weiss KL, Golnik KC. Neuro-ophthalmic sarcoidosis. Eye and brain. 2012:4():13-25. doi: 10.2147/EB.S29401. Epub 2012 Mar 13 [PubMed PMID: 28539778]
Ogino MH, Tadi P. Neuroanatomy, Trigeminal Reflexes. StatPearls. 2023 Jan:(): [PubMed PMID: 31869105]
Freilinger C, Auffenberg E, Lipski C, Freilinger T. Testing cranial nerve VII: It is all in the wording. eNeurologicalSci. 2016 Mar:2():14-16. doi: 10.1016/j.ensci.2016.02.003. Epub 2016 Feb 11 [PubMed PMID: 29473056]
Wiater JM, Bigliani LU. Spinal accessory nerve injury. Clinical orthopaedics and related research. 1999 Nov:(368):5-16 [PubMed PMID: 10613148]
Tuang GJ, Nik Hussin NR, Zainal Abidin ZA. Unilateral rhinorrhoea and button battery: a case report. Family medicine and community health. 2019:7(3):e000137. doi: 10.1136/fmch-2019-000137. Epub 2019 Jul 5 [PubMed PMID: 32148716]
Level 3 (low-level) evidenceXian LLS, Nallaluthan V, De Jun Y, Lin-Wei O, Halim SA, Chuan CY, Idris Z, Ghani ARI, Abdullah JM. Examination Techniques of the First Cranial Nerve: What Neurosurgical Residents Should Know. The Malaysian journal of medical sciences : MJMS. 2020 Oct:27(5):124-129. doi: 10.21315/mjms2020.27.5.12. Epub 2020 Oct 27 [PubMed PMID: 33154708]
Rombaux P, Mouraux A, Collet S, Eloy P, Bertrand B. Usefulness and feasibility of psychophysical and electrophysiological olfactory testing in the rhinology clinic. Rhinology. 2009 Mar:47(1):28-35 [PubMed PMID: 19382491]
Level 2 (mid-level) evidenceShiga H, Taki J, Okuda K, Watanabe N, Tonami H, Nakagawa H, Kinuya S, Miwa T. Prognostic value of olfactory nerve damage measured with thallium-based olfactory imaging in patients with idiopathic olfactory dysfunction. Scientific reports. 2017 Jun 15:7(1):3581. doi: 10.1038/s41598-017-03894-4. Epub 2017 Jun 15 [PubMed PMID: 28620194]
Sue S. Test distance vision using a Snellen chart. Community eye health. 2007 Sep:20(63):52 [PubMed PMID: 17971914]
Motlagh M, Geetha R. Physiology, Accommodation. StatPearls. 2024 Jan:(): [PubMed PMID: 31194346]
Heckmann JG, Vachalova I, Lang CJG, Pitz S. Neuro-Ophthalmology at the Bedside: A Clinical Guide. Journal of neurosciences in rural practice. 2018 Oct-Dec:9(4):561-573. doi: 10.4103/jnrp.jnrp_145_18. Epub [PubMed PMID: 30271051]
Spears W, Mian A, Greer D. Brain death: a clinical overview. Journal of intensive care. 2022 Mar 16:10(1):16. doi: 10.1186/s40560-022-00609-4. Epub 2022 Mar 16 [PubMed PMID: 35292111]
Level 3 (low-level) evidenceSmith AM, Czyz CN. Neuroanatomy, Cranial Nerve 2 (Optic). StatPearls. 2023 Jan:(): [PubMed PMID: 29939684]
Bidot S, Bruce BB, Newman NJ, Biousse V. Nonmydriatic retinal photography in the evaluation of acute neurologic conditions. Neurology. Clinical practice. 2013 Dec:3(6):527-531 [PubMed PMID: 24353924]
Iqbal U. Smartphone fundus photography: a narrative review. International journal of retina and vitreous. 2021 Jun 8:7(1):44. doi: 10.1186/s40942-021-00313-9. Epub 2021 Jun 8 [PubMed PMID: 34103075]
Level 3 (low-level) evidenceHelwany M, Bordoni B. Neuroanatomy, Cranial Nerve 1 (Olfactory). StatPearls. 2023 Jan:(): [PubMed PMID: 32310511]
Khanam S, Sood G. Trochlear Nerve Palsy. StatPearls. 2023 Jan:(): [PubMed PMID: 33351409]
Graham C, Mohseni M. Abducens Nerve Palsy. StatPearls. 2023 Jan:(): [PubMed PMID: 29489275]
Khan ZA. Revisiting the Corneal and Blink Reflexes for Primary and Secondary Trigeminal Facial Pain Differentiation. Pain research & management. 2021:2021():6664736. doi: 10.1155/2021/6664736. Epub 2021 Feb 9 [PubMed PMID: 33628353]
Muzyka IM, Estephan B. Electrophysiology of Cranial Nerve Testing: Trigeminal and Facial Nerves. Journal of clinical neurophysiology : official publication of the American Electroencephalographic Society. 2018 Jan:35(1):16-24. doi: 10.1097/WNP.0000000000000445. Epub [PubMed PMID: 29298209]
Javed K, Daly DT. Neuroanatomy, Lower Motor Neuron Lesion. StatPearls. 2023 Jan:(): [PubMed PMID: 30969636]
Kim JS, Kim DH, Jeon EJ, Kim BG, Yu J, Shin HI. Taste test using an edible taste film kit: a randomised controlled trial. BMJ open. 2019 Sep 27:9(9):e029077. doi: 10.1136/bmjopen-2019-029077. Epub 2019 Sep 27 [PubMed PMID: 31562147]
Level 1 (high-level) evidenceLiu DT, Besser G, Oeller F, Mueller CA, Renner B. Bitter Taste Perception of the Human Tongue Mediated by Quinine and Caffeine Impregnated Taste Strips. The Annals of otology, rhinology, and laryngology. 2020 Aug:129(8):813-820. doi: 10.1177/0003489420906187. Epub 2020 Feb 6 [PubMed PMID: 32028784]
Payne T, Kronenbuerger M, Wong G. Gustatory Testing. StatPearls. 2023 Jan:(): [PubMed PMID: 33620811]
Kong EL, Fowler JB. Rinne Test. StatPearls. 2023 Jan:(): [PubMed PMID: 28613725]
García-Romo E, Blanco R, Nicholls C, Hernández-Tejero A, Fernández-de-Arévalo B. COVID-19 presenting with nystagmus. Archivos de la Sociedad Espanola de Oftalmologia. 2021 Apr:96(4):224-226. doi: 10.1016/j.oftal.2020.09.008. Epub 2020 Nov 6 [PubMed PMID: 33279355]
Sivakumar S, Prabhu A. Physiology, Gag Reflex. StatPearls. 2023 Jan:(): [PubMed PMID: 32119389]
Walker HK, Hall WD, Hurst JW. Clinical Methods: The History, Physical, and Laboratory Examinations. 1990:(): [PubMed PMID: 21250045]
AlShareef S, Newton BW. Accessory Nerve Injury. StatPearls. 2023 Jan:(): [PubMed PMID: 30335278]
Greer DM, Shemie SD, Lewis A, Torrance S, Varelas P, Goldenberg FD, Bernat JL, Souter M, Topcuoglu MA, Alexandrov AW, Baldisseri M, Bleck T, Citerio G, Dawson R, Hoppe A, Jacobe S, Manara A, Nakagawa TA, Pope TM, Silvester W, Thomson D, Al Rahma H, Badenes R, Baker AJ, Cerny V, Chang C, Chang TR, Gnedovskaya E, Han MK, Honeybul S, Jimenez E, Kuroda Y, Liu G, Mallick UK, Marquevich V, Mejia-Mantilla J, Piradov M, Quayyum S, Shrestha GS, Su YY, Timmons SD, Teitelbaum J, Videtta W, Zirpe K, Sung G. Determination of Brain Death/Death by Neurologic Criteria: The World Brain Death Project. JAMA. 2020 Sep 15:324(11):1078-1097. doi: 10.1001/jama.2020.11586. Epub [PubMed PMID: 32761206]
Schmidt WU, Lutz M, Ploner CJ, Braun M. The diagnostic value of the neurological examination in coma of unknown etiology. Journal of neurology. 2021 Oct:268(10):3826-3834. doi: 10.1007/s00415-021-10527-4. Epub 2021 Apr 1 [PubMed PMID: 33796895]
Sharshar T, Citerio G, Andrews PJ, Chieregato A, Latronico N, Menon DK, Puybasset L, Sandroni C, Stevens RD. Neurological examination of critically ill patients: a pragmatic approach. Report of an ESICM expert panel. Intensive care medicine. 2014 Apr:40(4):484-95. doi: 10.1007/s00134-014-3214-y. Epub 2014 Feb 13 [PubMed PMID: 24522878]
Lacomis D. Neurosarcoidosis. Current neuropharmacology. 2011 Sep:9(3):429-36. doi: 10.2174/157015911796557975. Epub [PubMed PMID: 22379457]
Howell J, Costanzo RM, Reiter ER. Head trauma and olfactory function. World journal of otorhinolaryngology - head and neck surgery. 2018 Mar:4(1):39-45. doi: 10.1016/j.wjorl.2018.02.001. Epub 2018 Mar 14 [PubMed PMID: 30035260]
Kim HM, Park YJ, Park KH, Woo SJ. Visual field defects and changes in central retinal artery occlusion. PloS one. 2019:14(1):e0209118. doi: 10.1371/journal.pone.0209118. Epub 2019 Jan 3 [PubMed PMID: 30605464]
Hayreh SS. Giant cell arteritis: Its ophthalmic manifestations. Indian journal of ophthalmology. 2021 Feb:69(2):227-235. doi: 10.4103/ijo.IJO_1681_20. Epub [PubMed PMID: 33463564]
Jahr SH, Koldéus-Falch J, Zarnovicky S. Oculomotor nerve palsy. Tidsskrift for den Norske laegeforening : tidsskrift for praktisk medicin, ny raekke. 2017 Oct 17:137(19):. doi: 10.4045/tidsskr.17.0431. Epub 2017 Oct 16 [PubMed PMID: 29043749]
Joyce C, Le PH, Peterson DC. Neuroanatomy, Cranial Nerve 3 (Oculomotor). StatPearls. 2023 Jan:(): [PubMed PMID: 30725811]
Davis A, Lloyd CM. Doctor, I'm Seeing Two of Everything. Cureus. 2020 Jan 8:12(1):e6602. doi: 10.7759/cureus.6602. Epub 2020 Jan 8 [PubMed PMID: 32064184]
Cornblath WT. Diplopia due to ocular motor cranial neuropathies. Continuum (Minneapolis, Minn.). 2014 Aug:20(4 Neuro-ophthalmology):966-80. doi: 10.1212/01.CON.0000453309.44766.b4. Epub [PubMed PMID: 25099103]
Level 3 (low-level) evidenceKim SY, Motlagh M, Naqvi IA. Neuroanatomy, Cranial Nerve 4 (Trochlear). StatPearls. 2023 Jan:(): [PubMed PMID: 30725929]
Tamhankar MA, Ying GS, Volpe NJ. Success of prisms in the management of diplopia due to fourth nerve palsy. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2011 Sep:31(3):206-9. doi: 10.1097/WNO.0b013e318211daa9. Epub [PubMed PMID: 21378578]
Level 2 (mid-level) evidenceLambru G, Zakrzewska J, Matharu M. Trigeminal neuralgia: a practical guide. Practical neurology. 2021 Oct:21(5):392-402. doi: 10.1136/practneurol-2020-002782. Epub 2021 Jun 9 [PubMed PMID: 34108244]
Huff T, Weisbrod LJ, Daly DT. Neuroanatomy, Cranial Nerve 5 (Trigeminal). StatPearls. 2024 Jan:(): [PubMed PMID: 29489263]
Bendtsen L, Zakrzewska JM, Abbott J, Braschinsky M, Di Stefano G, Donnet A, Eide PK, Leal PRL, Maarbjerg S, May A, Nurmikko T, Obermann M, Jensen TS, Cruccu G. European Academy of Neurology guideline on trigeminal neuralgia. European journal of neurology. 2019 Jun:26(6):831-849. doi: 10.1111/ene.13950. Epub 2019 Apr 8 [PubMed PMID: 30860637]
Ma C, Tian F, Zhou L, Gu J, Zhang X, Quan J, Qu J, Yan X. Blink reflex: A practical test to evaluate the trigeminal nerve injury following percutaneous balloon compression for the treatment of trigeminal neuralgia. Headache. 2022 Mar:62(3):363-373. doi: 10.1111/head.14269. Epub 2022 Feb 18 [PubMed PMID: 35181896]
Lui F, Tadi P, Anilkumar AC. Wallenberg Syndrome. StatPearls. 2023 Jan:(): [PubMed PMID: 29262144]
Kumar K, Ahmed R, Bajantri B, Singh A, Abbas H, Dejesus E, Khan RR, Niazi M, Chilimuri S. Tumors Presenting as Multiple Cranial Nerve Palsies. Case reports in neurology. 2017 Jan-Apr:9(1):54-61. doi: 10.1159/000456538. Epub 2017 Apr 3 [PubMed PMID: 28553221]
Level 3 (low-level) evidenceWalker NR, Mistry RK, Mazzoni T. Facial Nerve Palsy. StatPearls. 2024 Jan:(): [PubMed PMID: 31747222]
Lima MA, Silva MTT, Soares CN, Coutinho R, Oliveira HS, Afonso L, Espíndola O, Leite AC, Araujo A. Peripheral facial nerve palsy associated with COVID-19. Journal of neurovirology. 2020 Dec:26(6):941-944. doi: 10.1007/s13365-020-00912-6. Epub 2020 Oct 2 [PubMed PMID: 33006717]
Finsterer J. Management of peripheral facial nerve palsy. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2008 Jul:265(7):743-52. doi: 10.1007/s00405-008-0646-4. Epub 2008 Mar 27 [PubMed PMID: 18368417]
Bordoni B, Mankowski NL, Daly DT. Neuroanatomy, Cranial Nerve 8 (Vestibulocochlear). StatPearls. 2024 Jan:(): [PubMed PMID: 30726044]
Swartz JD. Pathology of the vestibulocochlear nerve. Neuroimaging clinics of North America. 2008 May:18(2):321-46, x-xi. doi: 10.1016/j.nic.2008.02.001. Epub [PubMed PMID: 18466835]
De Foer B, Kenis C, Van Melkebeke D, Vercruysse JP, Somers T, Pouillon M, Offeciers E, Casselman JW. Pathology of the vestibulocochlear nerve. European journal of radiology. 2010 May:74(2):349-58. doi: 10.1016/j.ejrad.2009.06.033. Epub 2010 Mar 27 [PubMed PMID: 20347243]
Haller S, Etienne L, Kövari E, Varoquaux AD, Urbach H, Becker M. Imaging of Neurovascular Compression Syndromes: Trigeminal Neuralgia, Hemifacial Spasm, Vestibular Paroxysmia, and Glossopharyngeal Neuralgia. AJNR. American journal of neuroradiology. 2016 Aug:37(8):1384-92. doi: 10.3174/ajnr.A4683. Epub 2016 Feb 18 [PubMed PMID: 26892985]
Heman-Ackah SE, Golfinos JG, Roland JT Jr. Management of surgical complications and failures in acoustic neuroma surgery. Otolaryngologic clinics of North America. 2012 Apr:45(2):455-70, x. doi: 10.1016/j.otc.2011.12.012. Epub [PubMed PMID: 22483827]
Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-Toker DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009 Nov:40(11):3504-10. doi: 10.1161/STROKEAHA.109.551234. Epub 2009 Sep 17 [PubMed PMID: 19762709]
Level 2 (mid-level) evidenceStrupp M, Dlugaiczyk J, Ertl-Wagner BB, Rujescu D, Westhofen M, Dieterich M. Vestibular Disorders. Deutsches Arzteblatt international. 2020 Apr 24:117(17):300-310. doi: 10.3238/arztebl.2020.0300. Epub [PubMed PMID: 32530417]
Shah RJ, Padalia D. Glossopharyngeal Neuralgia. StatPearls. 2024 Jan:(): [PubMed PMID: 31082085]
Thomas K, Minutello K, M Das J. Neuroanatomy, Cranial Nerve 9 (Glossopharyngeal). StatPearls. 2023 Jan:(): [PubMed PMID: 30969699]
Khan M, Nishi SE, Hassan SN, Islam MA, Gan SH. Trigeminal Neuralgia, Glossopharyngeal Neuralgia, and Myofascial Pain Dysfunction Syndrome: An Update. Pain research & management. 2017:2017():7438326. doi: 10.1155/2017/7438326. Epub 2017 Jul 30 [PubMed PMID: 28827979]
Kandan SR, Khan S, Jeyaretna DS, Lhatoo S, Patel NK, Coakham HB. Neuralgia of the glossopharyngeal and vagal nerves: long-term outcome following surgical treatment and literature review. British journal of neurosurgery. 2010 Aug:24(4):441-6. doi: 10.3109/02688697.2010.487131. Epub [PubMed PMID: 20726751]
AbuRahma AF, Choueiri MA. Cranial and cervical nerve injuries after repeat carotid endarterectomy. Journal of vascular surgery. 2000 Oct:32(4):649-54 [PubMed PMID: 11013026]
Saleem F, M Das J. Lateral Medullary Syndrome. StatPearls. 2023 Jan:(): [PubMed PMID: 31869134]
Kim H, Lee HJ, Park JW. Clinical course and outcome in patients with severe dysphagia after lateral medullary syndrome. Therapeutic advances in neurological disorders. 2018:11():1756286418759864. doi: 10.1177/1756286418759864. Epub 2018 Feb 28 [PubMed PMID: 29511384]
Level 3 (low-level) evidenceBattel I, Koch I, Biddau F, Carollo C, Piccione F, Meneghello F, Merico A, Palmer K, Marchese Ragona R. Efficacy of botulinum toxin type-A and swallowing treatment for oropharyngeal dysphagia recovery in a patient with lateral medullary syndrome. European journal of physical and rehabilitation medicine. 2017 Oct:53(5):798-801. doi: 10.23736/S1973-9087.17.04499-9. Epub 2017 Mar 6 [PubMed PMID: 28264544]
Howland RH. Vagus Nerve Stimulation. Current behavioral neuroscience reports. 2014 Jun:1(2):64-73 [PubMed PMID: 24834378]
Yuan H, Silberstein SD. Vagus Nerve and Vagus Nerve Stimulation, a Comprehensive Review: Part II. Headache. 2016 Feb:56(2):259-66. doi: 10.1111/head.12650. Epub 2015 Sep 18 [PubMed PMID: 26381725]
Kenny BJ, Bordoni B. Neuroanatomy, Cranial Nerve 10 (Vagus Nerve). StatPearls. 2023 Jan:(): [PubMed PMID: 30725856]
Bordoni B, Reed RR, Tadi P, Varacallo M. Neuroanatomy, Cranial Nerve 11 (Accessory). StatPearls. 2023 Jan:(): [PubMed PMID: 29939544]
Olarte M, Adams D. Accessory nerve palsy. Journal of neurology, neurosurgery, and psychiatry. 1977 Nov:40(11):1113-6 [PubMed PMID: 202681]
Level 3 (low-level) evidenceKim DH, Cho YJ, Tiel RL, Kline DG. Surgical outcomes of 111 spinal accessory nerve injuries. Neurosurgery. 2003 Nov:53(5):1106-12; discussion 1102-3 [PubMed PMID: 14580277]
Level 2 (mid-level) evidenceKim SY, Naqvi IA. Neuroanatomy, Cranial Nerve 12 (Hypoglossal). StatPearls. 2023 Jan:(): [PubMed PMID: 30422464]
Robaina Bordón JM, González Hernández A, Curutchet Mesner L, Gil Díaz A. Isolated hypoglossal nerve palsy. Neurologia. 2019 Mar:34(2):125-127. doi: 10.1016/j.nrl.2016.08.004. Epub 2016 Oct 21 [PubMed PMID: 27776963]
Finsterer J, Grisold W. Disorders of the lower cranial nerves. Journal of neurosciences in rural practice. 2015 Jul-Sep:6(3):377-91. doi: 10.4103/0976-3147.158768. Epub [PubMed PMID: 26167022]
Fleury Curado T, Oliven A, Sennes LU, Polotsky VY, Eisele D, Schwartz AR. Neurostimulation Treatment of OSA. Chest. 2018 Dec:154(6):1435-1447. doi: 10.1016/j.chest.2018.08.1070. Epub 2018 Sep 14 [PubMed PMID: 30222959]
Ratneswaran D, Guni A, Pengo MF, Al-Sherif M, He B, Cheng MC, Steier J, Schwarz EI. Electrical stimulation as a therapeutic approach in obstructive sleep apnea - a meta-analysis. Sleep & breathing = Schlaf & Atmung. 2021 Mar:25(1):207-218. doi: 10.1007/s11325-020-02069-2. Epub 2020 May 9 [PubMed PMID: 32388780]
Level 1 (high-level) evidence