Introduction
More than 100 distinct types of cancer have been identified. Cancer often involves multiple organs due to uncontrolled cell division, migration, and invasion. Understanding the pathogenesis of cancer is crucial because of the growing number of cases and the inadequacy of standard treatment options, resulting in poor overall survival and disease-free survival rates for a significant population of patients.[1][2] Despite advances in survival rates in developed countries, the incidence of cancer continues to increase sharply. It is projected that by 2022, over 3 million cancer deaths occur in China, and in the United States, more than 600,000 deaths directly attributed to cancer.[3]
Over 90% of cancers are directly attributable to environmental factors and lifestyle, making them largely preventable. Over the past decade, significant advancements have been made in understanding cancer, including the processes of carcinogenesis (the initiation of cancer), oncogenesis, and the progression of initiated cells to malignant metastasis. Understanding the molecular and cellular mechanisms that drive carcinogenesis is essential for developing effective cancer prevention, diagnosis, and treatment strategies.
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
The epidemiology and history of cancer reveal a complex and evolving landscape of disease that has affected humanity for millennia. From ancient documentation in historical texts to modern epidemiological studies, the understanding of cancer has expanded dramatically. Today, insights into the prevalence, causes, and progression of cancer continue to shape public health strategies and medical advancements, highlighting the importance of historical context in the fight against cancer.
Epidemiology
Lung cancer is projected to become the leading cause of death in both males and females by 2040.[4] According to the current statistics from the Global Cancer Observatory (GLOBOCAN), it is estimated that there will be 28.4 million cancer cases by 2040, an almost 50% increase from 2020.[5] Cancer severity is mainly related to age, with middle-aged individuals having the highest risk of developing a malignant tumor. Heredity accounts for 5% to 10% of cancers and results from pathogenic variations in cancer-predisposing genes.[6] In addition, cancers can arise somatically, where genomic variation occurs de novo and cannot be passed down to offspring.[7] Therefore, understanding the biology of the disease, its pathogenesis, and the mechanisms by which normal cells transform into malignant cells is crucial.
Institutional History
Historical records, such as the Babylonian Code of Hammurabi, the ancient Egyptian Papyrus of Ebers and Smith, the Chinese Rites of the Zhou Dynasty, Medieval Islamic books, and the ancient Indian Ramayana, have documented references to cancer dating back to 1700 BC.[8][9]
In the United States, a definitive and structured initiative to reduce cancer and promote scientific research emerged with the establishment of the National Cancer Institute in 1937 and the signing of the National Cancer Act in 1971.[10]
Scientific Advancement History
Since time immemorial, humankind has been investigating benign and malignant tumors and hypothesizing about the causes of the disease. According to a recent finding by Randolph-Quinney et al, the discovery of osteosarcoma in a hominin foot bone over 1 million years old in South Africa provides evidence that cancer is as old as humanity.[11]
Until the 18th century, various hypotheses about the origins of carcinogenesis ranged from superstitious beliefs to the theory of lymph as the cause of the disease. Percival Pott was the first to identify cancerous growths in the scrotums of individuals exposed to chimney sweeps, providing an early indication of the agents that cause cancer.[12]
The cellular origin of the disease was established in 1858 with Rudolf Virchow's cell theory. Following this, several hypotheses emerged as initiating agents in cancer, including Warburg's theory of enhanced aerobic glycolysis in 1956, Knudson's two-hit hypothesis in 1971, chromosomal aberrations, and somatic mutation theory.
In 1914, Boveri proposed that cancer originated from a single cell and spread to neighboring cells, leading to abnormal proliferation. Building upon this, in 1917, Yamagiwa did a functional study supporting Percival's hypotheses. He was the first to demonstrate the direct relationship between chemicals and cancer occurrence using coal tar, a mixture of different chemicals. Subsequently, scientists began to identify the single chemicals responsible for cancer. Later, Cook et al discovered polycyclic hydrocarbons as activators of cancer-causing chemicals.[11]
The somatic mutation theory gained widespread acceptance as the primary cause of cancer. However, some exceptions emphasize the importance of considering all molecular interactions (EoMI) in understanding carcinogenesis. EoMI encompasses various processes, such as synthetic, mechanical, electrical, concentration, and heat generation–related processes, and should be considered when researching cancer.[13]
Currently, the most widely accepted theory is the multistep theory, which elucidates the events occurring between the corruption of a single cell and the emergence of numerous corrupted cells with the specific goal of metastasis. In addition, the emergence of the hallmarks of cancer has modified the understanding of carcinogenesis as a process, leading to the accumulation of these hallmarks to establish the full picture of cancer.[14][15]
Causes
Cancer typically originates from the transformation of normal cells into malignant tumor cells, often progressing from a precancerous condition to a fully developed tumor. This transition is influenced by an individual's genetics and exposure to 3 main categories of external factors, including:
Physical Carcinogens
- Ultraviolet and ionizing radiation
Chemical Carcinogens
- Polycyclic hydrocarbons
- Asbestos
- Components of tobacco smoke
- Alcohol
- Aflatoxin (a grain contaminant)
- Arsenic (a drinking water contaminant)
Biological Carcinogens
- Infections from certain viruses, such as hepatitis B and Human papillomavirus; bacteria, such as Helicobacter pylori; or parasites, such as Schistosoma haematobium.
Biochemical and Genetic Pathology
Cancer can be understood as a collection of diseases characterized by significant diversity in their molecular signatures, yet they all share common cellular phenotypic characteristics and functional capabilities. Hanahan and Weinberg elucidated these characteristics and identified them as the hallmarks of cancer. These hallmarks encompass increased rates of cell proliferation, achieved through sustained proliferative signaling or evasion of growth suppressors.
In addition, cancer cells exhibit mechanisms to evade destruction or halt their proliferation, such as resistance to cell death, avoidance of immune destruction, and the ability to achieve replicative immortality. Further aiding tumor progression and spread, cancer cells induce or access vasculature and activate invasion and metastasis. Two enabling characteristics facilitate the acquisition of additional traits—genome instability and tumor-promoting inflammation.
Lastly, deregulation of tumor metabolism has emerged as a characteristic feature of cancer cells. Carcinogenesis can be viewed as a series of consequential events involving genetic and epigenetic changes that gradually enable cells to acquire these hallmarks until they reach full cancer status.[16]
The process of carcinogenesis involves multiple events of genetic DNA damage (mutations) and epigenetic gene expression modifications that accumulate sequentially. These changes lead to the gradual acquisition of the hallmarks of cancer, ultimately resulting in fully developed, metastatic cancer.
DNA Damage
The role of DNA damage in carcinogenesis is crucial. The proper functioning of cells relies on the precise regulation of various cellular processes, such as DNA replication and repair. When DNA becomes damaged, it typically triggers repair mechanisms. As DNA is the genetic blueprint for all living organisms, damage can severely impact the affected cell and its future progeny. Consequently, cells possess highly efficient built-in DNA repair systems that prevent harmful mutations.
The 2015 Nobel Prize in Chemistry was awarded to Lindahl and Sancar for their groundbreaking work identifying fundamental DNA repair mechanisms, including mismatch, base excision, and nucleotide excision repair. If DNA damage proves irreparable, the affected cells undergo programmed cell death, also known as apoptosis. In cases where apoptosis fails, the cells activate a third line of defense—the immune system. The immune system can recognize and attack cells with mutated genes at this stage. However, if the immune system falters or is overwhelmed by the mutated cells, these cells persist with the potential to progress to full-blown cancer under favorable conditions.
Recent studies have estimated the rate of mutation accumulation to range from 13 to 44 per genome per year. This rate increases with exposure to environmental factors that induce DNA abnormalities, such as radiation, smoking, pollutants, and alcohol. Endogenous factors, such as abnormalities in DNA repair machinery, hormonal imbalances, and oxidative stress, can also influence mutation accumulation. These studies have revealed that even normal tissues contain clonal expansion of cells carrying carcinogenic driver mutations. These clonal expansions increase with age and exposure to exogenous and endogenous mutagenic factors. Some of these mutated cell clones are more prone to progressing to cancer compared to others, influenced by factors such as the nature of the cancer driver mutation within the clone itself. Beyond cancer, these clones of mutated cells also play a role in other diseases, such as inflammatory and cardiovascular diseases.
DNA damage can occur through endogenous factors, extrinsic factors, or a cumulative effect of both. Endogenous factors include normal metabolic processes, such as oxidative stress, whereas extrinsic factors encompass environmental exposures, such as radiation and toxins.
Endogenous DNA damage: Endogenous factors typically stem from spontaneous disruptions in normal metabolic processes, such as defects in DNA replication and disruptions in cellular signaling, leading to errors. These factors often result from hereditary predispositions. The most prevalent forms typically arise from oxidative and methylation reactions that naturally occur within cells and occasionally coincide with external environmental factors that elevate cancer risk, such as xeroderma pigmentosum.
Normal cellular processes, such as the Fenton reaction, combine hydrogen peroxide (H2O2) with iron to generate highly reactive hydroxyl (OH) free radicals, which can cause severe DNA damage and result in massive mutations. Moreover, DNA damage can occur internally through methylation, typically via physiological methylation reactions facilitated by methyltransferase enzymes utilizing S-adenosylmethionine as a methyl donor. This process leads to the formation of over 4600 methylated guanine and adenine residues, which hinder DNA repair and polymerization. In addition, these residues can disrupt the normal regulation of transcription.[17]
Exogenous DNA damage: Endogenous factors such as chemicals, toxins, radiation, and environmental hazards are common sources of DNA damage and, for the most part, are preventable agents of carcinogenesis. Combinations of chemicals, such as those found in tobacco smoke, industrial processes, and chemotherapy drugs, induce DNA aberrations through alkylation. Alkylating agents incorporate an alkyl group into the guanine base of DNA, disrupting normal protein synthesis and causing mutations. Tobacco smoke, fossil fuels, industrial-grade chemicals such as pesticides, and excessively high-temperature cooking can also produce aromatic amines. These aromatic amines can undergo conversion to alkylating agents, esters, and sulfates, which then attack guanine bases in DNA building blocks, causing damage that may initiate cancer.
Recent studies show that environmental pollution is an integral exogenous source of DNA damage. Extremes of air pollution, notably high levels of particulate matter, have been linked to a significant increase in the incidence of lung cancer. The long-term effects of extreme temperatures caused by global warming also contribute to DNA damage. Environmental pollution now ranks as the second most common cause of cancer, trailing only behind tobacco smoke. Since 2007, deaths from lung cancer have risen by approximately 30%, a trend likely attributed to increased environmental pollution, given the dramatic decrease in tobacco smoking rates. Particulate matter with a diameter of 2.5 µm, consisting of solid and liquid gaseous mixtures, is particularly concerning. Inhaled particles penetrate deep into the terminal bronchioles, where they are absorbed, leading to both local and systemic inflammation. Chronic inflammation can induce significant DNA damage through reactive oxygen species and oxygen free radicals.[18]
Epigenetics
Morphology
Morphologically, a cell undergoing initiation in the carcinogenesis process exhibits noticeable microscopic changes at the cellular level. The initiated cell often appears irregular, with a significant increase in the nuclear-to-cytoplasmic ratio (N/C ratio), meaning the nucleus occupies more of the cell's volume compared to the cytoplasm. This N/O ratio, also referred to as the karyoplasmic ratio, has emerged as a diagnostic parameter for cancer and is closely linked to cancer progression and the likelihood of metastasis.[19]
Mechanisms
Carcinogenesis is a multistep process involving several molecular and cellular events at the genetic and epigenetic levels. This process unfolds through the interplay of environmental, genetic, and metabolic factors. The process of carcinogenesis is generally divided into three main steps, regardless of the specific sequence of genetic or epigenetic events. These steps are initiation, promotion, and progression.
Initiation
Cancer initiation is marked by the onset of mutation, a pivotal event in carcinogenesis. This mutation can be hereditary or acquired. If repair mechanisms or apoptosis fail to rectify the mutation in response to the occurrence of a driver gene mutation, an initiated clone of cells emerges. Thus, the initiation phase begins with irreparable DNA damage. Initiation grants the cell proliferative capabilities that may or may not progress to the promotion stage, depending on factors such as the dose of exposure to an initiator. In addition, initiation is a reversible stage that can be corrected by removing the initiator. Therefore, understanding initiation is crucial for both prophylactic and therapeutic purposes.
Upon exposure to chemical, physical, or biological factors, the cell undergoes an irreversible transformation in its genetic material, potentially evolving into a neoplastic cell clone. The process occurs rapidly, spanning minutes to hours. The initiated cell can persist in this state indefinitely without incurring harm or being detected by the organism's defense systems, as it does not exhibit any discernible phenotypic changes. Sporadic mutations, which constitute approximately 75% of colorectal tumors, are common.
Critical factors in cancer initiation include DNA damage, genetic mutations, and inflammation. However, it is pertinent to note that there may be significant overlap in the mechanism through which genetic alteration, DNA damage, and inflammation contribute to the initiation process.
The mutated cell, known as an initiated cell or initiated clone, subsequently undergoes clonal expansion. This process involves the replication of the mutated cell and its progeny, creating a population of cells with identical genetic alterations.
Following initiation, carcinogenesis includes the promotion and progression stages. Understanding the initiation mechanisms in carcinogenesis is crucial for developing strategies to prevent, detect, and treat cancer. Identifying specific carcinogens and comprehending the genetic alterations associated with initiation have facilitated advances in cancer research and the development of targeted therapies.
Recent research suggests that cell initiation may occur due to epigenetic changes rather than genetic ones.[20] Environmental factors such as exposure to a nonmutagenic carcinogen could trigger these epigenetic events. In such cases, these epigenetic mutations could initiate the first modification in cell properties, leading to the appearance of the first hallmark of cancer. Alternatively, these epigenetic modifications could prime the cells to respond to a genetic mutation that occurs later on. Conversely, the epigenetic mutation could result from a mutation in one of the genes that control epigenetic processes, such as the DNA methyltransferase genes DNMT1 and DNMT3A.[21]
Promotion and Progression
Promotion involves the expansion of initiated cells into a detectable tumor, whereas progression involves acquiring additional genetic changes that render the tumor more aggressive. In addition, progression may include the development of metastatic capabilities, enabling the tumor to spread to distant sites in the body.
The term promotion pertains to the stage of cancer development in which altered or damaged cells, having evaded apoptosis, become immortal and are engineered to acquire all the necessary mutations to proliferate and survive from a single progenitor cell. Promotion is still considered premalignant and reversible. During this stage, the growth and constitutive survival of these transformed cells are stimulated, ultimately forming a discernible tumor.
This intricate process involves various intrinsic and extrinsic factors, including hormones; growth factors, such as transformation growth factor beta; and chronic exposure to certain chemicals or irritants, collectively constituting the tumor microenvironment. These agents facilitate and enhance the carcinogenic process by improving the growth of initiated cells. The promotion stage of carcinogenesis is contingent upon the duration and intensity of exposure to promoting agents, which can accelerate the progression of initiated cells toward distant tissue invasion.
Some events overlap between promotion and progression. Hormones, such as estrogen, can function as stimulatory agents in certain types of cancers. For instance, estrogen promotes the growth of breast tissue and is associated with the promotion and progression of specific breast cancers. The promotion process stimulates cellular proliferation, particularly in cells with acquired genetic alterations that trigger uncontrolled growth and are highly responsive to promoting signals.
Epigenetic modifications, including changes in DNA methylation patterns and histone modifications, can also promote cancer development by influencing the expression of genes involved in regulating the cell cycle and apoptosis, ultimately affecting cell behavior. Furthermore, chronic inflammation can contribute to cancer development by creating a microenvironment rich in growth-promoting signals and factors that support cell survival.
Inflammatory cells release cytokines and growth factors that promote the survival and proliferation of cancer cells. Angiogenesis, the formation of new blood vessels, is often associated with tumor growth during the promotion stage. Tumors rely on a blood supply for their growth and development, and angiogenic factors secreted by tumor cells or surrounding stromal cells stimulate the formation of blood vessels within the tumor. The tumor microenvironment, which encompasses immune cells, fibroblasts, and extracellular matrix components, promotes tumor growth. The interaction and cooperation between tumor cells and their surrounding environment are essential for the survival and proliferation of cancer cells.
Understanding the progression stage is crucial for devising interventions to halt the carcinogenic process. By targeting elements that promote cancer cell growth and disrupting the pathways that support them, it may be feasible to impede or slow the transformation of precancerous cells into malignancies. Moreover, identifying and addressing modifiable risk factors that contribute to cancer progression is essential for cancer prevention and control. In the final stage of carcinogenesis, cells initiated and promoted acquire all the necessary mutations to invade the tissue, marking the onset of invasive cancer.
Clinicopathologic Correlations
Inflammation and mutation are critical factors in the development and progression of cancer. Chronic inflammation, often resulting from persistent infections, autoimmune disorders, or long-term exposure to irritants, creates an environment conducive to DNA damage and cellular changes. Whether inherited or acquired, mutations disrupt normal cellular functions and can lead to uncontrolled cell proliferation. Together, these processes contribute to the initiation and progression of cancer by altering the genetic and epigenetic landscape of affected cells, promoting their survival and aggressive behavior. Understanding the interplay between inflammation and mutation is essential for developing targeted interventions and preventive strategies against cancer.
Inflammation
The link between inflammation and cancer has been recognized since Rudolf Virchow first made the connection in the 19th century. The debate persists—Is inflammation the root cause of cancer, or is cancer the result of ongoing inflammation? Chronic inflammation typically involves cellular damage, leading to cellular proliferation and the activation of repair pathways.
In the early stages of chronic inflammatory conditions, individuals face an increased risk of various types of cancers, particularly in rapidly dividing cells such as those found in the skin, gut, and blood. Chronic inflammation is estimated to contribute to approximately 15% of all cancers globally.
Inflammation can arise from various factors, including microorganisms such as Helicobacter pylori. Chronic infection with H pylori leads to a substantial increase in nitric oxide production, which damages DNA within the nuclei of the gastric mucosa by activating transcriptional regulation through the actions of DNA methyltransferase. In addition, H pylori promotes the proliferation of epithelial cells by stimulating the release of various inflammatory cytokines.[22]
Mutation
Mutations in the gene suppressor of mothers against decapentaplegic 4 (SMAD4), which plays a pivotal role in the transforming growth factor-beta (TGF-β) signaling pathway, are implicated in over 50% of pancreatic adenocarcinomas. Several studies have demonstrated its involvement in both initiation and progression depending on the tumor type.[17] As previously mentioned, SMAD4 is involved in TGF-β signaling, which regulates cellular migration, proliferation, and apoptosis. In cholangiocarcinoma, SMAD4 acts as a tumor initiator and correlates with the clinical severity of disease in over 40% of intrahepatic cholangiocarcinoma. Murine model studies show that SMAD4 facilitates pancreatic cancer progression initiated by KRAS mutations. Similarly, significant evidence indicates that SMAD4 facilitates colorectal cancer progression in adenomatous polyposis coli–mutated cancers (adenomatous polyposis coli initiated).
The TP53 gene on chromosome 17p13 is often called the guardian of the cell due to its crucial role in preventing aberrant proliferation. TP53 is responsible for expressing the P53 protein, one of the most elucidated proteins in carcinogenesis. The role of P53 in initiation, promotion, or progression varies depending on the context. P53 plays a significant role in expressing genes responsible for DNA repairs, apoptosis, and cell cycle arrest. In breast and ovarian cancers expressing cytokeratins, commonly known as basal cancers, the TP53 gene plays a vital role in initiation. Germline P53 mutations are also heavily involved in tumor initiation. Li Fraumeni syndrome, inherited in an autosomal dominant manner, involves numerous carcinomas, early-onset breast cancers, and sarcomas.[23]
Clinical Significance
Carcinogenesis refers to the process of transforming normal cells into cancerous ones, which holds significant clinical implications. It is clinically significant due to its profound impact on human health, including diagnosis, treatment, and prevention of cancer. Understanding the mechanisms and stages of carcinogenesis is vital for healthcare professionals across various fields, including oncology, pathology, pharmacy, family medicine, and public health. Below are some essential clinical significances of carcinogenesis.
Early Detection and Diagnosis
Understanding carcinogenesis enables the identification of risk factors and the detection of early warning signs for cancer. This knowledge also facilitates the development of screening tests and diagnostic biomarkers for early detection. In clinical practice, imaging techniques such as mammography, colonoscopy, and Pap smears identify early-stage tumors before progressing to advanced stages.
Treatment Selection and Personalized Medicine
Molecular profiling empowers clinicians to identify specific genetic abnormalities involved in cancer development. This knowledge is crucial for selecting targeted therapies that disrupt essential tumor growth and survival pathways. Personalized medicine strategies aim to customize treatments based on the unique genetic characteristics of individual tumors, thereby improving treatment efficacy and minimizing potential adverse effects.
Prognostic Stratification
In clinical practice, clinicians can classify patients into distinct risk categories by assessing the molecular traits of tumors influenced by carcinogenesis. Healthcare providers can obtain crucial prognostic insights by detecting biomarkers associated with different carcinogenesis stages. These insights can then inform well-informed therapeutic decisions and the development of appropriate post-treatment care plans.
Prevention and Risk Reduction
Comprehending carcinogenesis is paramount in developing effective public health strategies to minimize cancer risk. By targeting modifiable risk factors, such as smoking cessation; adopting a balanced diet; and maintaining regular physical activity, the likelihood of carcinogenesis and subsequent cancer development can be notably diminished. In addition, vaccination against cancer-causing viruses, such as the human papillomavirus and hepatitis B virus, which play a role in carcinogenesis, stands as a critical component of cancer prevention efforts.
Development of Therapeutic Agents
Understanding the critical steps of carcinogenesis is essential for innovating new therapeutic agents that target fundamental pathways involved in tumor initiation, promotion, and progression. Agents, such as selective estrogen receptor modulators, are engineered to disrupt precise phases of the carcinogenic process, consequently reducing the probability of cancer formation.
A promising avenue in cancer research involves the development of cancer vaccines. Researchers are currently exploring an E75 peptide vaccine, also known as nelipepimut-S, as a prospective treatment for breast cancer. This vaccine utilizes a nine-amino acid peptide derived from the extracellular domain of the human epidermal growth factor receptor 2 (HER2) protein, effectively arresting tumor development at the promotion stage.[24]
Monitoring Disease Progression and Recurrence
Monitoring molecular changes during carcinogenesis enables clinicians to track the disease progression and evaluate treatment effectiveness. Assessing biomarkers for residual or minimal residual disease following treatment offers valuable insights for monitoring recurrence and shaping post-treatment surveillance plans. Understanding the clinical implications of carcinogenesis is crucial for advancing cancer diagnosis, treatment, and prevention methods. By deciphering the complex molecular mechanisms involved in carcinogenesis, healthcare providers can develop tailored interventions that effectively minimize the impact of cancer on individuals and communities.
Media
(Click Image to Enlarge)
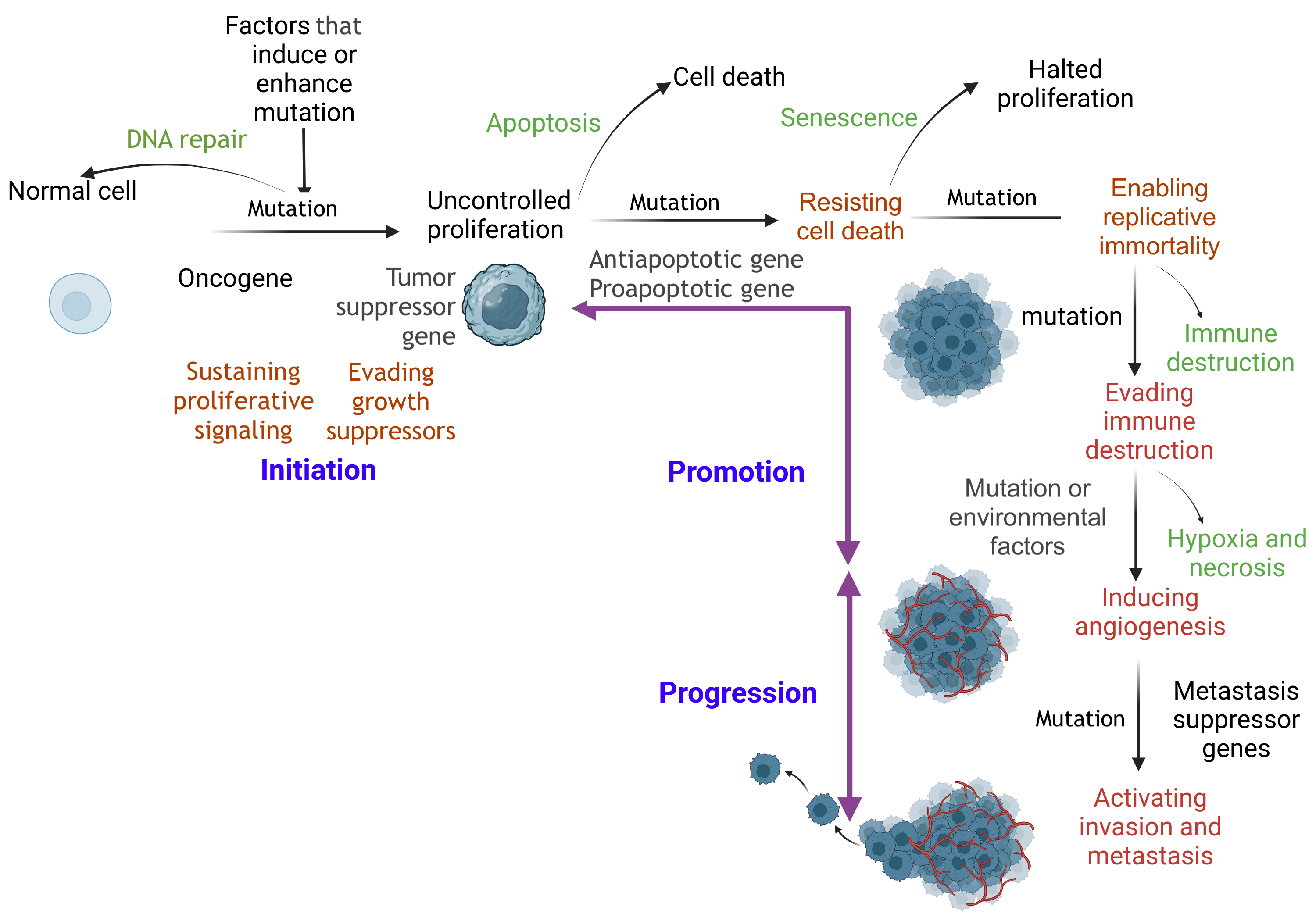
Carcinogenesis Pathway. Prolonged disruption of physiological cellular function can damage DNA. A damaged cell attempts DNA repair, such as base excision repair, nucleotide excision repair, mismatch repair, homologous recombination, and nonhomologous end joining. If repair fails, programmed cell death, also known as apoptosis, is typically initiated to eliminate the corrupt cell without inflammation and prevent the progression of carcinogenesis. Defective DNA repair and apoptosis are hallmarks of the initiation state. An initiated cell evades tumor suppressor genes and activates constitutional proliferation (auto-proliferation). Subsequently, the initiated cell is promoted through the acquisition of additional mutations. This mutation enables the cell to be immortal by skipping the normal immune checkpoints. Additional mutations are acquired, facilitating growth by creating a self-nutritional supply, eventually resulting in progression to distant tissue invasion (metastasis).
SA Ibrahim, MBBCh, MSc, PhD
References
Huang Y, Lu H, Li H. DNA methyltransferase 3a-induced hypermethylation of the fructose-1,6-bisphosphatase-2 promoter contributes to gastric carcinogenesis. Molecular biology reports. 2024 Jan 6:51(1):78. doi: 10.1007/s11033-023-08966-5. Epub 2024 Jan 6 [PubMed PMID: 38183507]
Delgado A, Guddati AK. Clinical endpoints in oncology - a primer. American journal of cancer research. 2021:11(4):1121-1131 [PubMed PMID: 33948349]
Martin DL. Developing nursing administration in Kyrgyzstan. The Kansas nurse. 1996 Jun-Jul:71(6):3-4 [PubMed PMID: 8920425]
Rahib L, Wehner MR, Matrisian LM, Nead KT. Estimated Projection of US Cancer Incidence and Death to 2040. JAMA network open. 2021 Apr 1:4(4):e214708. doi: 10.1001/jamanetworkopen.2021.4708. Epub 2021 Apr 1 [PubMed PMID: 33825840]
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians. 2021 May:71(3):209-249. doi: 10.3322/caac.21660. Epub 2021 Feb 4 [PubMed PMID: 33538338]
Hart SN, Polley EC, Yussuf A, Yadav S, Goldgar DE, Hu C, LaDuca H, Smith LP, Fujimoto J, Li S, Couch FJ, Dolinsky JS. Mutation prevalence tables for hereditary cancer derived from multigene panel testing. Human mutation. 2020 Aug:41(8):e1-e6. doi: 10.1002/humu.24053. Epub 2020 Jul 9 [PubMed PMID: 32442341]
Qing T, Mohsen H, Marczyk M, Ye Y, O'Meara T, Zhao H, Townsend JP, Gerstein M, Hatzis C, Kluger Y, Pusztai L. Germline variant burden in cancer genes correlates with age at diagnosis and somatic mutation burden. Nature communications. 2020 May 15:11(1):2438. doi: 10.1038/s41467-020-16293-7. Epub 2020 May 15 [PubMed PMID: 32415133]
Level 2 (mid-level) evidenceJavier RT, Butel JS. The history of tumor virology. Cancer research. 2008 Oct 1:68(19):7693-706. doi: 10.1158/0008-5472.CAN-08-3301. Epub [PubMed PMID: 18829521]
Hajdu SI. A note from history: landmarks in history of cancer, part 1. Cancer. 2011 Mar 1:117(5):1097-102. doi: 10.1002/cncr.25553. Epub 2010 Oct 19 [PubMed PMID: 20960499]
Parascandola M, Pearlman PC, Eldridge L, Gopal S. The Development of Global Cancer Research at the United States National Cancer Institute. Journal of the National Cancer Institute. 2022 Sep 9:114(9):1228-1237. doi: 10.1093/jnci/djac104. Epub [PubMed PMID: 35640108]
Peters JM, Gonzalez FJ. The Evolution of Carcinogenesis. Toxicological sciences : an official journal of the Society of Toxicology. 2018 Oct 1:165(2):272-276. doi: 10.1093/toxsci/kfy184. Epub [PubMed PMID: 30629266]
Lipsick J. A History of Cancer Research: Carcinogens and Mutagens. Cold Spring Harbor perspectives in medicine. 2021 Mar 1:11(3):. doi: 10.1101/cshperspect.a035857. Epub 2021 Mar 1 [PubMed PMID: 33649023]
Level 3 (low-level) evidenceHanselmann RG, Welter C. Origin of Cancer: Cell work is the Key to Understanding Cancer Initiation and Progression. Frontiers in cell and developmental biology. 2022:10():787995. doi: 10.3389/fcell.2022.787995. Epub 2022 Mar 1 [PubMed PMID: 35300431]
Level 3 (low-level) evidenceHanahan D. Hallmarks of Cancer: New Dimensions. Cancer discovery. 2022 Jan:12(1):31-46. doi: 10.1158/2159-8290.CD-21-1059. Epub [PubMed PMID: 35022204]
Loh JJ, Ma S. Hallmarks of cancer stemness. Cell stem cell. 2024 May 2:31(5):617-639. doi: 10.1016/j.stem.2024.04.004. Epub [PubMed PMID: 38701757]
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011 Mar 4:144(5):646-74. doi: 10.1016/j.cell.2011.02.013. Epub [PubMed PMID: 21376230]
Level 3 (low-level) evidenceChatterjee N, Walker GC. Mechanisms of DNA damage, repair, and mutagenesis. Environmental and molecular mutagenesis. 2017 Jun:58(5):235-263. doi: 10.1002/em.22087. Epub 2017 May 9 [PubMed PMID: 28485537]
Berg CD, Schiller JH, Boffetta P, Cai J, Connolly C, Kerpel-Fronius A, Kitts AB, Lam DCL, Mohan A, Myers R, Suri T, Tammemagi MC, Yang D, Lam S, International Association for the Study of Lung Cancer (IASLC) Early Detection and Screening Committee. Air Pollution and Lung Cancer: A Review by International Association for the Study of Lung Cancer Early Detection and Screening Committee. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2023 Oct:18(10):1277-1289. doi: 10.1016/j.jtho.2023.05.024. Epub 2023 Jun 3 [PubMed PMID: 37277094]
Schirmer EC, Latonen L, Tollis S. Nuclear size rectification: A potential new therapeutic approach to reduce metastasis in cancer. Frontiers in cell and developmental biology. 2022:10():1022723. doi: 10.3389/fcell.2022.1022723. Epub 2022 Oct 10 [PubMed PMID: 36299481]
Bhartiya D, Raouf S, Pansare K, Tripathi A, Tripathi A. Initiation of Cancer: The Journey From Mutations in Somatic Cells to Epigenetic Changes in Tissue-resident VSELs. Stem cell reviews and reports. 2024 May:20(4):857-880. doi: 10.1007/s12015-024-10694-7. Epub 2024 Mar 8 [PubMed PMID: 38457060]
You JS, Jones PA. Cancer genetics and epigenetics: two sides of the same coin? Cancer cell. 2012 Jul 10:22(1):9-20. doi: 10.1016/j.ccr.2012.06.008. Epub [PubMed PMID: 22789535]
Khandia R, Munjal A. Interplay between inflammation and cancer. Advances in protein chemistry and structural biology. 2020:119():199-245. doi: 10.1016/bs.apcsb.2019.09.004. Epub 2019 Nov 26 [PubMed PMID: 31997769]
Level 3 (low-level) evidenceMalkin D. The role of p53 in human cancer. Journal of neuro-oncology. 2001 Feb:51(3):231-43 [PubMed PMID: 11407595]
Lau KH, Tan AM, Shi Y. New and Emerging Targeted Therapies for Advanced Breast Cancer. International journal of molecular sciences. 2022 Feb 18:23(4):. doi: 10.3390/ijms23042288. Epub 2022 Feb 18 [PubMed PMID: 35216405]