Indications
Active Moderate to Severe Thyroid Eye Disease
Teprotumumab received fast track designation in April 2015 and Breakthrough Therapy designation in July 2016. Teprotumumab was granted orphan designation for the treatment of active thyroid eye disease on June 19, 2019 (12-3878/DRU201203878).
Teprotumumab was approved by the US FDA in 2020 for thyroid eye disease based on efficacy and safety evaluation of two randomized, double-masked, placebo-controlled, multicenter trials, which showed that teprotumumab was superior to a placebo in a combined total of 170 patients with active, moderate to severe thyroid orbitopathy, defined as having a Clinical Activity Score (CAS) of 4 or more out of 7 points and < 9 months of symptoms onset, over 24 weeks.[1][2]
In phase 2 randomized controlled trial (NCT01868997), Smith et al. demonstrated that 69% of patients in the teprotumumab group compared to 20% in the placebo group had a positive response at 24 weeks.[1] A response was defined as having a ≥ 2-point reduction in the CAS and a ≥ 2mm reduction in proptosis. The mean reduction of proptosis and CAS was significantly greater in the teprotumumab group compared to the placebo, at 2.46 mm and 3.43 points, respectively. Secondary outcomes were assessed by the improvement of diplopia by the Gorman diplopia score and quality of life by Graves' orbitopathy-Quality of Life (GO-QOL) score by 24 weeks. Diplopia score significantly improved in 68% of patients in the teprotumumab group compared to 26% in the placebo group, with a similar trend for both GO-QOL visual function and appearance scores.
Phase 3 teprotumumab trial (NCT03298867) validated the results of the phase 2 trial. In the phase 3 (OPTIC) trial, Douglas et al. showed that 83% of patients in the teprotumumab group, compared with only 10% in the placebo group, had a reduction of proptosis (≥ 2 mm) at 24 weeks.[2] Furthermore, all secondary outcomes were significantly better with teprotumumab than with placebo, including overall response (78% vs. 7%), CAS of 0 or 1 (59% vs. 21% ), the mean change in proptosis (−2.82 mm vs. −0.54 mm), diplopia response (68% vs. 29%), and the mean improvement in Graves' ophthalmopathy-specific quality-of-life (GO-QO) overall score (13.79 points vs. 4.43 points).
In the OPTIC trial, six patients who received teprotumumab underwent orbital magnetic resonance imaging (MRI) at baseline and week 24. Imaging showed volume reduction either in extraocular muscle, orbital fat, or both, with a mean reduction in extraocular muscle and orbital fat volumes of 35% and 17%, respectively.[2] Volumetric analysis of the MRI orbit of six patients from the OPTIC trial retrospectively showed the total extraocular muscle and orbital fat volume nearly double that of normal control. Teprotumumab treatment in these six cases led to 33% extraocular muscle volume reduction and 29% orbital fat volume reduction, with the inferior rectus, followed by the medial rectus showing the greatest response.[3]
In a separate retrospective study using orbital echography pre- and post-teprotumumab treatment, Tran et al. found a mean reduction of extraocular muscle diameter from 27.4 mm to 23.4 mm, with inferior recti showing the greatest reduction.[4]
Pooled and Extended Outcome Analysis in Active Moderate to Severe Thyroid Eye Disease
The extended outcome of the two teprotumumab randomized controlled trials was reported, with up to 18 months of follow-up (72 weeks). By pooling the intention-to-treat population from the phase 2 and phase 3 teprotumumab randomized controlled trials, significant efficacy for teprotumumab was revealed where numbers-needed-to-treat were 1.6 for proptosis response, 2.5 for diplopia response, 1.7 for the overall response, and 2.5 for disease inactivation.[5]
Proptosis reduction of at least 2 mm was achieved in 77% (65 of 84 patients) treated with teprotumumab, compared to 15% (13 of 87) in placebo at 24 weeks. By 51 weeks post final drug infusion, the maintenance of proptosis response was 67% (38 of 57 patients) in patients with long-term data. Diplopia score improvement by one grade or more was reported in 70% (46 of 66 patients) of the teprotumumab group, compared to 31% (18 of 59 patients) in the placebo group at week 24, with reports of complete resolution of diplopia in 53% in the teprotumumab group compared with 25% in the placebo group. By 51 weeks, 69% (33 of 48 patients) had maintained their diplopia response.[5]
An extension of the OPTIC trial, as an open-label study (OPTIC-X) (NCT03461211), examined the treatment responses in patients who did not have a proptosis response, either from the placebo group with TED of longer duration or from the teprotumumab treated group, or who had a flare-up (≥2 mm increase in proptosis, ≥2-point increase in CAS, or both) from either of the two groups in the initial OPTIC trial.[6] This included 37 patients who were previously given a placebo and 14 patients who were prescribed teprotumumab.
The non-responders subsequently received eight additional infusions of teprotumumab in OPTIC-X over 24 weeks. The OPTIC-X study showed patients with a longer duration of TED (mean of 12.3 months) still respond favorably to teprotumumab treatment, with 89.2% of placebo-treated OPTIC patients becoming proptosis responders, 60.9% showing improvement in diplopia score and 56.5% achieving complete resolution of diplopia at week 24. The proptosis response appeared durable in patients with a longer duration of thyroid eye disease, with 89% maintaining proptosis reduction by week 48 and 64% still showing a diplopia response by week 48. Of the five OPTIC teprotumumab non-responders, re-treatment led to a modest response (40% proptosis responders, with a mean reduction of -1.5 mm).[6]
Of the OPTIC trial teprotumumab arm, 34 responded with proptosis reduction at week 24. Of note, 10 (29.4%) of 34 patients experienced flare-up, either showing proptosis increase (5 patients), both proptosis and CAS flare (4 patients), or isolated CAS flare (1 patient), mostly at week 48, with few at week 60 and week 72.[6] Five of 8 patients (62.5%) of the retreated cases showed proptosis reduction with teprotumumab re-treatment. The teprotumumab re-treatment numbers were too small to draw firm conclusions on re-treatment efficacy.
In the absence of a head-to-head comparison between teprotumumab and intravenous methylprednisolone (IVMP) at the time, a meta-analysis of non-randomized studies compared the use of teprotumumab with IVMP in patients with moderate to severe thyroid orbitopathy.[7]
In the matching-adjusted indirect comparisons (MAICs) between teprotumumab, IVMP, and placebo, teprotumumab showed a significantly greater change in proptosis from baseline compared with IVMP (MD -2.31 mm), while proptosis change for IVMP compared with placebo was modest and statistically not significant (MD -0.16 mm). Teprotumumab diplopia response was greater when compared with IVMP (OR 2.32), while IVMP also showed increased odds of diplopia response compared with placebo (OR 2.69).[7]
This meta-analysis suggested teprotumumab was correlated with greater improvements in proptosis and maybe twice as likely to have a 1 grade or greater reduction in diplopia than IVMP. As the MAICs method cannot correct for confounding factors between different arms of the comparator, further studies, in particular, masked randomized controlled trials, are warranted to compare teprotumumab with IVMP.
A recent paper reported on the proptosis and diplopia response with teprotumumab and placebo vs. the recommended treatment regimen with intravenous methylprednisolone in moderate to severe thyroid eye disease. They showed an association with small improvements in proptosis from baseline for IVMP vs. placebo (-0.16 mm); associated proptosis improvements were statistically significantly greater with teprotumumab vs. IVMP (treatment difference, -2.31 mm). For diplopia response, IVMP was not favored over placebo, while teprotumumab was favored over IVMP.[7]
Chronic, Low CAS Thyroid Eye Disease
Given the positive responses to teprotumumab in patients with thyroid eye disease of prolonged duration from the OPTIC-X trial and an exploratory study showing an increased IGF-1R expression in the orbital fat tissue of patients with both inflammatory and non-inflammatory thyroid orbitopathy when compared to normal controls, increasing attention was given to the role of teprotumumab in inactive, chronic thyroid eye disease.[6][8]
Early studies suggest teprotumumab has also shown promising results in treating chronic, non-progressive thyroid eye disease. In a retrospective series, nine patients with chronic thyroid orbitopathy (> 9 months) and low CAS (≤ 1) were treated with eight infusions of teprotumumab and had a clinical and statistical improvement in proptosis.[9] Ugradar et al. demonstrated a reduction in proptosis in 4 patients with non-inflammatory thyroid eye disease (CAS ≤ 1, for at least four months) following eight infusions of teprotumumab over 24 weeks.[8]
Ozzello et al. reported one of the earliest cases of large proptosis response (5 to 6 mm proptosis reduction) in a chronic thyroid orbitopathy patient of 3 years duration.[10] The same study center further conducted a retrospective study where 21 patients met the eligibility criteria of chronic thyroid eye disease (> 9 months) and low CAS (≤ 1), and 9 of the 21 patients were included in the efficacy analysis after completing eight infusions of teprotumumab. The duration of chronic thyroid eye disease in this cohort was 6.25 years.
The follow-up duration post-completion of treatment was 16 weeks for 5 of the nine patients. The mean proptosis reduction was 4mm in all patients immediately post-treatment and 4.2 mm in the worse eye at 16 weeks, while the better eye proptosis reduction was 3 mm.[9] Nevertheless, 4 of the nine patients had rehabilitative surgeries after teprotumumab therapy. Ugradar et al. demonstrated a reduction in proptosis in 4 patients with non-inflammatory thyroid eye disease (CAS ≤ 1, for at least four months) following eight infusions of teprotumumab over 24 weeks.[8]
In another retrospective review of 31 consecutive patients with chronic stable thyroid eye disease > 2 years, Ugradar et al. demonstrated a mean reduction of proptosis (3.5mm), CAS response (90%) with a mean reduction by 1.8 points, diplopia improvement (67%), resolution of diplopia (47%) and a mean reduction of orbital muscle (2011mm^3), and fat volume (2101 mm^3) following ≥3 infusions of teprotumumab.[11] This study suggested teprotumumab significantly impacts patients with stable chronic thyroid orbitopathy. Diniz et al. used teprotumumab non-selectively in a heterogeneous group of 21 patients and found an improvement in proptosis, extraocular motility, and CAS.[12]
Dysthyroid Optic Neuropathy (DON)
More recently, case reports and series have been published showing that teprotumumab is effective in treating DON secondary to thyroid orbitopathy. Sears et al. described a case of a 45-year-old man with active progressive thyroid orbitopathy despite weekly pulsed intravenous steroids and right orbital radiation and represented with worsening left DON.[13]
Teprotumumab was used as an intravenous corticosteroid, and orbital decompression was contraindicated for medical reasons. After two infusions of teprotumumab, the patient's visual acuity, relative afferent pupillary defect, Humphrey visual fields, proptosis, and extraocular muscle size improved. Slentz et al. reported a case of a 62-year-old man with right-sided optic nerve hemorrhages, edema, and visual field defects. The patient improved after a single teprotumumab infusion. With sustained improvement at 25 weeks following the initial dose, he completed all eight inputs.[14]
Chiou et al. presented two patients with active thyroid orbitopathy and mild DON with corresponding visual field defects failing to improve with intravenous corticosteroid therapy.[15] Both patients were treated with teprotumumab and rapidly responded with complete resolution of DON and field defect. Hwang et al. reported a case of an 81-year-old woman with dense bilateral DON.[16]
The patient declined conventional treatment with intravenous corticosteroids or orbital decompression and proceeded with teprotumumab infusions. She showed an early vision response after two infusions. Visual acuity improved from 20/100 in the right eye and counting fingers in the left eye to 20/40 in the right and 20/30 in the left eye, with mild residual field defect after the eighth infusion.
Teprotumumab is indicated for DON when conventional therapies have failed, and patients are considered poor surgical candidates for orbital decompression. In a multicenter observational case series, patients were treated with teprotumumab who either had acute or chronic thyroid orbitopathy, as they had failed to respond to oral and intravenous corticosteroid, orbital radiation, and/or prior orbital decompression and progressed to DON.[17]
Ten patients with DON were eligible for inclusion in this study. Seventy percent of patients treated with teprotumumab had an objective improvement in DON after two infusions, measured as significant improvement in visual acuity, resolution of RAPD, or both.[17] Seven patients who presented with color vision deficits had subsequent normalization or improvement of color vision, and three patients with long-standing DON and hand movement vision had minimal objective improvement.
Asymmetric Thyroid Eye Disease
Teprotumumab may also improve asymmetric thyroid eye disease. Post-hoc analysis of the combined phase 2 and 3 teprotumumab trials (NCT01868997 and NCT03298867) showed the reduction in proptosis and CAS was significantly greater in the worse affected orbit, from 4.6 mm baseline asymmetric proptosis to 1.8mm in the treated group, while no significant reduction in asymmetry was noted in the placebo group.[18] Furthermore, there is variability in the time taken to manifest a clinically significant response to the drug. Of the 24 in the teprotumumab group who had no improvement in proptosis (≥2 mm from baseline) at 12 weeks, 63% went on to have a significant change at 24 weeks.[19]
Cosmetic Facial Changes
Thyroid eye disease may result in swelling and soft tissue expansion. Using 3D facial imaging, Ugradar et al. demonstrated a reduction in the mean volume of the upper face, periorbital region, temples, midface, and lower face, as well as mean reflex distances, after using teprotumumab in 23 patients with thyroid orbitopathy.[20]
Mechanism of Action
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Mechanism of Action
The rationale for choosing teprotumumab for the treatment trials in thyroid eye disease was based entirely on in-vitro studies of IGF-1R expressing orbital fibroblast and CD34+ fibrocytes and their critical contribution toward pathophysiology of thyroid orbitopathy, including upregulation of pro-inflammatory cytokine expression, T cell chemoattractant production including IL-16 and RANTES, hyaluronic acid synthesis and enhanced adipogenesis.[21][22][23]
IGF-1R was overexpressed in orbital fibroblasts, monocyte progenitor cells, also known as fibrocytes in patients with thyroid orbitopathy, and peripheral B and T cells in patients with Graves disease.[24][25][26] Moreover, over-expression of IGF-1R in orbital tissues from patients with active and inactive thyroid eye disease compared to normal controls was observed.[11] Immunoglobulins from patients with Graves disease have been shown to interact with IGF-1 binding sites on orbital fibroblasts and induce hyaluronan synthesis, an effect that was reproducible by IGF-1 through IGF-1R.[24][27]
Furthermore, TSHR and IGF-1R receptors are found to co-localize and form functional complexes on orbital fibroblasts.[28] A study suggested IGF-1R activation by Graves’ immunoglobulin occurs partly via TSHR/IGF-1R cross-talk rather than direct binding to IGF-1R.[29] The TSHR/IGF-1R cross-talk is enabled through GRK/arrestin-b,[30]
in addition to the known IGF-1R signaling through the canonical pathway (Shc/SOS, RAF, MAPK, MEK and ERK, and IRS-induced PI3K/AKT) and non-canonical pathway where IGF-1R uses G protein signaling.[31] This cascade ultimately results in orbital fat expansion, extra-ocular muscle enlargement, and inflammation with resultant soft tissue changes, diplopia, and proptosis.
Teprotumumab is a 150 kDa fully human monoclonal anti-IGF-IR antibody that does not display cross-reactivity towards the insulin receptor.[31] Teprotumumab binds with the cysteine-rich region of the IGF-1R extracellular domain, and the complex is internalized and degraded. In vitro, teprotumumab reduces TSHR and IGF-1F display on fibrocytes and attenuates TSH-dependent IL-6 and IL-8 mRNA expression and AKT phosphorylation, which infer the IGF-1R inhibitor interferes with both IGF-1R and TSHR-mediated effects.[32]
Administration
In the clinical trials, teprotumumab was given as an intravenous infusion with an initial dose of 10 mg/kg (over 90 minutes) followed by 20 mg/kg (over 90 minutes for infusion 2 and over 60 minutes for infusions 3 to 8), at a 3-week interval.
No dose-finding studies have been performed for teprotumumab in the setting of thyroid eye disease. Teprotumumab pharmacokinetics is linear in patients with thyroid orbitopathy with low systemic clearance and distribution volume, as well as a long elimination half-life.[33] Modeling studies have demonstrated that a 20 mg/ml trough blood level should maintain more than 90% IGF-1R saturation. Hence, the above dosing regime was chosen to maintain elevated trough concentrations.
Reconstitution and Dosage Preparation
Each vial contains 500 mg of teprotumumab. Using an aseptic technique, reconstitute the dose with 10mL of sterile water for injection. Ensure the stream of diluent is not directed onto the lyophilized powder, and gently swirl the solution until the powder is dissolved. The reconstituted solution has a volume of 10.5 mL, and the final concentration is 47.6 mg/mL. The reconstituted teprotumumab solution must be further diluted in 0.9% sodium chloride prior to infusion. Withdraw the required volume from the reconstituted vial based on the patient’s weight and transfer it into an intravenous bag to a total volume of 100 mL (for less than 1800 mg dose) or 250 mL (for 1800 mg or greater).
The reconstituted teprotumumab or the diluted solution in the intravenous bag does not contain preservatives and, therefore, must be used within 4 hours at room temperature 20 to 25 degrees or up to 48 hours under refrigerated conditions 2 to 8 degrees, protected from light. If refrigerated, allow the diluted solution to reach room temperature before infusion.
Reconstituted teprotumumab is either a colorless or slightly brown, clear solution. Discard the drug solution if there is any particulate matter or if the discoloration is observed.
Adverse Effects
Teprotumumab is generally well tolerated, with most reported adverse events classified as mild, mostly grade 1 or 2. Common adverse reactions>5% were reported in teprotumumab clinical trials. According to pooled data analysis from phase 2 and 3 clinical trials.[5] The most common adverse effects include:
- Infections and infestations (35%)
- Muscle spasm (25%)
- Nausea (17%)
- Diarrhea (13%)
- Hearing impairment (10%)
- Alopecia (13%)
- Weight loss (9%)
- Hyperglycemia (8%)
- Dry skin (8%)
- Headache (8%)
- Dysgeusia (8%)
- Paraesthesia (7%)
Serious adverse events occurred in 5 of 43 patients (12%) in the teprotumumab group in the phase 2 trial, which comprised one case of severe diarrhea in a patient with pre-existing ulcerative colitis, inflammatory bowel disease, Escherichia sepsis, Hashimoto encephalopathy, urinary retention, and one infusion-related reaction.[1][2]
In the phase 3 trial, serious adverse events occurred in 2 of 41 (5%) patients in the teprotumumab group, which was a grade 2 infusion reaction that led to discontinuation of trial, and pneumothorax which was considered unrelated to the trial drug. No deaths occurred during teprotumumab clinical trials.[2]
Hoang et al. described a case of teprotumumab-induced encephalopathy in a 76-year-old man with a resolution of symptoms following plasmapheresis.[34] However, this patient's exact mechanisms for the rapidly progressive cognitive decline remain unclear.
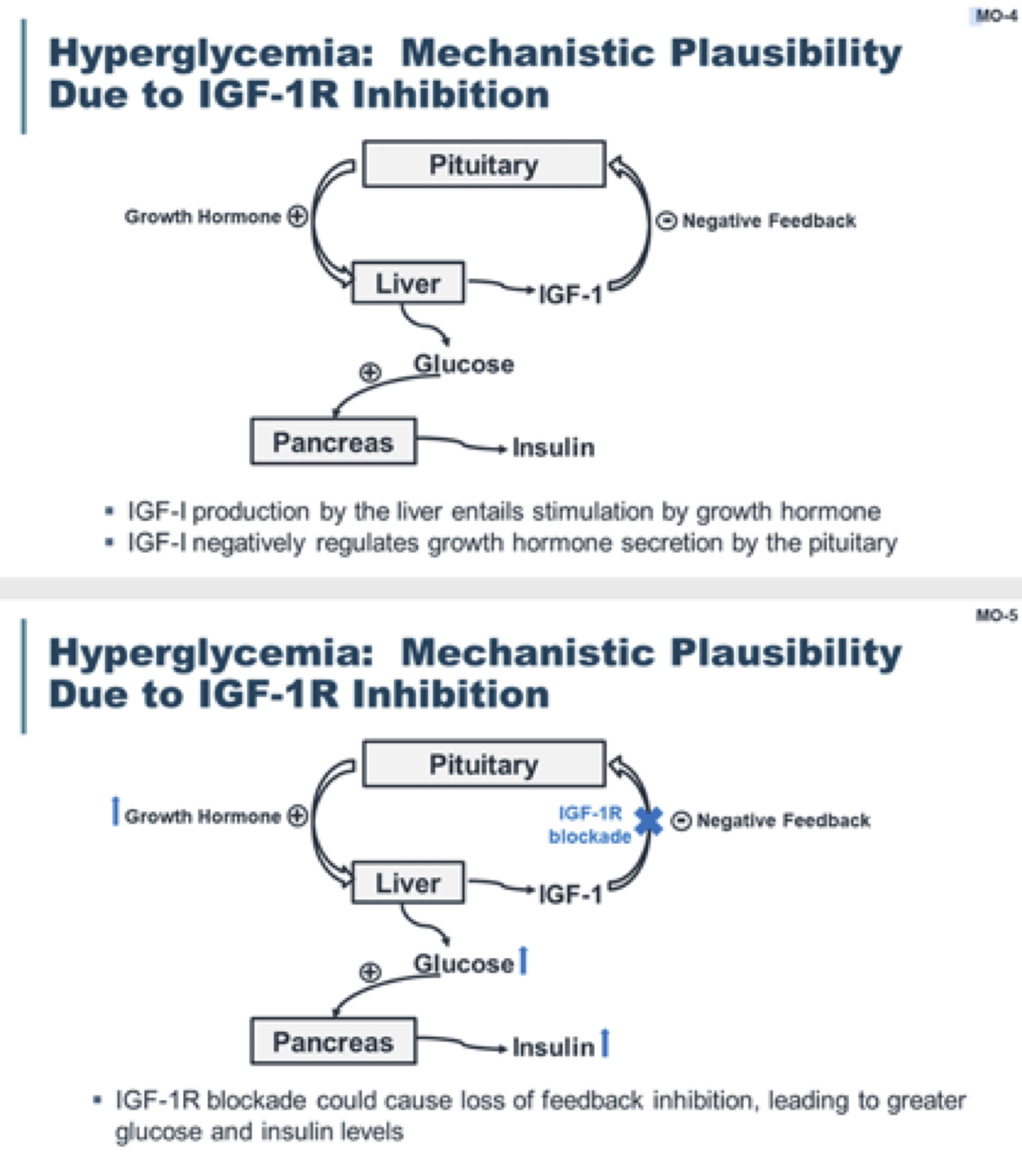
Hyperglycemia
Hyperglycemia is identified as related to drug action for teprotumumab. In patients who did not have underlying diabetes, hyperglycemia was mild (55 to 70 mg/dl), intermittent, and occurred at similar rates in the placebo and teprotumumab groups. Moderate or severe hyperglycemia occurred in some patients with diabetes who received teprotumumab, which was well controlled after adjusting the medication for diabetes. Hemoglobin A1c levels returned to baseline after cessation of therapy. The OPTIC trial reported two grade 1 hyperglycemia cases (<5%) which resolved spontaneously.[2]
OPTIC-X study reported three other cases of hyperglycemia (8.1%) in the OPTIC placebo group who later received teprotumumab, two with new-onset type 2 diabetes, and one with a transient increase in blood glucose.[6] Teprotumumab shows no detectable affinity for the insulin receptor; thus, the secondary hyperglycemia is likely linked to IGF-IR inhibition.[35][36][37]
Hearing Impairment
Hearing impairment is a common adverse reaction of teprotumumab. Hearing complications range from deafness, eustachian tube dysfunction, hyper and hypoacusis, tinnitus, and autophony at a rate of 10% in phase 2 and 3 teprotumumab clinical trials. These adverse events were noted to resolve either while on therapy or after completion of treatment.[5] In the phase 2 trial, three patients experienced mild hearing impairment and tinnitus.[1]
In the phase 3 trial, five patients reported hearing disturbances in the teprotumumab group (2 hypoacusis, 1 deafness, 1 autophony, and 1 patulous eustachian tube), which all subsequently resolved.[2]In the OPTIC-X trial, four patients had ongoing mild hearing disturbances at the time of reporting to the FDA. The onset and duration of the hearing impairment were all disparate. However, baseline audiograms were also not part of the screening protocol in these trials to quantify the severity and completeness of hearing recovery.
Insulin-like growth factor 1 (IGF-1) plays an essential role in developing the inner ear and cochlear hair cells.[38] Animal studies have shown that IGF-1 may protect the cochlea from excessive noise, aminoglycoside antibiotics, and ischemic injury.[39][40][41] IGF-1 appears to be neuroprotective and has the potential to facilitate regeneration of inner hair cells, activate growth, and limit cell death within the spiral ganglion neuron in cochlear explants; IGF-1 deficiency has been associated with sensorineural hearing loss in humans and animal studies.[42][43]
In a large English longitudinal study, higher IGF-1 levels correlated with lower odds of hearing impairment in people aged 50 to 60.[44] Patients with thyroid eye disease are also more likely to develop hearing loss.[45][46]
The post-clinical trials' use of teprotumumab raises concerns that the incidence of hearing complications may be higher and, in some instances, does not reverse after cessation of teprotumumab therapy. Two of the earliest reported TED cases developed hearing deficits after the third and fifth teprotumumab infusions, respectively, with severe hearing loss after the seventh infusion, after which treatment was ceased. Routine audiograms with teprotumumab therapy have been suggested.[43][42]
Both cases showed moderate to severe sensorineural hearing loss, with no significant recovery of neurosensory loss 4 and 9 months after discontinuation of teprotumumab treatment. Another TED patient developed irreversible hearing loss in the setting of rifle shooting without ear protection two months into teprotumumab treatment. He continued to require hearing aids 12 months following completion of teprotumumab infusions.[47]
In a compiled case series, 4 cases out of 28 teprotumumab-treated thyroid eye disease patients (16%) developed neurosensory hearing loss based on audiometry. Severity ranges from mild and transient to severe and chronic. The milder form of ototoxicity is only detected on high-frequency testing.[48]
In a prospective observational study, 27 patients underwent otologic assessment at baseline after second, fourth, and eighth teprotumumab infusions. 22 of the 27 (81.5%) of the patients developed new subjective otologic symptoms after a mean of 3.8 infusions, ranging from mild to declines in word recognition. While tinnitus, ear fullness, and autophony resolved well (83% to 100%) by 39 weeks, only 5 of 11 (45.5%) patients with subjective hearing loss/decreased word comprehension experienced resolution.
Subjectively, most patients were not functionally impacted by their otologic symptoms, but four did require hearing aids. Six patients had baseline and post-treatment audiometry, 5 met the criteria for ototoxicity due to teprotumumab-related sensorineural hearing loss, and one patient also had a patulous eustachian tube. Importantly the patients experiencing sensorineural hearing loss were older, and all had baseline hearing loss compared to the asymptomatic group (p<0.05).[49]
Given the ototoxicity potential, baseline audiometry and tympanometry with repeat audiology testing during the course of teprotumumab are recommended for early detection and management of otologic symptoms. This practice would help with pre-treatment patient counseling, as having a baseline hearing impairment is a predictor of hearing loss with ototoxic medication. Patients should be counseled to avoid loud noise exposure and other ototoxic medications during teprotumumab treatment.
Clinical trials have revealed encouraging results with the use of topical IGF-1 to treat sudden sensorineural hearing loss.[50][51] Topical IGF-1 rescue or as a preventive treatment remains to be explored in teprotumumab-related ototoxicity.
Inflammatory Bowel Disease (IBD)
Pre-existing inflammatory bowel disease is a labeled precaution for the use of teprotumumab. Greater care is needed while using teprotumumab in these patients. In the phase 2 teprotumumab trial, a patient in the teprotumumab group with pre-existing ulcerative colitis for six months experienced severe diarrhea, which necessitated discontinuation of intervention after the sixth infusion. Another participant in the trial with a 7-month history of abdominal pain and bloody diarrhea before commencing teprotumumab was diagnosed with exacerbation of inflammatory bowel disease (ulcerative colitis) 3 months into the study and was withdrawn from the study.[1]
In another recent case report, a patient with thyroid eye disease was treated with teprotumumab and developed symptoms suggestive of IBD after her sixth infusion. Colonoscopy confirmed the diagnosis of ulcerative colitis, and the patient required subsequent treatment with infliximab to control her IBD.[52]
IGF-1R is expressed on intestinal epithelium and smooth muscle cells. It is thought that its stimulation with IGF-1 contributes to mucosal proliferation and repair and reduces intestinal inflammation.[53][54] Using an IGF-1R antibody such as teprotumumab may trigger or exacerbate IBD.
Contraindications
Warnings and Precautions
Teprotumumab has no listed contraindications on the label. However, warnings and precautions are for poorly controlled diabetics, pregnant or breastfeeding women, and prepubertal children. Based on its mechanism of action, it is likely that teprotumumab will have teratogenic effects during pregnancy. Therefore, it is recommended that women of child-bearing age use adequate contraception before starting therapy, during the course of treatment, and for six months following the last dose of teprotumumab.
Teprotumumab for thyroid eye disease should be used cautiously in patients with IBD. In the phase 2 study for thyroid orbitopathy, two patients with pre-existing colitis showed severe exacerbations while receiving teprotumumab. These adverse events led the researchers to exclude patients with IBD from the phase 3 study and for the FDA to list IBD as a relative contraindication. It can be considered in selected patients; however, co-management with a gastroenterologist is strongly recommended.
Simultaneous use of teprotumumab with another biological agent is strongly discouraged. No data currently exists regarding potential infectious risks with concomitant treatment. Rituximab-treated patients may have depleted B-cell counts for six months following their last treatment; hence a 6-month break between the last rituximab dose and the start of teprotumumab is strongly advised. The peripheral B-cell count can be checked beforehand.
Monitoring
The efficacy and therapeutic index of teprotumumab are assessable with subjective and objective findings. Before starting the drug, the following should be undertaken:
- A thorough history (in particular, a history of diabetes and IBD).
- Physical examination (e.g., measurement of weight and blood pressure).
- Complete ophthalmologic examination (including visual acuity, color vision, exophthalmometry, extraocular motility, anterior segment, and fundus examination).
- Standard clinical laboratory evaluation (e.g., complete blood count, liver function tests, fasting blood glucose, and hemoglobin A1c).
- Objective audiology testing (if available).[55]
Patients with diabetes who receive teprotumumab are advised to have monitoring of blood glucose and glycated hemoglobin levels and should be co-managed with an endocrinologist. Tests recommended include fasting blood glucose after each of the first two infusions and self-monitoring at least twice a day.
Dosing in both clinical trials involved eight infusions over six months. However, the duration and total infusion numbers are debatable, as an improvement has been reported by injection 2 or 3. Smith et al. showed that the therapeutic effects were rapid, with 43% of patients in the teprotumumab group and 4% of patients in the placebo group responding at week 6.[1]
Patients with severe disease who continue demonstrating response at 24 weeks to teprotumumab can be considered for additional infusions using the same dosing schedule.[56] In contrast, early discontinuation should be considered if there is no improvement at the 4th to 6th infusion.
Toxicity
There is a paucity of data regarding the toxicity and overdosage of teprotumumab. Symptoms of overdose are likely to be consistent with its adverse effect profile.
Enhancing Healthcare Team Outcomes
Teprotumumab is a novel targeted treatment for thyroid eye disease that has shown encouraging results in decreasing the inflammatory process, reversing disease endpoints such as diplopia and proptosis, and reducing the need for surgical intervention.[57] The drug may be considered first-line therapy for patients with clinically significant ophthalmopathy. The CAS may be valid for longitudinal monitoring but should not be used to establish treatment eligibility.[56]
The use of teprotumumab requires an interprofessional team of healthcare professionals, which may include:
- An endocrinologist to render the patient euthyroid and manage patients who may develop secondary hyperglycemia
- An ophthalmologist to adequately examine the eye, ascertain which patients may be good candidates for teprotumumab, and monitor the progression
- A rheumatologist for medical workups, drug prescription, and managing drug infusion reactions in a day center
- A pharmacist to assist with dosing
- A radiologist to assess treatment response (e.g., possible reduction of muscle or fat volume, improvement of apex crowding)
- A gastroenterologist to manage patients who may develop IBD
- Psychosocial support
Teprotumumab has shown great promise to change the landscape of managing thyroid eye disease, and working in an interprofessional team will better patient outcomes.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
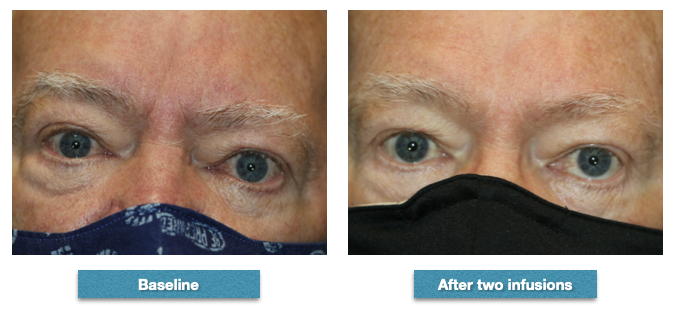
At baseline prior to infusions, the patient had proptosis of 24 mm right eye and 25 mm left eye. His clinical activity score was 5. He received two infusions of teprotumumab but did not complete the course because of hyperglycemia episodes. However, his proptosis improved by 1 mm in each eye and his clinical activity score was 1 after his second infusion. This patient, like many others in our experience, noticed an improvement in his general well-being (concerning his orbits and eyes) even though the improvement in appearance was moderate Contributed from the clinical series of patients of Prof. Bhupendra C. K. Patel MD, FRCS
(Click Image to Enlarge)
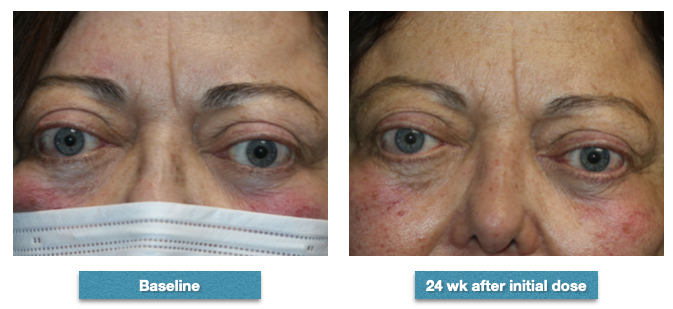
Patient before-and-after completing the series of 8 infusions of teprotumumab. Baseline, she had right proptosis of 27 mm and left proptosis of 28 mm. Her clinical activity score was 3. After completing the course of infusions, her right proptosis improved to 25 mm and her left proptosis to 26 mm. Her clinical activity score improved to 2. She subjectively noted an improvement in how her eyes felt overall but especially when she awoke in the morning when she used to experience swelling and discomfort. Although she did not have diplopia prior to the injections, she subjectively noted that it was easier for her "to move the eyes." She also noticed a subjective improvement in the "thickness" of her skin around the face and noted that her frown line was less marked. Clinically, one can see the decrease in the corrugated line and also the improvement in the fullness of her brow fat pads Contributed from the clinical series of patients of Prof. Bhupendra C. K. Patel MD, FRCS
(Click Image to Enlarge)
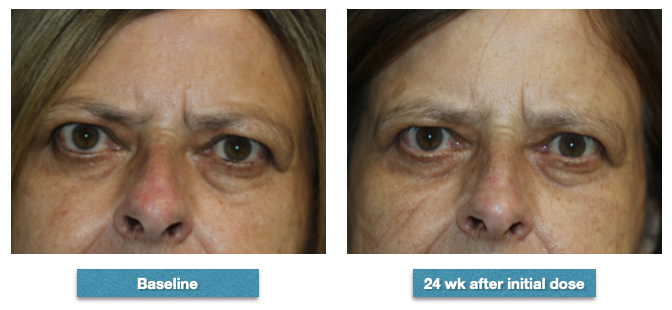
Description: 62-year-old female whose proptosis measured 26 mm right and 25 mm left prior to commencing infusions with teprotumumab. Her clinical activity score was 4. After completing the series of 8 infusions, her right proptosis measured 25 mm and her left proptosis measured 24 mm. Her clinical activity score was 2 after the completion of her infusions. She had mild double vision in up gaze prior to the infusions and noticed a marked improvement but the double vision was still there in up gaze. She also noted that her eyes felt better. She also noted the reduction in the fullness of her face, forehead and eyelids Contributed from the clinical series of patients of Prof. Bhupendra C. K. Patel MD, FRCS
(Click Image to Enlarge)
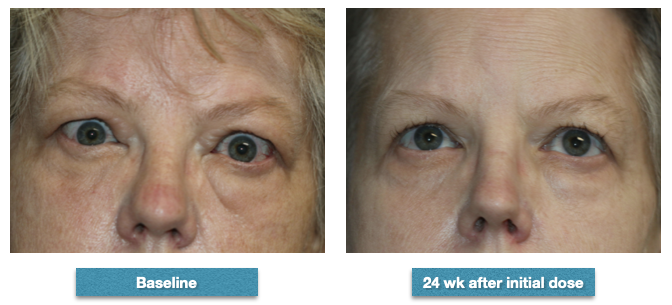
A 52-year-old female whose measurements prior to commencing infusions with teprotumumab were right proptosis of 26 mm and left proptosis of 27 mm. Her clinical activity score was 6. After completing the course of 8 infusions of teprotumumab, her proptosis measured 24 mm right and 25 mm left. Her double vision in up gaze was considerably improved but was still there in extreme up gaze. Her clinical activity score was improved to 2. Note the marked improvement in the fullness at the bridge of the nose, the brow fat fullness, the prominence of the lower eyelid fat, and the overall improvement in the texture of her skin. She noticed all of these changes as have many of our patients. Contributed from the clinical series of patients of Prof. Bhupendra C. K. Patel MD, FRCS
References
Smith TJ, Kahaly GJ, Ezra DG, Fleming JC, Dailey RA, Tang RA, Harris GJ, Antonelli A, Salvi M, Goldberg RA, Gigantelli JW, Couch SM, Shriver EM, Hayek BR, Hink EM, Woodward RM, Gabriel K, Magni G, Douglas RS. Teprotumumab for Thyroid-Associated Ophthalmopathy. The New England journal of medicine. 2017 May 4:376(18):1748-1761. doi: 10.1056/NEJMoa1614949. Epub [PubMed PMID: 28467880]
Douglas RS, Kahaly GJ, Patel A, Sile S, Thompson EHZ, Perdok R, Fleming JC, Fowler BT, Marcocci C, Marinò M, Antonelli A, Dailey R, Harris GJ, Eckstein A, Schiffman J, Tang R, Nelson C, Salvi M, Wester S, Sherman JW, Vescio T, Holt RJ, Smith TJ. Teprotumumab for the Treatment of Active Thyroid Eye Disease. The New England journal of medicine. 2020 Jan 23:382(4):341-352. doi: 10.1056/NEJMoa1910434. Epub [PubMed PMID: 31971679]
Jain AP, Gellada N, Ugradar S, Kumar A, Kahaly G, Douglas R. Teprotumumab reduces extraocular muscle and orbital fat volume in thyroid eye disease. The British journal of ophthalmology. 2022 Feb:106(2):165-171. doi: 10.1136/bjophthalmol-2020-317806. Epub 2020 Nov 10 [PubMed PMID: 33172865]
Tran C, Pham CM, Simmons BA, Warner LL, Fuhrmeister LJ, Shriver EM. Echographic Assessment of Extraocular Muscle Response to Teprotumumab. Ophthalmic plastic and reconstructive surgery. 2022 Jul-Aug 01:38(4):336-339. doi: 10.1097/IOP.0000000000002072. Epub 2022 Oct 13 [PubMed PMID: 34652310]
Kahaly GJ, Douglas RS, Holt RJ, Sile S, Smith TJ. Teprotumumab for patients with active thyroid eye disease: a pooled data analysis, subgroup analyses, and off-treatment follow-up results from two randomised, double-masked, placebo-controlled, multicentre trials. The lancet. Diabetes & endocrinology. 2021 Jun:9(6):360-372. doi: 10.1016/S2213-8587(21)00056-5. Epub 2021 Apr 15 [PubMed PMID: 33865501]
Level 1 (high-level) evidenceDouglas RS, Kahaly GJ, Ugradar S, Elflein H, Ponto KA, Fowler BT, Dailey R, Harris GJ, Schiffman J, Tang R, Wester S, Jain AP, Marcocci C, Marinò M, Antonelli A, Eckstein A, Führer-Sakel D, Salvi M, Sile S, Francis-Sedlak M, Holt RJ, Smith TJ. Teprotumumab Efficacy, Safety, and Durability in Longer-Duration Thyroid Eye Disease and Re-treatment: OPTIC-X Study. Ophthalmology. 2022 Apr:129(4):438-449. doi: 10.1016/j.ophtha.2021.10.017. Epub 2021 Oct 21 [PubMed PMID: 34688699]
Douglas RS, Dailey R, Subramanian PS, Barbesino G, Ugradar S, Batten R, Qadeer RA, Cameron C. Proptosis and Diplopia Response With Teprotumumab and Placebo vs the Recommended Treatment Regimen With Intravenous Methylprednisolone in Moderate to Severe Thyroid Eye Disease: A Meta-analysis and Matching-Adjusted Indirect Comparison. JAMA ophthalmology. 2022 Apr 1:140(4):328-335. doi: 10.1001/jamaophthalmol.2021.6284. Epub [PubMed PMID: 35175308]
Level 1 (high-level) evidenceUgradar S, Shi L, Wang Y, Mester T, Yang H, Douglas RS. Teprotumumab for non-inflammatory thyroid eye disease (TED): evidence for increased IGF-1R expression. Eye (London, England). 2021 Sep:35(9):2607-2612. doi: 10.1038/s41433-020-01297-w. Epub 2020 Nov 21 [PubMed PMID: 33221815]
Ozzello DJ, Dallalzadeh LO, Liu CY. Teprotumumab for chronic thyroid eye disease. Orbit (Amsterdam, Netherlands). 2022 Oct:41(5):539-546. doi: 10.1080/01676830.2021.1933081. Epub 2021 Jun 1 [PubMed PMID: 34060414]
Ozzello DJ, Kikkawa DO, Korn BS. Early experience with teprotumumab for chronic thyroid eye disease. American journal of ophthalmology case reports. 2020 Sep:19():100744. doi: 10.1016/j.ajoc.2020.100744. Epub 2020 May 15 [PubMed PMID: 32462101]
Level 3 (low-level) evidenceUgradar S, Kang J, Kossler AL, Zimmerman E, Braun J, Harrison AR, Bose S, Cockerham K, Douglas RS. Teprotumumab for the treatment of chronic thyroid eye disease. Eye (London, England). 2022 Aug:36(8):1553-1559. doi: 10.1038/s41433-021-01593-z. Epub 2021 Jul 9 [PubMed PMID: 34244669]
Diniz SB, Cohen LM, Roelofs KA, Rootman DB. Early Experience With the Clinical Use of Teprotumumab in a Heterogenous Thyroid Eye Disease Population. Ophthalmic plastic and reconstructive surgery. 2021 Nov-Dec 01:37(6):583-591. doi: 10.1097/IOP.0000000000001959. Epub [PubMed PMID: 33710036]
Sears CM, Azad AD, Dosiou C, Kossler AL. Teprotumumab for Dysthyroid Optic Neuropathy: Early Response to Therapy. Ophthalmic plastic and reconstructive surgery. 2021 May-Jun 01:37(3S):S157-S160. doi: 10.1097/IOP.0000000000001831. Epub [PubMed PMID: 32976335]
Slentz DH, Smith TJ, Kim DS, Joseph SS. Teprotumumab for Optic Neuropathy in Thyroid Eye Disease. JAMA ophthalmology. 2021 Feb 1:139(2):244-247. doi: 10.1001/jamaophthalmol.2020.5296. Epub [PubMed PMID: 33270090]
Chiou CA, Reshef ER, Freitag SK. Teprotumumab for the treatment of mild compressive optic neuropathy in thyroid eye disease: A report of two cases. American journal of ophthalmology case reports. 2021 Jun:22():101075. doi: 10.1016/j.ajoc.2021.101075. Epub 2021 Mar 17 [PubMed PMID: 33889787]
Level 3 (low-level) evidenceHwang CJ, Nichols EE, Chon BH, Perry JD. Bilateral dysthyroid compressive optic neuropathy responsive to teprotumumab. European journal of ophthalmology. 2022 May:32(3):NP46-NP49. doi: 10.1177/1120672121991042. Epub 2021 Feb 1 [PubMed PMID: 33525898]
Sears CM, Wang Y, Bailey LA, Turbin R, Subramanian PS, Douglas R, Cockerham K, Kossler AL. Early efficacy of teprotumumab for the treatment of dysthyroid optic neuropathy: A multicenter study. American journal of ophthalmology case reports. 2021 Sep:23():101111. doi: 10.1016/j.ajoc.2021.101111. Epub 2021 May 14 [PubMed PMID: 34113737]
Level 2 (mid-level) evidenceUgradar S, Wang Y, Mester T, Kahaly GJ, Douglas R. Improvement of asymmetric thyroid eye disease with teprotumumab. The British journal of ophthalmology. 2022 Jun:106(6):755-759. doi: 10.1136/bjophthalmol-2020-318314. Epub 2021 Feb 12 [PubMed PMID: 33579690]
Ugradar S, Wang Y, Mester T, Kahaly GJ, Douglas RS. Teprotumumab for thyroid eye disease: early response is not required for benefit. Eye (London, England). 2022 Jul:36(7):1403-1408. doi: 10.1038/s41433-021-01539-5. Epub 2021 Jun 28 [PubMed PMID: 34183792]
Ugradar S, Braun J, Wang Y, Zimmerman E, Douglas RS. Facial and Eyelid Changes in Thyroid Eye Disease Are Reversed by Teprotumumab. Plastic and reconstructive surgery. Global open. 2021 Sep:9(9):e3809. doi: 10.1097/GOX.0000000000003809. Epub 2021 Sep 15 [PubMed PMID: 34549003]
Pritchard J, Han R, Horst N, Cruikshank WW, Smith TJ. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves' disease is mediated through the insulin-like growth factor I receptor pathway. Journal of immunology (Baltimore, Md. : 1950). 2003 Jun 15:170(12):6348-54 [PubMed PMID: 12794168]
Pritchard J, Horst N, Cruikshank W, Smith TJ. Igs from patients with Graves' disease induce the expression of T cell chemoattractants in their fibroblasts. Journal of immunology (Baltimore, Md. : 1950). 2002 Jan 15:168(2):942-50 [PubMed PMID: 11777993]
Smith TJ, Janssen JAMJL. Insulin-like Growth Factor-I Receptor and Thyroid-Associated Ophthalmopathy. Endocrine reviews. 2019 Feb 1:40(1):236-267. doi: 10.1210/er.2018-00066. Epub [PubMed PMID: 30215690]
Weightman DR, Perros P, Sherif IH, Kendall-Taylor P. Autoantibodies to IGF-1 binding sites in thyroid associated ophthalmopathy. Autoimmunity. 1993:16(4):251-7 [PubMed PMID: 7517705]
Douglas RS, Gianoukakis AG, Kamat S, Smith TJ. Aberrant expression of the insulin-like growth factor-1 receptor by T cells from patients with Graves' disease may carry functional consequences for disease pathogenesis. Journal of immunology (Baltimore, Md. : 1950). 2007 Mar 1:178(5):3281-7 [PubMed PMID: 17312178]
Douglas RS, Naik V, Hwang CJ, Afifiyan NF, Gianoukakis AG, Sand D, Kamat S, Smith TJ. B cells from patients with Graves' disease aberrantly express the IGF-1 receptor: implications for disease pathogenesis. Journal of immunology (Baltimore, Md. : 1950). 2008 Oct 15:181(8):5768-74 [PubMed PMID: 18832736]
Smith TJ, Hoa N. Immunoglobulins from patients with Graves' disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, insulin-like growth factor-I receptor. The Journal of clinical endocrinology and metabolism. 2004 Oct:89(10):5076-80 [PubMed PMID: 15472208]
Tsui S, Naik V, Hoa N, Hwang CJ, Afifiyan NF, Sinha Hikim A, Gianoukakis AG, Douglas RS, Smith TJ. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves' disease. Journal of immunology (Baltimore, Md. : 1950). 2008 Sep 15:181(6):4397-405 [PubMed PMID: 18768899]
Krieger CC, Place RF, Bevilacqua C, Marcus-Samuels B, Abel BS, Skarulis MC, Kahaly GJ, Neumann S, Gershengorn MC. TSH/IGF-1 Receptor Cross Talk in Graves' Ophthalmopathy Pathogenesis. The Journal of clinical endocrinology and metabolism. 2016 Jun:101(6):2340-7. doi: 10.1210/jc.2016-1315. Epub 2016 Apr 4 [PubMed PMID: 27043163]
Krieger CC, Boutin A, Jang D, Morgan SJ, Banga JP, Kahaly GJ, Klubo-Gwiezdzinska J, Neumann S, Gershengorn MC. Arrestin-β-1 Physically Scaffolds TSH and IGF1 Receptors to Enable Crosstalk. Endocrinology. 2019 Jun 1:160(6):1468-1479. doi: 10.1210/en.2019-00055. Epub [PubMed PMID: 31127272]
Girnita L, Smith TJ, Janssen JAMJL. It Takes Two to Tango: IGF-I and TSH Receptors in Thyroid Eye Disease. The Journal of clinical endocrinology and metabolism. 2022 Aug 8:107(Suppl_1):S1-S12. doi: 10.1210/clinem/dgac045. Epub [PubMed PMID: 35167695]
Chen H, Mester T, Raychaudhuri N, Kauh CY, Gupta S, Smith TJ, Douglas RS. Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes. The Journal of clinical endocrinology and metabolism. 2014 Sep:99(9):E1635-40. doi: 10.1210/jc.2014-1580. Epub 2014 May 30 [PubMed PMID: 24878056]
Xin Y, Xu F, Gao Y, Bhatt N, Chamberlain J, Sile S, Hammel S, Holt RJ, Ramanathan S. Pharmacokinetics and Exposure-Response Relationship of Teprotumumab, an Insulin-Like Growth Factor-1 Receptor-Blocking Antibody, in Thyroid Eye Disease. Clinical pharmacokinetics. 2021 Aug:60(8):1029-1040. doi: 10.1007/s40262-021-01003-3. Epub 2021 Mar 26 [PubMed PMID: 33768488]
Hoang TD, Nguyen NT, Chou E, Shakir MK. Rapidly progressive cognitive decline associated with teprotumumab in thyroid eye disease. BMJ case reports. 2021 May 10:14(5):. doi: 10.1136/bcr-2021-242153. Epub 2021 May 10 [PubMed PMID: 33972303]
Level 3 (low-level) evidenceSmith TJ. Challenges in Orphan Drug Development: Identification of Effective Therapy for Thyroid-Associated Ophthalmopathy. Annual review of pharmacology and toxicology. 2019 Jan 6:59():129-148. doi: 10.1146/annurev-pharmtox-010617-052509. Epub 2018 Jul 25 [PubMed PMID: 30044728]
Pollak M. Metformin and pancreatic cancer: a clue requiring investigation. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012 May 15:18(10):2723-5. doi: 10.1158/1078-0432.CCR-12-0694. Epub 2012 Mar 31 [PubMed PMID: 22465829]
Level 2 (mid-level) evidenceGualberto A, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions. Oncogene. 2009 Aug 27:28(34):3009-21. doi: 10.1038/onc.2009.172. Epub 2009 Jul 6 [PubMed PMID: 19581933]
Level 3 (low-level) evidenceGao L, Nakagawa T. Insulin-like growth factor 1: role in the auditory system and therapeutic potential in otology. Current opinion in otolaryngology & head and neck surgery. 2020 Oct:28(5):286-290. doi: 10.1097/MOO.0000000000000652. Epub [PubMed PMID: 32796270]
Level 3 (low-level) evidenceIwai K, Nakagawa T, Endo T, Matsuoka Y, Kita T, Kim TS, Tabata Y, Ito J. Cochlear protection by local insulin-like growth factor-1 application using biodegradable hydrogel. The Laryngoscope. 2006 Apr:116(4):529-33 [PubMed PMID: 16585854]
Level 3 (low-level) evidenceHayashi Y, Yamamoto N, Nakagawa T, Omori K, Ito J. Activation of IGF1 Signaling in the Cochlea Induces the Transcription of Its Mediators During the Protection of Cochlear Hair Cells Against Aminoglycoside. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2017 Feb:38(2):278-282. doi: 10.1097/MAO.0000000000001276. Epub [PubMed PMID: 27846039]
Fujiwara T, Hato N, Nakagawa T, Tabata Y, Yoshida T, Komobuchi H, Takeda S, Hyodo J, Hakuba N, Gyo K. Insulin-like growth factor 1 treatment via hydrogels rescues cochlear hair cells from ischemic injury. Neuroreport. 2008 Oct 29:19(16):1585-8. doi: 10.1097/WNR.0b013e328311ca4b. Epub [PubMed PMID: 18845939]
Level 3 (low-level) evidenceDing AS, Mahoney NR, Campbell AA, Creighton FX. Sensorineural Hearing Loss After Teprotumumab Therapy for Thyroid Eye Disease: A Case Report. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2022 Feb 1:43(2):e148-e152. doi: 10.1097/MAO.0000000000003428. Epub [PubMed PMID: 34789694]
Level 3 (low-level) evidenceHighland J, Gordon S, Reddy D, Patel N. Ototoxicity and Teprotumumab. The Annals of otology, rhinology, and laryngology. 2022 Aug:131(8):910-913. doi: 10.1177/00034894211042740. Epub 2021 Aug 27 [PubMed PMID: 34448414]
Lassale C, Batty GD, Steptoe A, Zaninotto P. Insulin-like Growth Factor 1 in relation to future hearing impairment: findings from the English Longitudinal Study of Ageing. Scientific reports. 2017 Jun 23:7(1):4212. doi: 10.1038/s41598-017-04526-7. Epub 2017 Jun 23 [PubMed PMID: 28646237]
Berker D, Karabulut H, Isik S, Tutuncu Y, Ozuguz U, Erden G, Aydin Y, Dagli M, Guler S. Evaluation of hearing loss in patients with Graves' disease. Endocrine. 2012 Feb:41(1):116-21. doi: 10.1007/s12020-011-9515-9. Epub 2011 Aug 11 [PubMed PMID: 21833679]
Level 2 (mid-level) evidenceTsai YT, Chang IJ, Hsu CM, Yang YH, Liu CY, Tsai MS, Chang GH, Lee YC, Huang EI, Lin MH, Luan CW. Association between Sudden Sensorineural Hearing Loss and Preexisting Thyroid Diseases: A Nationwide Case-Control Study in Taiwan. International journal of environmental research and public health. 2020 Jan 29:17(3):. doi: 10.3390/ijerph17030834. Epub 2020 Jan 29 [PubMed PMID: 32013113]
Level 2 (mid-level) evidenceReed DS, Kostosky N, Davies BW, Epstein A, Durairaj VD. Rifle Blast Exacerbating Hearing Loss in a Patient Treated With Teprotumumab for Thyroid Eye Disease. Ophthalmic plastic and reconstructive surgery. 2022 Mar-Apr 01:38(2):e41-e43. doi: 10.1097/IOP.0000000000002078. Epub [PubMed PMID: 34652314]
Belinsky I, Creighton FX Jr, Mahoney N, Petris CK, Callahan AB, Campbell AA, Kazim M, Lee HBH, Yoon MK, Dagi Glass LR. Teprotumumab and Hearing Loss: Case Series and Proposal for Audiologic Monitoring. Ophthalmic plastic and reconstructive surgery. 2022 Jan-Feb 01:38(1):73-78. doi: 10.1097/IOP.0000000000001995. Epub [PubMed PMID: 34085994]
Level 2 (mid-level) evidenceSears CM, Azad AD, Amarikwa L, Pham BH, Men CJ, Kaplan DN, Liu J, Hoffman AR, Swanson A, Alyono J, Lee JY, Dosiou C, Kossler AL. Hearing Dysfunction After Treatment With Teprotumumab for Thyroid Eye Disease. American journal of ophthalmology. 2022 Aug:240():1-13. doi: 10.1016/j.ajo.2022.02.015. Epub 2022 Feb 25 [PubMed PMID: 35227694]
Nakagawa T, Sakamoto T, Hiraumi H, Kikkawa YS, Yamamoto N, Hamaguchi K, Ono K, Yamamoto M, Tabata Y, Teramukai S, Tanaka S, Tada H, Onodera R, Yonezawa A, Inui K, Ito J. Topical insulin-like growth factor 1 treatment using gelatin hydrogels for glucocorticoid-resistant sudden sensorineural hearing loss: a prospective clinical trial. BMC medicine. 2010 Nov 25:8():76. doi: 10.1186/1741-7015-8-76. Epub 2010 Nov 25 [PubMed PMID: 21108784]
Nakagawa T, Kumakawa K, Usami S, Hato N, Tabuchi K, Takahashi M, Fujiwara K, Sasaki A, Komune S, Sakamoto T, Hiraumi H, Yamamoto N, Tanaka S, Tada H, Yamamoto M, Yonezawa A, Ito-Ihara T, Ikeda T, Shimizu A, Tabata Y, Ito J. A randomized controlled clinical trial of topical insulin-like growth factor-1 therapy for sudden deafness refractory to systemic corticosteroid treatment. BMC medicine. 2014 Nov 19:12():219. doi: 10.1186/s12916-014-0219-x. Epub 2014 Nov 19 [PubMed PMID: 25406953]
Level 1 (high-level) evidenceAshraf DC, Jankovic I, El-Nachef N, Winn BJ, Kim GE, Kersten RC. New-Onset of Inflammatory Bowel Disease in a Patient Treated With Teprotumumab for Thyroid Associated Ophthalmopathy. Ophthalmic plastic and reconstructive surgery. 2021 Sep-Oct 01:37(5):e160-e164. doi: 10.1097/IOP.0000000000001943. Epub [PubMed PMID: 33710035]
Chen T, Zheng F, Tao J, Tan S, Zeng L, Peng X, Wu B. Insulin-Like Growth Factor-1 Contributes to Mucosal Repair by β-Arrestin2-Mediated Extracellular Signal-Related Kinase Signaling in Experimental Colitis. The American journal of pathology. 2015 Sep:185(9):2441-53. doi: 10.1016/j.ajpath.2015.05.020. Epub [PubMed PMID: 26362717]
Barahona-Garrido J, Hernández-Calleros J, García-Juárez I, Yamamoto-Furusho JK. Growth factors as treatment for inflammatory bowel disease: a concise review of the evidence toward their potential clinical utility. Saudi journal of gastroenterology : official journal of the Saudi Gastroenterology Association. 2009 Jul-Sep:15(3):208-12. doi: 10.4103/1319-3767.54742. Epub [PubMed PMID: 19636186]
Yu CY, Correa T, Simmons BA, Hansen MR, Shriver EM. Audiology findings in patients with teprotumumab associated otologic symptoms. American journal of ophthalmology case reports. 2021 Dec:24():101202. doi: 10.1016/j.ajoc.2021.101202. Epub 2021 Sep 16 [PubMed PMID: 34585021]
Level 3 (low-level) evidenceDouglas RS, Wang Y, Dailey RA, Harris GJ, Wester ST, Schiffman JS, Tang RA, Fowler B, Fleming J, Smith TJ. Teprotumumab in Clinical Practice: Recommendations and Considerations From the OPTIC Trial Investigators. Journal of neuro-ophthalmology : the official journal of the North American Neuro-Ophthalmology Society. 2021 Dec 1:41(4):461-468. doi: 10.1097/WNO.0000000000001134. Epub [PubMed PMID: 33417417]
Khong JJ, McNab A. Medical treatment in thyroid eye disease in 2020. The British journal of ophthalmology. 2021 Mar:105(3):299-305. doi: 10.1136/bjophthalmol-2020-316051. Epub 2020 May 23 [PubMed PMID: 32447327]