Introduction
Sclerotherapy is a group of techniques characterized by the injection of an irritating substance into a blood vessel to damage the vessel endothelium, induce inflammation with subsequent endofibrosis, and destroy the vessel.[1][2][3] The irritating substances are termed sclerosing agents and include osmotic agents such as glycerin and hypertonic saline, detergents like polidocanol and sodium tetradecyl sulfate (STS), and iodinated substances.[2][4] Sclerotherapy is most commonly a primary or adjunctive therapy for cosmetically distressing or symptomatic venous varicosities of the lower extremities, but it also may be employed when treating certain hemorrhoids.[5] Sclerotherapy remains the gold standard therapeutic intervention for lower extremity superficial varicose veins, reticular veins, and telangiectasia.
The treatment of venous insufficiency and varicosities dates back to Galen, Celsus, and Hippocrates.[6] However, the modern medical references to sclerotherapy appeared in the 1850s. High infection rates secondary to treatments with perchloride of iron of mercury at that time resulted in the abandonment of sclerosing interventions until the early 1900s with the introduction of hypertonic saline as a sclerosing agent.[7] Reiner introduced STS as a sclerosing agent in the 1940s, and the modern era of sclerotherapy was born.[7]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Venous varicosities of the lower extremities are large, tortuous veins frequently visible beneath the skin, giving the extremities a lumpy, irregular contour and causing itching, pain, swelling, a feeling of heaviness, and occasionally bleeding.[1] Venous varicosities affect nearly one-third of the population, of which 60% are women.[8][9] The incidence of venous varicosities increases with age.[10]
Venous varicosities are often related to venous insufficiency of the deep or superficial venous systems or their connecting perforating vessels.[10] Incompetence of the superficial venous system leads to visible varicosities, while deep venous insufficiency can cause lower extremity edema with subsequent hyperpigmentation, induration, and ulceration of the skin.[10]
Family history is the most significant predisposing factor for developing venous varicosities. However, activities or conditions that increase intraabdominal pressure and impede venous blood flow toward the heart, such as obesity, constipation, pregnancy, standing for long periods, and estrogen and progesterone supplementation, promote venous dilatation.[10] Trauma may damage perforating veins and increase blood flow from the deep to the superficial circulatory system, promoting varicosity formation. Telangiectasias may occur secondary to localized trauma or in response to sun exposure or radiation damage.[10]
Approximately 90% of the venous drainage in the lower extremities is via the deep venous system; incompetence of this system is usually due to insufficient venous valves that permit inappropriate backflow, increasing pressure on the superficial system and predisposing to superficial varicosities.[10] Superficial venous varicosities contain increased collagen and decreased elastin, resulting in weakness of the vein wall and valves. Increased pressure from incompetent deep veins, especially when standing or walking, causes superficial venous distension.[11]
Venous varicosities are tortuous veins that measure greater than 4 to 5 mm in diameter; reticular veins measure from 1 to 4 mm in diameter. Telangiectasias are bluish vessels frequently found branching off reticular veins in fan-shaped arrays; they may be greater than 1 mm in diameter. Spider veins are reddish in color and measure less than 1 mm in diameter. [3][6]
Telangiectatic and venous varicosities are signs of venous insufficiency, a process characterized by the reversal of venous blood flow, which predisposes to the development of venous hypertension, an increase in venous diameter, and resultant valvular insufficiency. Incompetent communicating veins cause blood to flow back toward the deep venous system, increasing pressure and inducing dilatation of the superficial veins to accommodate this increased blood flow. The naturally occurring elevation in venous pressure inside the calf-muscle pump during movement of the lower limbs is transmitted directly to the superficial veins and subcutaneous tissues connected with these communicating veins. Telangiectasias also result from increased venous pressure in the superficial vascular system [10].
Treating telangiectasias, reticular veins, and venous varicosities requires a multifaceted approach involving medical therapies, lifestyle modifications such as exercise to improve the muscular pumps within the legs, weight loss to decrease central venous pressure, limb elevation, and compression garments which can be knee-high, thigh-high, or full-body.[8] The classification of venous insufficiency comprises clinical, etiologic, anatomic, and pathologic features (CEAP), as shown in Table 1. Invasive treatment is indicated in C2 disease or greater, with surgical options including vein stripping or ligation, endovascular options such as ablation with radiofrequency devices, thermal or steam damage, or nonsurgical treatments such as sclerotherapy.[8]
Table 1. CEAP Classification
| CEAP Classification | Features |
| C1 | Reticular veins, telangiectasias, or spider veins of purely cosmetic concern |
| C2a | Asymptomatic varicose veins |
| C2b | Symptomatic varicose veins (itching, pain, thrombosis, bleeding) |
| C3 | Edema due to varicose veins |
| C4 | Skin changes such as lipodermatosclerosis or hyperpigmentation |
| C5 | Healed venous ulcers |
| C6 | Active venous ulcers |
Sclerosants
The sclerosants commonly employed in current clinical practice can be classified as irritants, osmotic agents, or detergents.[6][12] Sclerosants damage the endothelial cells of the tunica intima and the luminal 300 μm of the tunica media.[1] Irritants and osmotic sclerosants are directly cytotoxic; detergents are less cytotoxic but disrupt intercellular connections.[1] In the United States, the most commonly employed sclerosants are the osmotic agent hypertonic saline and the detergents STS and polidocanol.[11]
Foam sclerosants were first described in the 1930s when air was incorporated into a vial of liquid detergent sclerosant via agitation to create a microfoam.[7] This technique has undergone multiple modifications; the current technique of employing 2 syringes and a multiway stopcock was first described in 1997 by Tesari and Frullini.[7] Foam sclerotherapy is favored over pure liquid sclerotherapy because foam bubbles increase the surface area of the liquid, prolonging contact with the vessel endothelium while minimizing the mixing of the sclerosant in the blood.[6][13] Foam is more viscous, has an exponentially larger surface area, and allows for treatment to occur with a smaller volume while simultaneously increasing the length of efficacy within the target vein.[2][14] Foam induces a vasospastic response that apposes vessel walls and increases the likelihood that the vein remains obliterated.[2]
The majority of sclerotherapy procedures are performed using room air; pure CO2 and 70/30 mixtures of CO2/O2 may be utilized. A ratio of 1-part liquid to 4- or 5-part gas is the most stable.[15] Carbon dioxide offers several benefits over room air: it forms smaller bubbles and dissipates more quickly, increasing the surface area of endothelial contact and subsequent fibrosis, while dissolving more readily into the blood and decreasing adverse effects.[13]
Irritant Sclerosants
Irritants are corrosive agents that destroy cellular membranes and lead to vessel fibrosis.[6] Typical irritants are ethanol, phenol, and polyiodinated iodine; polyiodinated iodines cause full-thickness vessel destruction. The historically but no longer utilized agents, perchloride of iron or mercury, are also irritants.[6]
Osmotic Sclerosants
Osmotic sclerosants destroy cells by promoting rapid diffusion of intracellular fluid into the vessel, resulting in cellular dehydration and membrane disruption.[6] The most commonly utilized osmotic sclerosants are 23% hypertonic saline (HTS) and glycerin.[6][16]
Hypertonic saline is an osmotic agent most commonly utilized when treating hyponatremia and cerebral edema. HTS is available in 3% or 23% concentrations; 23% HTS is used when treating varicose veins. The use of 23% HTS initially fell out of favor due to its adverse effect of extravasation necrosis, but its use was revived in the 1970s with the advent of fine-gauge, disposable syringes.[17] This osmotic agent uses the concentration gradient between the endothelial cell and the intravascular space to cause fibrosis by denaturing the endothelial cell membrane. However, HTS is easily diluted in blood, rendering it ineffective unless a sufficient solution is injected to displace blood from the treated vein.[17] HTS has a low toxicity profile and poses virtually no risk of anaphylaxis. However, the injection of HTS can cause localized transient pain, muscle cramping, or hyperpigmentation.[11]
Glycerin (glycerol; 1,2,3-propanetriol) is a potent osmotic agent most frequently utilized to treat intracranial hypertension.[16] The sclerosing effects of glycerol were first elucidated in 1925 by Jausion et al; glycerin induces mild endosclerosis within 45 minutes of administration.[16]
Detergent Sclerosants
Detergent sclerosants disrupt cell membranes via protein denaturation.[6] Detergent sclerosants also form micelles at certain concentrations and will form a foam when mixed with gas. Unlike other sclerosants, detergents in liquid and foam configurations cause endothelial damage within several minutes after administration; cellular injury can spread away from the injection site.[6] Detergent sclerosants employed during sclerotherapy include STS, polidocanol, morrhuate sodium, and ethanolamine oleate.
STS is a small, synthetic organic sodium salt; 2% benzyl alcohol is added to the formulation for anesthetic purposes in medical use.[12] STS is a clear, nonviscous solution that evenly distributes within the blood following injection and rapidly clears as it attaches to red blood cells and causes hemolysis [12].
Polidocanol is a synthetic polyethylene glycol initially marketed as a local anesthetic.[7] However, injection of polidocanol was found to cause venous sclerosis. While it is still used as a topical anesthetic, its primary use is as a sclerosant.[18] Polidocanol for sclerotherapy is dissolved in distilled water and ethanol to achieve a 5% concentration that facilitates micelle formation.[12]
Polidocanol and STS come in various concentrations, each with recommendations for the size of the vein that can be treated (see Table 2. Size Requirements for STS and Polidocanol Based on Concentration). The incidence of allergic or anaphylactic reaction following STS or polidocanol injection is less than 1%.[11]The 3% STS foam is more potent than 3% Polidocanol, rendering 3% STS foam more appropriate for use on larger veins. Polidocanol is more appropriate for telangiectasias or reticular veins due to its anesthetic properties and decreased risk of thrombophlebitis.[19]
Table 2. Size requirements for STS and Polidocanol based on concentration.
| Concentration | Vein type and diameter |
| STS liquid 0.1%-0.3% | Telangiectatic veins 0.2-1 mm |
| STS liquid 0.5%-1% | Uncomplicated varicose veins 2-4 mm |
| STS foam 0.25%-1% (1:4 ratio) | Large reticular and varicose veins 3-6 mm |
| STS foam 3% (1:4 ratio) | Great saphenous vein or veins up to 10 mm |
| Polidocanol 0.5% | Telangiectasias <1 mm |
| Polidocanol 1% | Veins 1-2 mm |
| Polidocanol 2% | Reticular and varicose veins 2-4 mm |
| Polidocanol 3% | Larger veins 4-8 mm |
Indications
Sclerotherapy techniques are indicated in the treatment of venous varicosities and certain hemorrhoids. The sclerosants utilized in these therapeutic interventions vary.
Liquid or foam sclerotherapy is an indicated treatment for cosmetically distressing or disturbing varicose veins, spider veins, reticular veins, and telangiectasias. These therapies are also employed when treating saphenous vein reflux, enlarged tributary and perforator veins, and vascular malformations.[11] Sclerotherapy can also be used to treat smaller collateral veins not amenable to stab phlebectomy or ablation.
A meta-analysis of foam sclerotherapy as a treatment for venous ulceration or C5-C6 venous insufficiency revealed that foam sclerotherapy may be superior to compression therapy alone. However, this meta-analysis evaluated a few markedly heterogeneous studies; more research is needed.[20]
Utilizing ultrasound guidance during sclerotherapy for venous insufficiency provides a simple, efficacious, targeted method to treat deep venous insufficiency.[21] Studies have reported extremely high satisfaction rates with ultrasound-guided sclerotherapy (UGS); one study reported that 100% of patients were satisfied with the degree of treatment, and more than 90% reported improved quality of life and appearance of the treated limb.[19] Ultrasound-guided sclerotherapy may be appropriate for any patient but is particularly useful when treating patients with obesity, insufficiency of the great saphenous vein, and whose comorbidities preclude more invasive surgery.[13][21][22]
Sclerotherapy is also indicated when treating patients with symptomatic hemorrhoids, active bleeding, risks of major bleeding, and those with HIV or AIDS. Phenol in almond oil and 3% polidocanol are the sclerosants recommended for treating grades 1 through 3 hemorrhoids; aluminum potassium sulfate and tannic acid (ALTA)are recommended for treating grades 2 through 4 hemorrhoids.[5]
Contraindications
There are several absolute and relative contraindications to sclerotherapy for venous varicosities; in patients for whom sclerotherapy is contraindicated, other treatment options, such as stab phlebectomy, should be considered.[11] The contraindications for patients with venous varicosities differ from those with hemorrhoids.
Absolute contraindications to sclerotherapy for the treatment of venous varicosities include but are not limited to specific sclerosant allergy, current systemic infection or that localized to the treatment area, and a personal history of deep venous thrombosis, severe peripheral arterial disease, advanced collagen vascular diseases, or cardiovascular or neurologic events following sclerosant injection.[11][6][23]
Relative contraindications to sclerotherapy for the treatment of venous varicosities include severely impaired mobility and a personal history of a marked allergic diathesis, asthma, severe deep venous incompetence, or thrombophilia. Sclerotherapy may also be relatively contraindicated in patients who are currently pregnant or breastfeeding.[19][11] Visual disturbances and transient ischemic attacks, possibly due to paradoxical emboli, have been reported following foam sclerotherapy in patients with a patent foramen ovale.[1][11] Large varices may be a relative contraindication to sclerotherapy due to the increased risk of recanalization.
Contraindications to the use of sclerotherapy in the treatment of hemorrhoids include thrombosed hemorrhoids and a personal history of asthma, specific sclerosant allergy, thrombophilia, inflammatory bowel disease, and cardiac, renal, or hepatic comorbidities.[5]
Equipment
The equipment required to perform sclerotherapy typically includes:
- Needle, 30-gauge or smaller
- Multiple 5-mL syringes
- Sclerosant of choice
- Local anesthetic
- Gauze dressings
- Compressive bandages
- Ultrasound (if performing ultrasound-guided sclerotherapy)
Personnel
The personnel required to perform sclerotherapy in an office-based setting typically include:
- Primary clinician trained in sclerotherapy
- Assistant
The personnel required to perform sclerotherapy in a hospital-based setting, perhaps as part of a more complex procedure, typically include:
- Primary clinician trained in sclerotherapy
- Surgical technician or operating room nurse
- Circulating or operating room nurse
- Anesthesia personnel
Preparation
The patient and primary clinician should engage in shared decision-making before the procedure to ensure an understanding of the possibility of adverse events and the risk of cosmetic complications such as hyperpigmentation and telangiectatic matting. These risks are increased in patients taking combined oral contraceptives, disulfiram, or minocycline.[23]
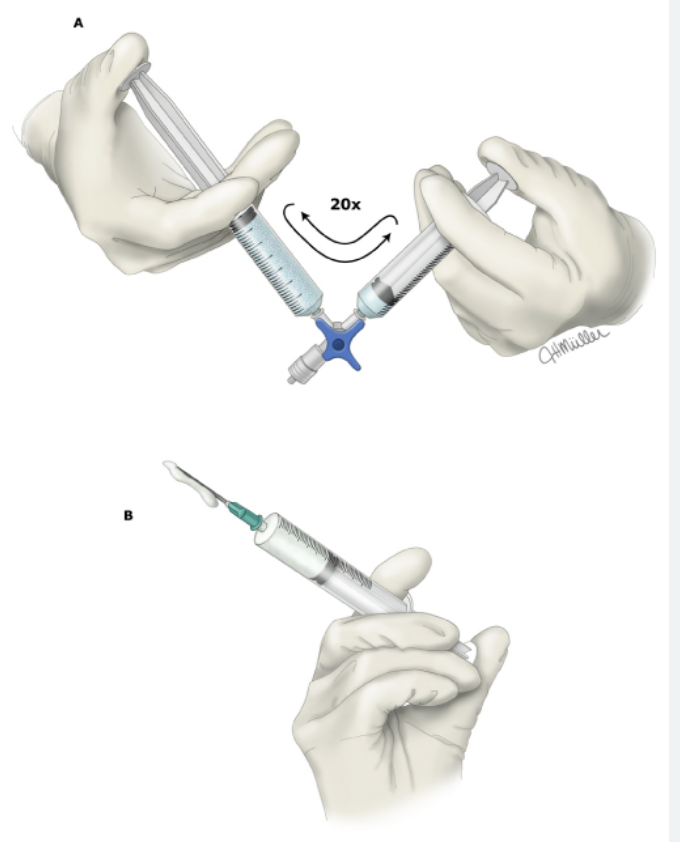
Sclerotherapy is typically performed with the patient in the supine or seated position, as dictated by optimum visualization of the target vessels. The identified procedural areas should be prepped and draped in a sterile fashion. If ultrasound guidance is employed, the machine should be set to vascular mode. Detergent sclerosants to be used as foam should be combined with air employing the Tessari or double-syringe technique (see Figure. Schematic of Tessari Technique).[24][15]
Technique or Treatment
Sclerosant injection should be carried out in a “downstream” fashion from the largest to the smallest vessels, such that the sclerosant will flow from the injection site into other target veins.[3][17] The entire superficial venous system of each leg should be treated in one setting to avoid recanalization or hyperpigmentation due to the extravasation of erythrocytes from the damaged vessel.
Cannulate the target vein with a 30-gauge or smaller needle; confirm correct intravascular placement using aspiration.[2][17] Once intravascular placement is confirmed, inject only enough sclerosant to displace the blood from the vessel.[3] For reticular veins, spider veins, and telangiectasias, inject only enough sclerosant to induce blanching of the system; blanching confirms successful treatment.[17] Using the lowest necessary volume and concentration for vessel injection reduces the risk of initiating new telangiectasias around the treated area.
Patients should remain supine or seated for several minutes following sclerosant injection. The treated extremity should be wrapped with a compressive dressing, or compression stockings should be worn. Dressings and stocking should not be removed for 7 days to promote compression of the treated vessels. Applying compression immediately following sclerotherapy minimizes thrombus formation. Patients should be encouraged to ambulate with the compressive wraps or garments in place.
Complications
Localized adverse effects of sclerotherapy for venous varicosities include but are not limited to pain with or following sclerosant injection, erythema, edema, pruritus, ulceration, increased hair growth, and telangiectatic matting characterized by the appearance of a complex of fine, red veins in the treated area.[6][13] Pain during sclerosant injection is especially common with HTS and glycerin.[6] Approximately 30% of patients may experience skin hyperpigmentation or discoloration from hemosiderin deposition 6 to 8 weeks following sclerotherapy.[11] A systematic review of patients who underwent sclerotherapy with polidocanol demonstrated that hyperpigmentation was more common with a higher sclerosant concentration or treatment focused on superficial venous systems such as the epifascial vessels.[25]
Systemic adverse effects of sclerotherapy include chest tightness, visual disturbances, transient ischemic attacks, dizziness, and migraine headaches; headaches are more likely to occur in patients with a history of migrainous events.[13] These adverse effects occur more frequently following unintentional injection of the deep venous system and ultrasound-guided treatment of the great saphenous vein.[6][13]
The incomplete destruction of the target vessel may lead to recanalization and the need for additional procedures; prolonged compression of the treated limb assists healing and minimizes the risk of recanalization.[6] A systematic review noted no significant differences in the rate of complications or optimal outcomes between foam and liquid sclerotherapy.[1]
The osmotic agents HTS and glycerin carry an increased risk for local tissue damage and ulceration if the solution extravasates or is injected into an arteriole.[6] Intraarterial injection of HTS or glycerin can potentially induce acute limb ischemia that requires amputation.[26] Additionally, glycerin may cause mild hematuria; adding a chromated alum to the glycerol molecule can mitigate this adverse effect. This modification increases the potency of the sclerosant.[16]
The most common complication of hemorrhoid sclerotherapy is an easily treatable localized infection; more severe infections and sepsis have been reported. Additionally, improper or excessive sclerosant injection may lead to surrounding tissue necrosis and complications such as rectovaginal fistulae.[5]
Clinical Significance
Sclerotherapy is a clinically significant treatment modality for venous varicosities, offering a minimally invasive approach with substantial therapeutic benefits via a controlled thrombophelbitic reaction. Sclerotherapy is effective in treating varicose veins, spider veins, and telangiectasia in a short amount of time. Injection sclerotherapy provides notable cosmetic improvement by reducing the appearance of unsightly veins and enhancing patients' confidence and quality of life. Moreover, injection sclerotherapy alleviates symptoms associated with venous insufficiency, such as pain, swelling, and discomfort, leading to improved overall vascular health.
Injection sclerotherapy is versatile, offers primary or adjunctive therapeutic options, is applicable in various settings, and can be performed on an outpatient basis without the need for extensive recovery time. Its minimally invasive nature makes it an attractive option for patients averse to surgery or for whom surgery is contraindicated. Furthermore, advancements in sclerosing agents and techniques have enhanced efficacy and safety, contributing to the widespread use of injection sclerotherapy in managing venous varicosities.
Overall, sclerotherapy offers cosmetic enhancement and symptomatic relief, making it an inherently valuable treatment option for patients with venous disease.
Enhancing Healthcare Team Outcomes
Patients with venous varicosities often require multimodality treatments ranging from compression therapy to surgical intervention to achieve optimal results.[6] Asymptomatic patients may wish only to improve cosmesis; symptomatic patients may additionally desire symptom relief. Patients with venous varicosities frequently present to various healthcare providers, including primary care providers, plastic surgeons, dermatologists, or vascular surgeons. If the venous disease has resulted in ulceration, wound care specialists may be involved. A comprehensive understanding of the multimodal therapies employed in venous varicosity treatment is needed to provide optimal patient care.
The Cochrane database reviews several available interventions for treating venous varicosities, including laser or radiofrequency endovenous ablation therapy, ultrasound-guided foam sclerotherapy, and traditional surgery. Low-to-moderate–quality evidence suggests that 1-year recanalization rates are lower following endovenous ablation therapy than conventional surgery. However, the review could not determine whether ultrasound-guided foam sclerotherapy was superior to traditional surgery.[27]
Achieving optimal outcomes following injection sclerotherapy necessitates a collaborative interprofessional approach that engages in shared decision-making with patients, ensuring alignment with individual preferences, understanding potential risks, and establishing realistic treatment expectations. Effective communication among team members and patients enhances treatment efficacy and patient satisfaction. Clinical staff, including nurses, assistants, and pharmacists, play a critical role in patient education, providing preprocedural guidance and postprocedural care instructions.
An interprofessional approach and shared decision-making ultimately foster patient-centered care, optimizing injection sclerotherapy outcomes. This approach ensures comprehensive evaluation, personalized treatment strategies, and patient empowerment, enhancing treatment adherence, improved outcomes, and overall patient satisfaction.
Media
(Click Image to Enlarge)
References
de Ávila Oliveira R, Riera R, Vasconcelos V, Baptista-Silva JC. Injection sclerotherapy for varicose veins. The Cochrane database of systematic reviews. 2021 Dec 10:12(12):CD001732. doi: 10.1002/14651858.CD001732.pub3. Epub 2021 Dec 10 [PubMed PMID: 34883526]
Level 1 (high-level) evidencePalm MD, Guiha IC, Goldman MP. Foam sclerotherapy for reticular veins and nontruncal varicose veins of the legs: a retrospective review of outcomes and adverse effects. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2010 Jun:36 Suppl 2():1026-33. doi: 10.1111/j.1524-4725.2010.01496.x. Epub [PubMed PMID: 20590709]
Level 2 (mid-level) evidenceGoldman MP. My sclerotherapy technique for telangiectasia and reticular veins. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2010 Jun:36 Suppl 2():1040-5. doi: 10.1111/j.1524-4725.2009.01408.x. Epub [PubMed PMID: 20590711]
Weiss MA, Hsu JT, Neuhaus I, Sadick NS, Duffy DM. Consensus for sclerotherapy. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2014 Dec:40(12):1309-18. doi: 10.1097/DSS.0000000000000225. Epub [PubMed PMID: 25418805]
Level 3 (low-level) evidenceHe A, Chen M. Sclerotherapy in Hemorrhoids. The Indian journal of surgery. 2023 Apr:85(2):228-232. doi: 10.1007/s12262-022-03414-3. Epub 2022 Apr 20 [PubMed PMID: 35469212]
Worthington-Kirsch RL. Injection sclerotherapy. Seminars in interventional radiology. 2005 Sep:22(3):209-17. doi: 10.1055/s-2005-921954. Epub [PubMed PMID: 21326695]
Myers K. A history of injection treatments - II sclerotherapy. Phlebology. 2019 Jun:34(5):303-310. doi: 10.1177/0268355518798283. Epub 2018 Oct 18 [PubMed PMID: 30336757]
Chwała M, Szczeklik W, Szczeklik M, Aleksiejew-Kleszczyński T, Jagielska-Chwała M. Varicose veins of lower extremities, hemodynamics and treatment methods. Advances in clinical and experimental medicine : official organ Wroclaw Medical University. 2015 Jan-Feb:24(1):5-14. doi: 10.17219/acem/31880. Epub [PubMed PMID: 25923081]
Level 3 (low-level) evidenceLorenz MB, Gkogkolou P, Goerge T. Sclerotherapy of varicose veins in dermatology. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology : JDDG. 2014 May:12(5):391-3. doi: 10.1111/ddg.12333. Epub [PubMed PMID: 24797742]
Goldman MP, Weiss RA, Bergan JJ. Diagnosis and treatment of varicose veins: a review. Journal of the American Academy of Dermatology. 1994 Sep:31(3 Pt 1):393-413; quiz 414-6 [PubMed PMID: 8077464]
Andrews RH, Dixon RG. Ambulatory Phlebectomy and Sclerotherapy as Tools for the Treatment of Varicose Veins and Telangiectasias. Seminars in interventional radiology. 2021 Jun:38(2):160-166. doi: 10.1055/s-0041-1727151. Epub 2021 Jun 3 [PubMed PMID: 34108801]
Rao J, Wildemore JK, Goldman MP. Double-blind prospective comparative trial between foamed and liquid polidocanol and sodium tetradecyl sulfate in the treatment of varicose and telangiectatic leg veins. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2005 Jun:31(6):631-5; discussion 635 [PubMed PMID: 15996411]
Level 1 (high-level) evidencePeterson JD, Goldman MP. An investigation of side-effects and efficacy of foam-based sclerotherapy with carbon dioxide or room air in the treatment of reticular leg veins: a pilot study. Phlebology. 2012 Mar:27(2):73-6. doi: 10.1258/phleb.2011.010073. Epub 2011 Sep 16 [PubMed PMID: 21926097]
Level 3 (low-level) evidenceTremaine AM, Friedmann DP, Goldman MP. Foam sclerotherapy for reticular veins of the dorsal hands: a retrospective review. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2014 Aug:40(8):892-8. doi: 10.1097/DSS.0000000000000076. Epub [PubMed PMID: 25022711]
Level 2 (mid-level) evidencePeterson JD, Goldman MP. An investigation into the influence of various gases and concentrations of sclerosants on foam stability. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2011 Jan:37(1):12-7. doi: 10.1111/j.1524-4725.2010.01832.x. Epub 2010 Dec 28 [PubMed PMID: 21199095]
Leach BC, Goldman MP. Comparative trial between sodium tetradecyl sulfate and glycerin in the treatment of telangiectatic leg veins. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2003 Jun:29(6):612-4; discussion 615 [PubMed PMID: 12786704]
Level 1 (high-level) evidenceMcCoy S, Evans A, Spurrier N. Sclerotherapy for leg telangiectasia--a blinded comparative trial of polidocanol and hypertonic saline. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 1999 May:25(5):381-5; discussion 385-6 [PubMed PMID: 10469077]
Level 1 (high-level) evidenceDuffy DM. Sclerosants: a comparative review. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2010 Jun:36 Suppl 2():1010-25. doi: 10.1111/j.1524-4725.2009.01469.x. Epub [PubMed PMID: 20590708]
Level 2 (mid-level) evidenceBarrett JM, Allen B, Ockelford A, Goldman MP. Microfoam ultrasound-guided sclerotherapy of varicose veins in 100 legs. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2004 Jan:30(1):6-12 [PubMed PMID: 14692919]
Level 2 (mid-level) evidenceJoyce DP, De Freitas S, Woo EY, Tang TY, Tubassam M, Walsh SR. Ultrasound-guided foam sclerotherapy as a therapeutic modality in venous ulceration. The surgeon : journal of the Royal Colleges of Surgeons of Edinburgh and Ireland. 2022 Oct:20(5):e206-e213. doi: 10.1016/j.surge.2021.08.008. Epub 2021 Oct 8 [PubMed PMID: 34629303]
Barrett JM, Allen B, Ockelford A, Goldman MP. Microfoam ultrasound-guided sclerotherapy treatment for varicose veins in a subgroup with diameters at the junction of 10 mm or greater compared with a subgroup of less than 10 mm. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2004 Nov:30(11):1386-90 [PubMed PMID: 15522019]
Level 2 (mid-level) evidenceBreu FX, Guggenbichler S, Wollmann JC, Second European Consensus Meeting on Foam Sclerotherapy. Duplex ultrasound and efficacy criteria in foam sclerotherapy from the 2nd European Consensus Meeting on Foam Sclerotherapy 2006, Tegernsee, Germany. VASA. Zeitschrift fur Gefasskrankheiten. 2008 Feb:37(1):90-5 [PubMed PMID: 18512547]
Level 3 (low-level) evidenceWong M, Parsi K, Myers K, De Maeseneer M, Caprini J, Cavezzi A, Connor DE, Davies AH, Gianesini S, Gillet JL, Grondin L, Guex JJ, Hamel-Desnos C, Morrison N, Mosti G, Orrego A, Partsch H, Rabe E, Raymond-Martimbeau P, Schadeck M, Simkin R, Tessari L, Thibault PK, Ulloa JH, Whiteley M, Yamaki T, Zimmet S, Kang M, Vuong S, Yang A, Zhang L. Sclerotherapy of lower limb veins: Indications, contraindications and treatment strategies to prevent complications - A consensus document of the International Union of Phlebology-2023. Phlebology. 2023 May:38(4):205-258. doi: 10.1177/02683555231151350. Epub 2023 Mar 14 [PubMed PMID: 36916540]
Level 3 (low-level) evidenceCarroll C, Hummel S, Leaviss J, Ren S, Stevens JW, Everson-Hock E, Cantrell A, Stevenson M, Michaels J. Clinical effectiveness and cost-effectiveness of minimally invasive techniques to manage varicose veins: a systematic review and economic evaluation. Health technology assessment (Winchester, England). 2013 Oct:17(48):i-xvi, 1-141. doi: 10.3310/hta17480. Epub [PubMed PMID: 24176098]
Level 1 (high-level) evidenceBossart S, Daneluzzi C, Cazzaniga S, Ramelet AA, Uthoff H, Seyed Jafari SM, Baumgartner M, Hunger RE, Heidemeyer K, Willenberg T. Skin hyperpigmentation after sclerotherapy with polidocanol: A systematic review. Journal of the European Academy of Dermatology and Venereology : JEADV. 2023 Feb:37(2):274-283. doi: 10.1111/jdv.18639. Epub 2022 Oct 17 [PubMed PMID: 36196455]
Level 1 (high-level) evidenceYiannakopoulou E. Safety Concerns for Sclerotherapy of Telangiectases, Reticular and Varicose Veins. Pharmacology. 2016:98(1-2):62-9. doi: 10.1159/000445436. Epub 2016 Apr 23 [PubMed PMID: 27104778]
Paravastu SC, Horne M, Dodd PD. Endovenous ablation therapy (laser or radiofrequency) or foam sclerotherapy versus conventional surgical repair for short saphenous varicose veins. The Cochrane database of systematic reviews. 2016 Nov 29:11(11):CD010878 [PubMed PMID: 27898181]
Level 1 (high-level) evidence