Introduction
Chronic venous obstruction (CVO) may arise from postthrombotic venous occlusion, acute traumatic or iatrogenic venous injuries, or in patients who have undergone removal of primary or metastatic malignant neoplasms invading the inferior vena cava (IVC) or iliac veins.[1] Recent advancements have been made in the treatment of chronic iliofemoral or iliocaval obstruction employing endovascular methods.[2] Endovascular venous stenting is currently the primary treatment option for benign occlusions of the IVC, iliac, iliofemoral, and iliocaval veins in patients for whom conservative therapy has failed.[3] However, patients who experience severe symptoms of CVO but are not suitable for or do not respond to endovascular interventions may be candidates for surgical venous reconstruction.[4]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Chronic venous insufficiency (CVI) presents with a constellation of symptoms, including leg edema, skin changes, and ulceration precipitated by impaired lower extremity venous drainage and venous forward flow.[5] Venous hypertension and functional abnormalities in the venous system, such as intrinsic valvular dysfunction and inadequate collateral venous circulation, are among the most common etiologies of CVI.[6] Risk factors for CVI may be modifiable or nonmodifiable. Nonmodifiable risk factors include the female sex, age younger than 50, and a family history of venous disease. Modifiable risk factors include obesity, smoking, occupation, pregnancy, and combined oral contraceptive (COC) use. [7]
Contrarily, the symptoms of CVO are caused by a mechanical blockage or obstruction rather than intrinsic vein wall or valvular insufficiency.[8] There are congenital, primary, and secondary etiologies of CVO.[1] Congenital venous anomalies include deep vein agenesis or hypoplasia.[9] Primary CVO originates from intrinsic structural and biochemical vein wall changes without a precipitating event.[10] Lastly, secondary CVO develops after an identifiable and precipitating event, such as acute deep venous thrombosis (DVT), blunt or penetrating accidental or iatrogenic trauma, benign or malignant neoplasms, or intervention or instrumentation such as IVC filter or central venous catheter placements.[10]
Indications
Presenting Symptoms
CVO presents with venous claudication consistent with exercise-induced limb swelling and pain relieved with leg elevation and rest.[6] Symptoms such as edema, varicosity, lipodermatosclerosis, eczema, and ulceration resemble those seen in CVI.[11] However, CVO presents with more pronounced claudication, edema, and relief with limb elevation or rest tends to be less effective.[12] CVO may lead to compromised limb viability depending on the extent of collateral circulation.[12]
History
A comprehensive medical history can often distinguish among congenital, primary, or secondary venous disorders resulting in CVO. Questioning should include a personal or family history of thrombophilia or venous disorders, previous DVT or thrombophlebitis, traumatic or iatrogenic injuries, obstetric history including the number of pregnancies, personal history of benign or malignant neoplasm, medication history, past abdominopelvic surgical or endovascular interventions, and social history involving smoking, illicit drug use, and occupation performed.
Physical Examination
General physical examination findings of CVO include lower extremity edema, varicose veins even when supine, skin hyperpigmentation, eczema, lipodermatosclerosis, and ulceration. Observation of the laterality of these findings is essential, as laterality indicates the extent of iliofemoral, caval, or systemic disease. An abdominal and inguinal examination should be performed to identify sources of extrinsic venous compression and outflow obstruction, such as an abdominal mass or lymphadenopathy. Signs of pelvic venous congestion, such as perineal, vulvar, or groin varicosities, should raise suspicion for iliac, internal iliac, and gonadal vein incompetence or obstruction.[13] Collateral vessels are often a result of outflow obstruction. Scrotal varicosities may also indicate gonadal vein incompetence secondary to nutcracker anatomy involving the compression of the left renal vein between the superior mesenteric artery and the aorta, caval lesions, or a neoplasm such as renal cell carcinoma invading the left renal vein.
Contraindications
Contraindications to surgical venous reconstruction include severe malnutrition, an immunocompromised state, uncorrected coagulopathy, pregnancy, and active infections at the surgical site. Surgical venous reconstruction is also contraindicated in medically unstable patients.
Equipment
Endovascular Procedures
The equipment required for endovascular treatment of venous obstruction includes that which is typically used in most endovascular procedures.
- Portable ultrasound unit to guide vascular access
- Access and working sheaths
- Guide wires
- Diagnostic catheters
- Contrast material
- Interventional devices, including angioplasty balloons, stents, and thrombectomy devices
- Intravascular ultrasound (IVUS) device
- Fluoroscopy unit, either mobile (C-arm) or fixed (ceiling- or floor-mounted)
- Personal protective equipment (PPE) for radiation exposure: lead apron, vest, gloves, thyroid collar, and safety goggles
Surgical Procedures
The equipment required for surgical venous reconstruction techniques includes that which is typically found in a basic or a major vascular instrument tray. Instruments that are specific for venous reconstruction include:
- Metzenbaum scissors
- Weitlaner retractor
- DeBakey and Gerald forceps
- Vessel loops
- DeBakey and Bulldog clamps
- Castro-Viejo needle driver
- Nerve hook
- Synthetic bypass graft, for use in cases where autologous vein graft is not suitable or available
Personnel
Essential personnel required for endovascular or surgical venous procedures typically includes:
- Interventionalist (vascular surgeon, interventional cardiologist, interventional radiologist)
- First assistant
- Anesthesia provider
- Circulating or operating room nurse
- Surgical technician or operating room nurse
Preparation
Venous duplex ultrasound scanning (VDUS) is the preferred initial diagnostic test for evaluating all patients with CVI or CVO, as it is safe, noninvasive, and inexpensive.[14] VDUS can define the location, cause, and severity of infrainguinal venous obstruction and valvular incompetence by measuring venous visibility, compressibility, flow, and augmentation.[15] However, evaluation of the proximal iliac veins and IVC is limited mainly by body habitus and overlying bowel gas.[15] In cases where VDUS is normal or nondiagnostic but a high clinical suspicion for CVI or CVO remains, adjunctive plethysmography has proven to add diagnostic value by evaluating reflux, obstruction, and calf muscle pump function.[16]When obstruction in the IVC or proximal iliac veins is suspected and is not well visualized with VDUS, computed tomographic venography (CTV) and magnetic resonance venography (MRV) can provide information about intrinsic venous anatomy, obstruction, or stenosis and identify extrinsic venous obstruction or compression such as neoplasm or May-Thurner syndrome.[17][18][19]
Although invasive and costly, intravascular ultrasonography (IVUS) is considered by many to be the gold standard for the assessment and diagnosis of CVO.[1] IVUS employs an ultrasound probe attached to the tip of an endovascular catheter, providing an intraluminal view of the vein and allowing better characterization of the lesion of interest.[20] Furthermore, recent studies have shown that IVUS is superior to venography in identifying clinically significant lesions undetected by other diagnostic modalities.[21]
In select patients undergoing surgical treatment, ascending and descending contrast venography can preoperatively map the superficial and deep veins.[22] Descending venography is the definitive test for evaluating valvular incompetence and differentiating between primary valve incompetence, postthrombotic valve incompetence, and valve aplasia.[23] Descending venography is performed by simultaneous injection of contrast into the proximal venous system and a Valsalva maneuver to increase venous pressure. Ascending venography can define the site of venous obstruction and image collateral venous circulation.[24]Ascending venography is performed by injecting contrast in the distal venous system to map the venous drainage of a limb.
Ancillary testing, including ambulatory venous and arm-foot pressure measurements, can quantify venous hypertension. Some clinicians have defined a resting arm-foot pressure differential greater than 4 mm Hg as sufficient evidence to warrant venous reconstruction.[25] Preoperative invasive femoral and central venous pressure measurements are also used to determine the degree of venous hypertension, where a difference greater than 5 mm Hg is considered a hemodynamically significant stenosis or obstruction.[26]
Technique or Treatment
Percutaneous Venous Stenting
Since the development of endovascular techniques, percutaneous venous stenting has quickly become the standard care for caval, iliocaval, and iliofemoral venous obstructions refractory to conservative therapy.[27] Additionally, percutaneous venous stenting has dramatically broadened the therapeutic options for patients with CVI or CVO who would otherwise be considered poor surgical candidates for traditional open surgical repairs.[3] Open surgical venous reconstruction is considered in select cases where endovascular intervention failed or was deemed inappropriate or when excision of malignant tumors invading the IVC or iliofemoral veins was performed. Determinants of candidacy for surgical venous reconstruction include but are not limited to location and length of venous occlusion, nature of extrinsic compression, age of the thrombus or obstruction, and evidence of underlying malignant disease.
Sapheno-Popliteal Bypass, or the May-Husni Procedure
Femoral and proximal popliteal vein obstructions that do not involve the saphenofemoral junction (SFJ) are rare; however, if these obstructions are present, they can be surgically treated with a saphenopopliteal bypass, known as the May-Husni Procedure. Popularized by May and Husni, the saphenopopliteal bypass consists of an end-to-side anastomosis between the ipsilateral distal great saphenous vein (GSV) and the distal popliteal vein.[28][29] Venous drainage from the lower extremity is shunted from the distal popliteal vein into the newly created GSV-popliteal vein anastomosis. Venous blood is returned to the central venous system via the native SFJ, bypassing the obstructed vein. If the ipsilateral GSV is inadequate for the procedure, the contralateral GSV or a cadaveric vein can be used as a conduit. Artificial conduits like polytetrafluoroethylene (PTFE) should be avoided when bypassing the knee joint due to inherent decreased patency rates.
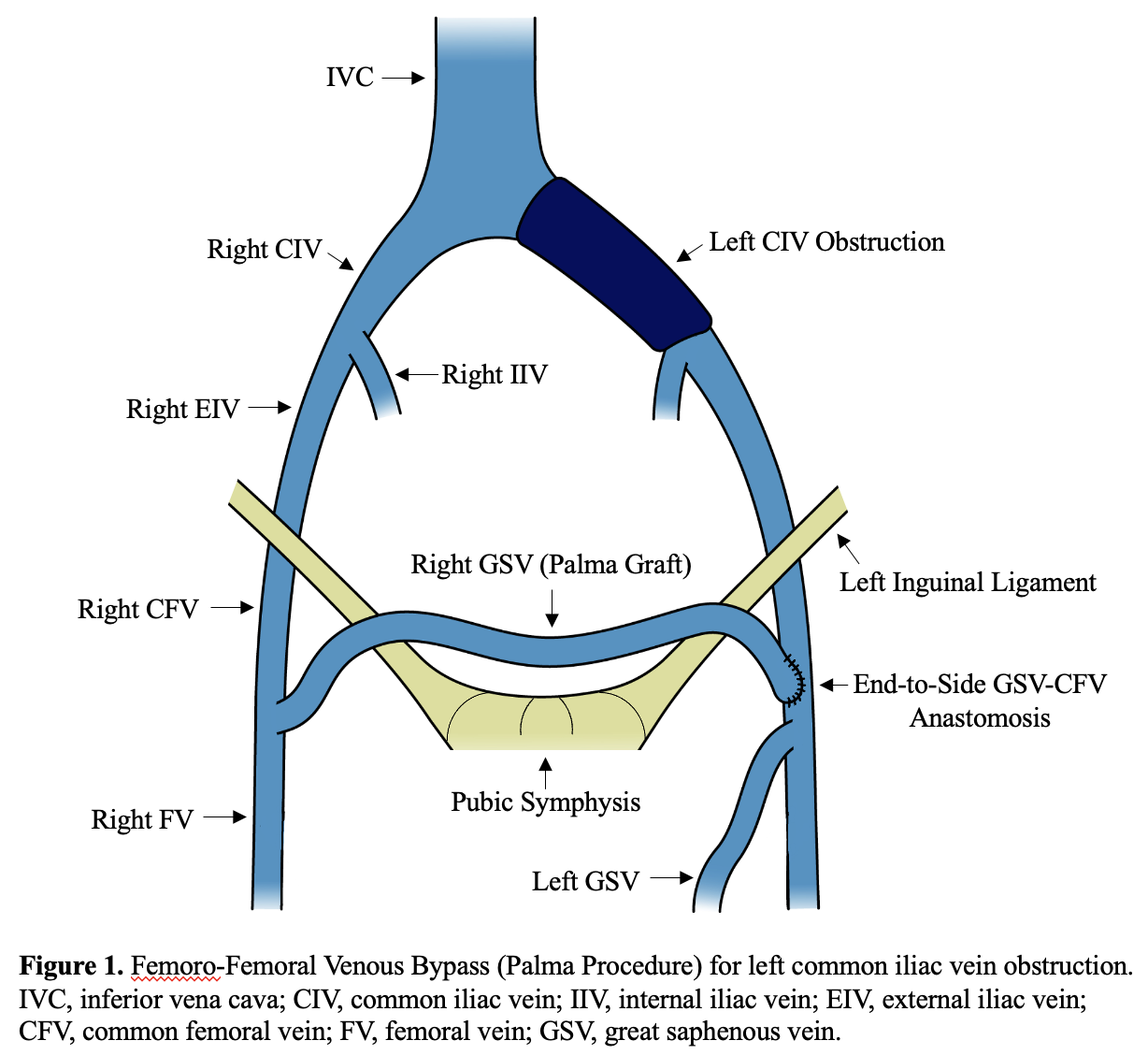
Femoro-femoral Venous Bypass, or the Palma Procedure
Unilateral iliofemoral venous obstructions with intact contralateral iliofemoral venous drainage can be surgically treated with a femoro-femoral venous bypass known as the Palma procedure (see Image. Femoro-femoral Venous Bypass). Initially described by Palma and later popularized by Dale, the femoro-femoral venous bypass employs the contralateral GSV as a conduit between the contralateral femoral and the ipsilateral femoral veins.[30] Effectively, venous return from the affected limb is redirected to the central venous system via the contralateral iliofemoral veins.
Preoperative contralateral GSV ultrasound scanning is performed to determine the suitability of the GSV to be used as a conduit. GSV varicosity or diameter <4 mm has been associated with decreased long-term conduit patency. Generally, a 25- to 30-cm conduit length is sufficient for this procedure. Endoscopic harvest provides the best cosmetic result but carries an increased risk of vein wall damage that can lead to decreased long-term conduit patency. Alternatively, open surgical harvest can be performed via 2 or 3 short skin incisions along the medial aspect of the thigh. Preoperative and intraoperative ultrasound scanning can guide the placement of these skin incisions.
The procedure begins with bilateral 3- to 5-cm incisions in the groin crease medial to the femoral pulses. The contralateral GSV, SFJ, and anterior portion of the common femoral vein (CFV) wall are thoroughly dissected to ensure no kinking or buckling of the GSV during later suprapubic tunneling. A second or third short incision is made in the distal thigh and calf to dissect the GSV to the desired length. GSV tributaries are ligated and divided during this process. Once the adequate length is achieved, the GSV is ligated distally and transected just proximal to the ligature. The fully mobilized GSV is gently pulled back through the subcutaneous tissue and brought out through the groin incision.
Next, a dissection of the ipsilateral CFV is performed. Dissection should be minimal and aim to expose only the anterior and medial aspects of the CFV wall while preserving as many tributaries as possible. The contralateral GSV is then gently tunneled in the suprapubic space and brought out through the groin incision on the affected side. To avoid thrombosis during the creation of the anastomosis, a small catheter can be placed in a tributary vein of the ipsilateral GSV to allow for an immediate injection of low-dose heparin before clamping the CFV. The ipsilateral CFV is then cross-clamped with either proximal and distal clamps or a side-biting clamp, a longitudinal 2-cm venotomy is performed, and an end-to-side contralateral GSV to ipsilateral CFV anastomosis is created in the standard fashion with a running 6-0 polypropylene suture.
In cases where significant contralateral GSV kinks are anticipated or noted during suprapubic tunneling, a free vein graft can be used by completely excising the contralateral GSV and reimplanting it to the proximal CFV. Other autogenous graft options include ipsilateral GSV or arm veins. If no suitable autogenous grafts are available, an externally supported 8- to 10-mm PTFE graft would be the next best alternative. When utilizing free vein or PTFE grafts, standard suprapubic tunneling is performed but will require a lateral end-to-side graft to CFV anastomosis and a routine temporary arteriovenous fistula (AVF) creation between the ipsilateral femoral artery and femoral vein to increase graft flow and improve patency rates.
Novel Valvular Repair Techniques
Management of CVI due to venous obstruction and poor calf muscle function has well-described and efficient therapeutic modalities. CVI, due to valvular incompetence, on the other hand, remains a therapeutic challenge. Novel techniques have been described using devices composed of a porcine aortic valve sewn onto a stainless-steel frame. These devices are surgically implanted into peripheral veins and have shown early clinical success in managing CVI due to valvular incompetence.[31]
Complications
Endovascular Procedures
Endovascular procedures are frequently the first intervention offered to patients with CVO, as they are less invasive and safer than open surgical procedures. Nonetheless, these procedures have unique complications that interventionalists must be aware of and prepared to manage.
Specific endovascular procedure-related complications include:
- Bleeding and hematoma at the venous access site
- Vein rupture secondary to angioplasty
- Vein perforation secondary to instrumentation
- Venous thrombosis
- Stent migration or thrombosis
- Arteriovenous fistula formation
Surgical Procedures
Complications from surgical venous reconstruction procedures are comparable to those seen in most open surgical interventions. These include bleeding, surgical site infection, and iatrogenic injury to adjacent structures.
Clinical Significance
Historically, CVI and CVO have been successfully managed with the conservative interventions of compression therapy and exercise or invasive surgical interventions. However, endovascular techniques have quickly become the mainstay treatment for CVI and CVO, and surgical management is now reserved for select patients who failed conservative or endovascular management. Although surgical management is offered less frequently, clinicians must maintain expertise in surgical options to provide comprehensive patient care.
Enhancing Healthcare Team Outcomes
Given the intrinsic pathological and therapeutic complexity of CVI and CVO, an interprofessional approach is integral to effectively treating patients with these conditions.[32] Clinical suspicion of CVI and CVO begins during routine health evaluation by primary care practitioners. It is imperative to build a multidisciplinary team consisting of a healthcare provider for the overall management of patients and prescription of medications, a specialized wound care team for tailored wound management, a social worker to aid in the coordination of visiting nurse services in the outpatient setting, and a physical therapist to ensure continued patient mobilization and activity. Radiology technologists and physicians are essential during the evaluation phase. Various physicians, including interventional cardiologists, radiologists, and vascular surgeons, can perform endovascular and office-based interventions. Lastly, a vascular surgeon can provide therapeutic options for patients needing surgical interventions.
An integrated, evidence-based approach among multidisciplinary healthcare professionals has been proven to improve patient outcomes, quality of life, and treatment cost-effectiveness.[33]
Media
(Click Image to Enlarge)
References
Harris M, Lim CS. Chronic venous outflow obstruction: An important cause of chronic venous disease. Cleveland Clinic journal of medicine. 2021 Dec 2:88(12):680-688. doi: 10.3949/ccjm.88a.21068. Epub 2021 Dec 2 [PubMed PMID: 34857606]
Lichtenberg M, Jalaie H. [Endovascular Therapy of Chronic Venous Outflow Obstruction]. Zentralblatt fur Chirurgie. 2017 Oct:142(5):481-486. doi: 10.1055/s-0043-119999. Epub 2017 Oct 27 [PubMed PMID: 29078243]
Taha MAH, Busuttil A, Bootun R, Thabet BAH, Badawy AEH, Hassan HA, Shalhoub J, Davies AH. A clinical guide to deep venous stenting for chronic iliofemoral venous obstruction. Journal of vascular surgery. Venous and lymphatic disorders. 2022 Jan:10(1):258-266.e1. doi: 10.1016/j.jvsv.2020.12.087. Epub 2021 May 18 [PubMed PMID: 34020107]
Wittens C, Davies AH, Bækgaard N, Broholm R, Cavezzi A, Chastanet S, de Wolf M, Eggen C, Giannoukas A, Gohel M, Kakkos S, Lawson J, Noppeney T, Onida S, Pittaluga P, Thomis S, Toonder I, Vuylsteke M, Esvs Guidelines Committee, Kolh P, de Borst GJ, Chakfé N, Debus S, Hinchliffe R, Koncar I, Lindholt J, de Ceniga MV, Vermassen F, Verzini F, Document Reviewers, De Maeseneer MG, Blomgren L, Hartung O, Kalodiki E, Korten E, Lugli M, Naylor R, Nicolini P, Rosales A. Editor's Choice - Management of Chronic Venous Disease: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2015 Jun:49(6):678-737. doi: 10.1016/j.ejvs.2015.02.007. Epub 2015 Apr 25 [PubMed PMID: 25920631]
Azar J, Rao A, Oropallo A. Chronic venous insufficiency: a comprehensive review of management. Journal of wound care. 2022 Jun 2:31(6):510-519. doi: 10.12968/jowc.2022.31.6.510. Epub [PubMed PMID: 35678787]
Raffetto JD, Mannello F. Pathophysiology of chronic venous disease. International angiology : a journal of the International Union of Angiology. 2014 Jun:33(3):212-21 [PubMed PMID: 24755829]
Level 3 (low-level) evidenceSermsathanasawadi N, Pruekprasert K, Pitaksantayothin W, Chinsakchai K, Wongwanit C, Ruangsetakit C, Mutirangura P. Prevalence, risk factors, and evaluation of iliocaval obstruction in advanced chronic venous insufficiency. Journal of vascular surgery. Venous and lymphatic disorders. 2019 May:7(3):441-447. doi: 10.1016/j.jvsv.2018.10.021. Epub 2019 Feb 11 [PubMed PMID: 30765330]
Salem AM, AbdelAzeem AboElNeel H, Fakhr ME. Long-term outcome of dedicated venous stents in management of chronic iliofemoral obstruction. Journal of vascular surgery. Venous and lymphatic disorders. 2022 Jan:10(1):52-59. doi: 10.1016/j.jvsv.2021.04.018. Epub 2021 May 18 [PubMed PMID: 34020109]
Pichon M, Hij A, Wifaq B, Abderrahmane M, El Jarrari M, Menn AM. [Deep venous thrombosis caused by congenital inferior vena cava agenesis]. Journal de medecine vasculaire. 2019 Feb:44(1):79-85. doi: 10.1016/j.jdmv.2018.11.005. Epub 2018 Dec 19 [PubMed PMID: 30770086]
Chopard R, Albertsen IE, Piazza G. Diagnosis and Treatment of Lower Extremity Venous Thromboembolism: A Review. JAMA. 2020 Nov 3:324(17):1765-1776. doi: 10.1001/jama.2020.17272. Epub [PubMed PMID: 33141212]
Comerota A, Lurie F. Pathogenesis of venous ulcer. Seminars in vascular surgery. 2015 Mar:28(1):6-14. doi: 10.1053/j.semvascsurg.2015.07.003. Epub 2015 Jul 17 [PubMed PMID: 26358304]
Lichtenberg M, de Graaf R, Erbel C. Standards for recanalisation of chronic venous outflow obstructions. VASA. Zeitschrift fur Gefasskrankheiten. 2018 Jun:47(4):259-266. doi: 10.1024/0301-1526/a000696. Epub 2018 Mar 8 [PubMed PMID: 29514591]
Bałabuszek K, Toborek M, Pietura R. Comprehensive overview of the venous disorder known as pelvic congestion syndrome. Annals of medicine. 2022 Dec:54(1):22-36. doi: 10.1080/07853890.2021.2014556. Epub [PubMed PMID: 34935563]
Level 3 (low-level) evidenceYoun YJ, Lee J. Chronic venous insufficiency and varicose veins of the lower extremities. The Korean journal of internal medicine. 2019 Mar:34(2):269-283. doi: 10.3904/kjim.2018.230. Epub 2018 Oct 26 [PubMed PMID: 30360023]
Needleman L, Cronan JJ, Lilly MP, Merli GJ, Adhikari S, Hertzberg BS, DeJong MR, Streiff MB, Meissner MH. Ultrasound for Lower Extremity Deep Venous Thrombosis: Multidisciplinary Recommendations From the Society of Radiologists in Ultrasound Consensus Conference. Circulation. 2018 Apr 3:137(14):1505-1515. doi: 10.1161/CIRCULATIONAHA.117.030687. Epub [PubMed PMID: 29610129]
Level 3 (low-level) evidenceSuehiro K, Morikage N, Ueda K, Samura M, Takeuchi Y, Nagase T, Mizoguchi T, Nakamura K, Hamano K. Venous hemodynamics assessed with air plethysmography in legs with lymphedema. Vascular medicine (London, England). 2018 Apr:23(2):139-142. doi: 10.1177/1358863X17745372. Epub 2018 Jan 11 [PubMed PMID: 29325501]
Coelho A, O'Sullivan G. Usefulness of Direct Computed Tomography Venography in Predicting Inflow for Venous Reconstruction in Chronic Post-thrombotic Syndrome. Cardiovascular and interventional radiology. 2019 May:42(5):677-684. doi: 10.1007/s00270-019-02161-5. Epub 2019 Jan 9 [PubMed PMID: 30627773]
Rossi FH, Kambara AM, Rodrigues TO, Rossi CBO, Izukawa NM, Pinto IMF, Thorpe PE. Comparison of computed tomography venography and intravascular ultrasound in screening and classification of iliac vein obstruction in patients with chronic venous disease. Journal of vascular surgery. Venous and lymphatic disorders. 2020 May:8(3):413-422. doi: 10.1016/j.jvsv.2019.09.015. Epub 2020 Mar 17 [PubMed PMID: 32197952]
Aurshina A, Huber S, Deng Y, Attaran R, Nassiri N, Dardik A, Ochoa Chaar CI. Correlation of venous symptoms with iliac vein stenosis on magnetic resonance imaging. Journal of vascular surgery. Venous and lymphatic disorders. 2021 Sep:9(5):1291-1296.e1. doi: 10.1016/j.jvsv.2020.12.077. Epub 2020 Dec 30 [PubMed PMID: 33387666]
Peng C, Wu H, Kim S, Dai X, Jiang X. Recent Advances in Transducers for Intravascular Ultrasound (IVUS) Imaging. Sensors (Basel, Switzerland). 2021 May 19:21(10):. doi: 10.3390/s21103540. Epub 2021 May 19 [PubMed PMID: 34069613]
Level 3 (low-level) evidenceGagne PJ, Tahara RW, Fastabend CP, Dzieciuchowicz L, Marston W, Vedantham S, Ting W, Iafrati MD. Venography versus intravascular ultrasound for diagnosing and treating iliofemoral vein obstruction. Journal of vascular surgery. Venous and lymphatic disorders. 2017 Sep:5(5):678-687. doi: 10.1016/j.jvsv.2017.04.007. Epub 2017 Jun 28 [PubMed PMID: 28818221]
Gloviczki P, Pairolero PC, Toomey BJ, Bower TC, Rooke TW, Stanson AW, Hallett JW Jr, Cherry KJ Jr. Reconstruction of large veins for nonmalignant venous occlusive disease. Journal of vascular surgery. 1992 Nov:16(5):750-61 [PubMed PMID: 1433663]
Papadakis KG, Christopoulos D, Hobbs JT, Nicolaides AN. Descending phlebography in patients with venous ulceration: hemodynamic implications. International angiology : a journal of the International Union of Angiology. 2015 Jun:34(3):263-8 [PubMed PMID: 25877427]
Neglén P, Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. Journal of vascular surgery. 2002 Apr:35(4):694-700 [PubMed PMID: 11932665]
Raju S. New approaches to the diagnosis and treatment of venous obstruction. Journal of vascular surgery. 1986 Jul:4(1):42-54 [PubMed PMID: 3522942]
Raju S, Knight A, Lamanilao L, Pace N, Jones T. Peripheral venous hypertension in chronic venous disease. Journal of vascular surgery. Venous and lymphatic disorders. 2019 Sep:7(5):706-714. doi: 10.1016/j.jvsv.2019.03.006. Epub 2019 Jun 10 [PubMed PMID: 31196767]
Oropallo A, Andersen CA. Venous Stenting. StatPearls. 2023 Jan:(): [PubMed PMID: 34662029]
Shaydakov E, Porembskaya O, Geroulakos G. The May-Husni Procedure: A Reappraisal. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2015 Oct:50(4):513-7. doi: 10.1016/j.ejvs.2015.05.010. Epub 2015 Jul 31 [PubMed PMID: 26238309]
Husni EA. Reconstruction of veins: the need for objectivity. The Journal of cardiovascular surgery. 1983 Sep-Oct:24(5):525-8 [PubMed PMID: 6654967]
PALMA EC, ESPERON R. Vein transplants and grafts in the surgical treatment of the postphlebitic syndrome. The Journal of cardiovascular surgery. 1960 Jul:1():94-107 [PubMed PMID: 14429961]
Ulloa JH, Glickman M. One-Year First-in-Human Success for VenoValve in Treating Patients With Severe Deep Venous Insufficiency. Vascular and endovascular surgery. 2022 Apr:56(3):277-283. doi: 10.1177/15385744211073730. Epub 2022 Feb 7 [PubMed PMID: 35129407]
Medical Advisory Secretariat. Community-based care for chronic wound management: an evidence-based analysis. Ontario health technology assessment series. 2009:9(18):1-24 [PubMed PMID: 23074522]
Vu T, Harris A, Duncan G, Sussman G. Cost-effectiveness of multidisciplinary wound care in nursing homes: a pseudo-randomized pragmatic cluster trial. Family practice. 2007 Sep:24(4):372-9 [PubMed PMID: 17602174]
Level 1 (high-level) evidence