Introduction
Osteonecrosis is ischemia of bone due to inadequate vascular supply. This condition results in bone tissue death and loss of structural integrity. If left untreated, it can lead to bone collapse. This condition is also known as avascular necrosis or aseptic necrosis when it involves the epiphysis and as bone infarct when it involves metadiaphyseal regions of bone.
Osteonecrosis is common and typically asymptomatic in its early stages. This condition affects various bones in the body, including hips, knees, shoulders, ankles, and small bones of hands and feet. Some of the most common causes of osteonecrosis include chronic corticosteroid use, alcohol use disorder, trauma, and idiopathic causes. Other common predispositions include systemic diseases such as sickle cell anemia and collagen vascular disease.[1][2]
Osteonecrosis may be asymptomatic in its early stage. This condition can also present with limited movement, inability to bear weight, significant pain, and swelling of the affected joint. Recognizing various presentations of osteonecrosis is crucial. In addition, understanding when and what further necessitates evaluation is important.
When osteonecrosis involves the small bones of hands and feet or epiphysis of long bones, it is essential to assess the risk of secondary complications such as subchondral collapse. Imaging modalities used to evaluate osteonecrosis include plain radiographs, computed tomography (CT), magnetic resonance imaging (MRI), and nuclear medicine studies. However, knowing which modality is most sensitive at a certain point in the patient's clinical presentation and when it is necessary is crucial. Early diagnosis and treatment of osteonecrosis affecting epiphyseal locations remains the key to joint preservation.
Anatomy
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy
The bone is a complex organ composed of cortical and trabecular bone structures. Osteonecrosis involves the trabecular and subcortical bone. The pathophysiology of osteonecrosis is characterized by cell death due to lack of blood supply, followed by a complex process of bone resorption and formation in an attempt to heal. The healing process includes walling off the dead bone by forming a fibrotic rim at the margin between the living and dead bone. At this interface, granulation tissue develops with osteoblasts encapsulating the dead trabeculae and osteoclasts that partially reabsorb the dead bone. In the subchondral trabeculae, the bone resorption rate exceeds the bone formation rate, leading to a net removal of bone and loss of structural integrity.[3] This area is then predisposed to increased compressive forces and subsequent subchondral collapse.
These histological changes can be observed on radiographs as serpentine areas of sclerosis and lucency. The lucent areas represent bone resorption, whereas the sclerotic areas represent mixed areas of living and dead bone trabeculae.[1][3] These changes are most prominently observed in the diaphysis and metaphysis of long bones. Osteonecrosis affecting the diaphysis and metaphysis is less clinically important because dead bone in long bones is not subject to the same compressive forces, and it is considered just as strong as living bone without the ability to remodel.[1] In these locations, it often goes underdiagnosed as it is typically asymptomatic and often incidentally found.
Osteonecrosis affecting small bones commonly has a history of antecedent trauma. This condition affects many bones of the hands and feet, the most commonly involved being the scaphoid bone, lunate, and talus. On plain film, it often appears as an increased area of sclerosis requiring MRI for further evaluation.
Most osteonecrosis sites are associated with a specific eponym (see Table 1. Common Osteonecrosis Eponyms).
Table 1. Common Osteonecrosis Eponyms
| Eponym | Site of Involvement |
| Adams disease | Medial epicondyle of the humerus |
| Ahlback disease | Femoral condyle in adults, ie, SONK |
| Breck disease | Medial malleolus |
| Chandler disease | Idiopathic osteonecrosis of the femoral head in adults |
| Dias disease | Trochlea of the talus |
| Freiberg infraction | Head of second or third metatarsal |
| Iselin disease | Base of the fifth metatarsal apophysis in children |
| Kienbock disease | Lunate |
| Kohler disease | Navicular in children |
| Kummel disease | Vertebral body |
| Madelung disease | Distal radial epiphysis |
| Mueller-Weiss disease | Navicular in adults |
| Osgood Schlatter disease | Tibial tubercle |
| Panner disease | Capitellum of the humerus |
| Perthes disease (Legg-Calve-Perthes) | Femoral head in children |
| Preiser disease | Scaphoid |
| Scheuermann disease | Ring epiphysis of the spine |
| Sever disease | Calcaneus |
| Sinding-Larsen-Johansson disease | Distal pole of the patella |
| Thiemann disease | Base of phalanges |
The etiologies of osteonecrosis are diverse. Trauma is a frequent cause of symptomatic osteonecrosis of the femoral head, scaphoid, and talus due to disruption of the end arterial supply. The most common nontraumatic causes of osteonecrosis include chronic corticosteroid use, excessive alcohol consumption, and idiopathic factors. Sickle cell disease is an important consideration of nontraumatic causes due to the precipitation of hemoglobin S in low-oxygen environments, leading to vasocclusion and bone ischemia.[2] Drug therapy with bisphosphonates has a well-known association with osteonecrosis of the jaw due to inhibition of bone turnover.[4] In the renal transplant population, there is also a high prevalence of this condition due to chronic high-dose steroid use and chronic immunosuppression. Nontraumatic causes are often bilateral and have multiple affected sites. A detailed clinical history, including the patient's predisposing risk factors, is crucial when interpreting diagnostic imaging studies, particularly for plain radiographs, to improve sensitivity in detecting subtle areas of osteonecrosis, particularly in the epiphysis.
Some of the more common causes of osteonecrosis include the following:
- Trauma
- Chronic high-dose corticosteroid use
- Alcohol use disorder
- Sickle cell disease or hemoglobinopathies
- Systemic lupus erythematosus
- Antiphospholipid antibody syndrome
- Gaucher disease
- Metabolic diseases such as hyperlipidemia
- Renal transplantation
- HIV
- Prior radiation therapy
- Chemotherapy
- Decompression sickness
- Bisphosphonate use
Plain Films
Plain films or radiographs are good initial imaging exams to begin the evaluation. Although not as sensitive for detecting early changes in osteonecrosis, plain radiographs are readily accessible, inexpensive, and can exclude other underlying causes of bone pain. Radiographs have imaging characteristics that may limit the need for additional imaging evaluations.[1]
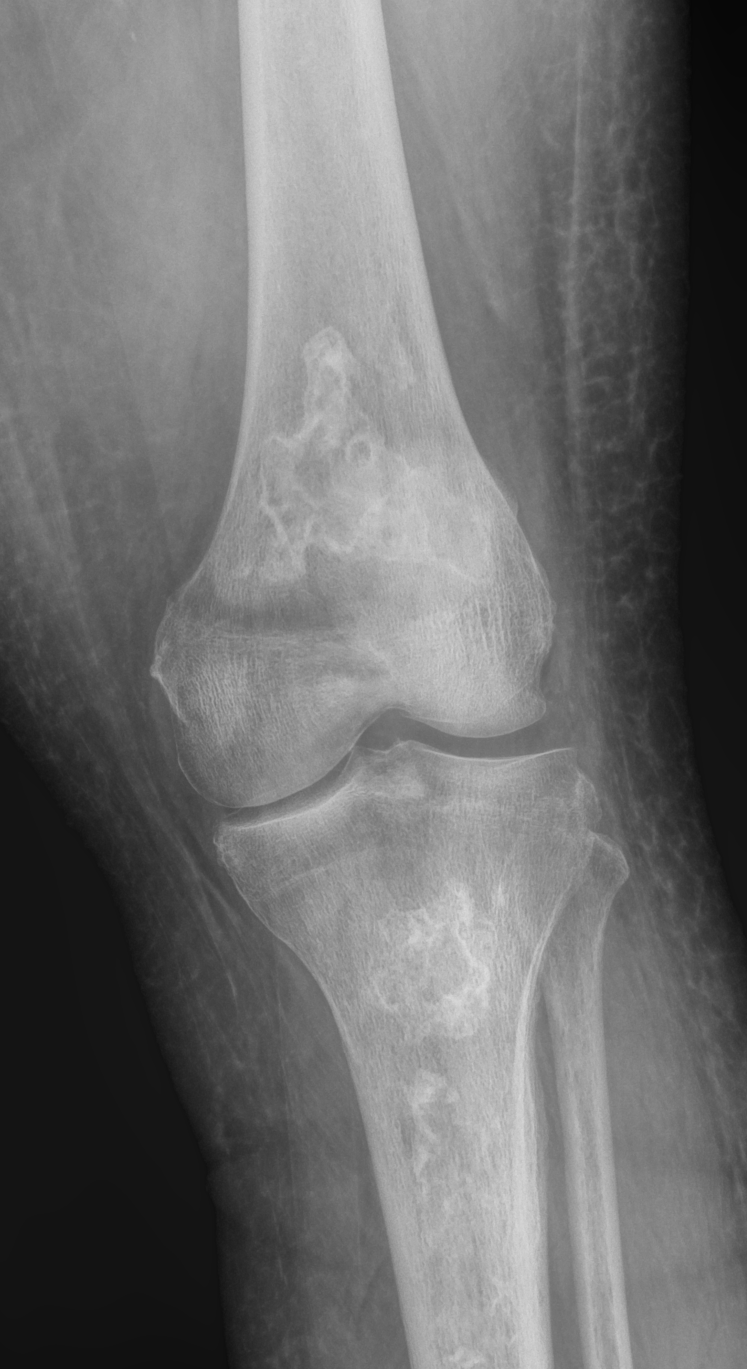
Early findings may show minor osteopenia compared to the contralateral bone. In small bones, osteonecrosis appears as areas of increased sclerosis compared to adjacent bones. In long bones, early to chronic characteristic imaging findings show patchy areas of lucency with a serpentine rim of sclerosis. The serpentine sclerotic rim is most conspicuous in meta-diaphyseal zones (see Image. Metaphyseal Osteonecrosis).
Early findings may show slight osteopenia in comparison to the opposite bone. In small bones, osteonecrosis will appear as areas with increased hardness compared to nearby bones. In long bones, characteristic imaging findings from early to chronic stages include scattered areas of hollowness surrounded by a winding rim of hardness. This winding hard rim is most noticeable in the areas between the metaphysis and diaphysis
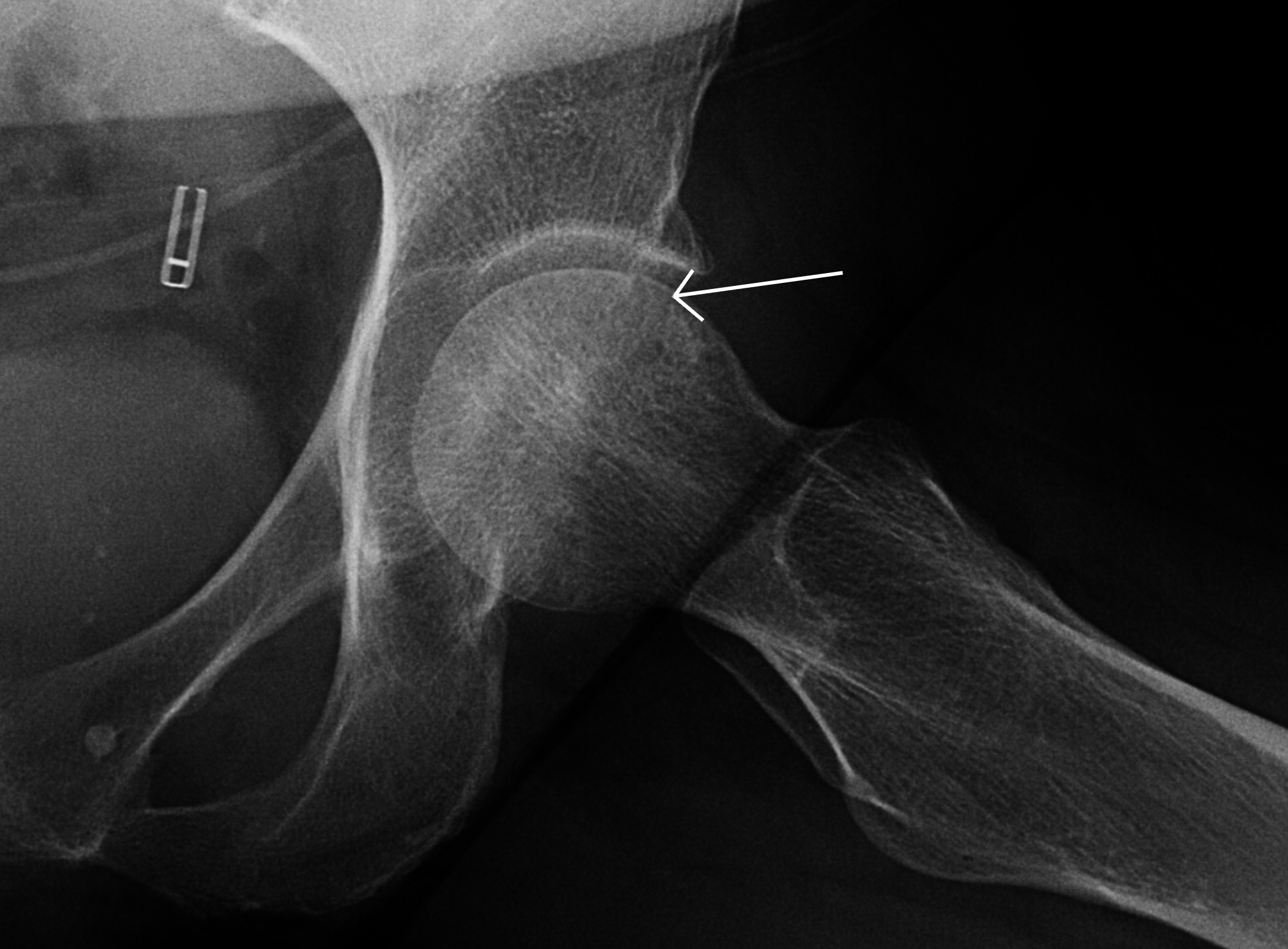
Plain radiographs also show late findings and complications involving the epiphysis, such as articular collapse. Articular collapse occurs when there is epiphyseal osteonecrosis with areas extending to the junction of the articular surface and subchondral bone (see Image. Articular Collapse). When stress is maximally exerted at these sites, the underlying necrotic subchondral bone collapses, creating a linear crescentic subchondral lucency known as the crescent sign (see Image. Crescent Sign).[5] Eventually, the bone cortex collapses and fragments, leading to secondary progressive degenerative changes.
Computed Tomography
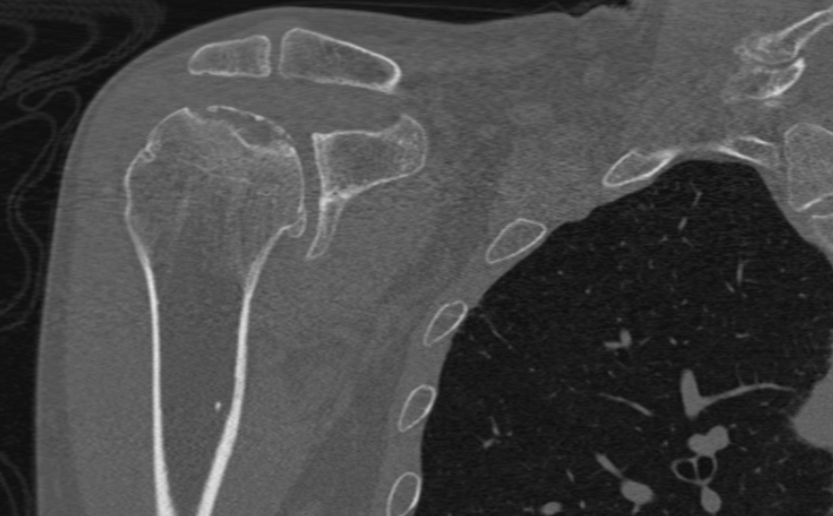
CT is useful in the advanced stages of osteonecrosis and for surgical planning once the diagnosis of osteonecrosis is established. This technique is valuable in evaluating bony details to delineate the location and extent of articular collapse, particularly when there is epiphyseal involvement. CT scans typically share similar imaging characteristics as observed in plain radiographs, showing a serpentine sclerotic rim around the area of osteonecrosis and detecting subchondral fractures with the crescent sign (see Image. Crescent Sign on CT scan).
There is limited use of CT for early detection, and it should not be used as first-line imaging when clinically suspecting osteonecrosis.[6] Consideration should be given to the risk of radiation exposure, particularly in younger patients. However, preoperative CT before total hip arthroplasty has shown improved sensitivity for femoral head collapse, ultimately upstaging femoral head osteonecrosis in 20% of patients, according to the Association Research Circulation Osseous (ARCO) classification.[7] Thus, the advantages and disadvantages of CT imaging before surgery should be considered with the patient individually.
Magnetic Resonance
MRI is the gold standard for imaging and characterizing osteonecrosis, with a high sensitivity and specificity of nearly 100%. MRI should be performed when there is clinical suspicion for osteonecrosis as the next line imaging study, after plain radiographs, if needed for further characterization. MRI allows for the evaluation of volume and associated inflammatory changes.[6] Imaging findings on MRI can manifest as early as 1 week from vascular injury.[1] The classic MRI pattern is a rim of low signal intensity on all pulse sequences followed by a second inner rim of high signal intensity observed on fluid-sensitive sequences, known as the double-line sign (see Image. MRI of Double-Line Sign with Subchondral Collapse).[5] The rim of low signal intensity observed on all sequences represents the outside rim of sclerosis, and the inner rim of the high signal represents the reactive interface or zone of creeping substitution.[1]
In small bones, a double-line sign may not be as apparent. Characteristic findings of osteonecrosis in small bones generally demonstrate low signal intensity on T1 sequences (compared to skeletal muscle) with variable T2 signal. Fluid-sensitive sequences show early increased signal in acute osteonecrosis and decreased signal in advanced stages of the disease. Other secondary signs may be observed, such as changes in morphology and malignment of, and relative to, the adjacent bones and the development of secondary osteoarthritis.[1]
Contrast is generally not necessary in evaluating osteonecrosis. Occasionally, contrast-enhanced MRI and dynamic contrast-enhanced studies may be used to distinguish between transient bone marrow edema syndrome and subchondral insufficiency fracture. Contrast-enhanced exams may demonstrate areas of reduced enhancement in osteonecrosis, thus confirming areas of devascularization.[8][9]
MRI is useful for assessing the volume and presence of bone marrow edema and joint effusions, which in turn help predict the risk of subchondral collapse. However, MRI has limitations in evaluating the extent of articular collapse required for surgical planning. In contrast, CT is better suited for assessing osseous details and visualizing fractures that extend to the cortex.[10] CT and MRI can be complementary to each other.
Nuclear Medicine
When MRI is inconclusive or when there are contraindications to MRI, bone scans are often used as an alternative route to image early changes of osteonecrosis and determine the risk of impeding bone collapse.[11] The most common bone scan is technetium-99m methylene diphosphonate (99mTc-MDP). This scan is often performed as a single delayed phase or in 3 phases—flow, blood pool, and delayed phase. MDP is a phosphate analog taken up by osteoblasts through chemisorption, binding to crystalline hydroxyapatite in the osseous matrix.
Osteonecrosis is a progressive condition, and its appearance in bone scans often depends on the stage of the disease. Osteonecrosis can be visualized on bone scans as soon as 7 to 10 days from an event, typically appearing as normal or as a general area of photopenia on delayed images due to a lack of radioisotope uptake in avascular bone.[5] Increased radiotracer activity is observed after 1 to 3 weeks due to increased osteoblastic activity at the reactive interface around the necrotic component as the tissue attempts to heal. On imaging, this gives the classic donut sign or ring sign with a hot area of increased activity around a central cold photopenic defect. Overall sensitivity in diagnosing osteonecrosis of the femoral head ranges from 78% to 91%, depending on the healing phase. However, it remains relatively nonspecific as many bone pathologies may present similarly. MDP bone scans are limited by spatial resolution; however, combining them with single-photon emission computed tomography/CT has improved accuracy at 3-dimensional localization.[11]
Positron emission tomography (PET) imaging using F18 glucose is a diagnostic imaging technique used to detect metabolic abnormalities. Although it is not ideal for examining the bone in isolation, it can detect areas of increased bone metabolism. Thus, it is useful for identifying osteoblastic activity and bone turnover. Studies on femoral head osteonecrosis have shown that F18 PET/CT has improved sensitivity and accuracy compared to MRI.[12] Evidence suggests that the metabolic information obtained may predict the risk of impeding femoral head collapse before changes become apparent on plain film.[13] Metabolic activity is quantified through maximum standardized uptake value (SUVmax) and increases with stage progression. Kubota et al concluded that SUVmax >6.45 leads to femoral head collapse within 12 months. When combined with plain radiographs or MRI for type classification, PET imaging has shown increased specificity for predicting femoral head collapse.[13] Thus, PET can be used as an adjunct in early clinical management for joint preservation but should not be used as first-line screening due to its excessive cost and radiation exposure.
Clinical Significance
Patients with osteonecrosis in epiphyseal locations in the femoral head and small bones, such as the lunate, are at an increased risk of subchondral collapse. Early diagnosis and treatment are crucial for joint preservation. The goal of imaging should be to identify osteonecrosis before it undergoes irreversible change. The care of patients with osteonecrosis necessitates a collaborative approach among healthcare professionals to ensure patient-centered care and improved overall outcomes. Radiograph and MRI classification systems have been developed in conjunction with clinical findings to help accurately describe the progression of osteonecrosis for further risk stratification. Below is a summary of commonly used classification systems in clinical practice for femoral head and lunate osteonecrosis.
Osteonecrosis affecting long bone metaphysis and diaphysis is often clinically occult and may go underdiagnosed. A rare complication of osteonecrosis that clinicians should be made aware of when new clinical findings arise is described.
Staging of Femoral Head Osteonecrosis
The most common site for osteonecrosis is the femoral head, with an increased prevalence observed among younger adults aged 20 to 40, accounting for 10% of annual total hip arthroplasties in the United States.[14] There are a few staging systems developed for adult femoral head osteonecrosis, the 3 main ones being—Ficat and Arlet (see Table 2. Ficat and Arlet Staging of Osteonecrosis of the Hip), Steinberg (see Table 3. Steinberg Staging System of Osteonecrosis of the Hip), and the Association Research Circulation Osseous (ARCO) classification (see Table 4. ARCO 2019 Revised Staging System). These systems are designed to stage osteonecrosis from clinically occult disease to positive imaging findings to subchondral collapse and the late findings of secondary osteoarthritis. These classifications are clinically significant for epiphyseal osteonecrosis as they predict the likelihood of articular collapse. Successful treatment depends on accurate staging. There is no consensus on the best classification system, as all have demonstrated interobserver and intraobserver reliability.[15]
Table 2. Ficat and Arlet Staging of Osteonecrosis of the Hip
| Stage | Clinical Features | Imaging Findings |
| 0 | Preclinical | Normal |
| I | No pain |
Normal radiographs Decreased or increased uptake on a bone scan |
| II | Variable pain | Preservation of femoral head shape with variable changes in trabecular bone with sclerosis and cyst formation |
| III | Pain | Cresent sign on imaging with collapse or fracturing of subchondral bone |
| IV | Pain | Marked collapse of subchondral bone with flattened contours and decreased joint space, accompanied by secondary osteoarthritis and acetabular changes |
Reference for the table.[16]
Table 3. Steinberg Staging System of Osteonecrosis of the Hip (the University of Philadephia System)
| Stage* | Imaging Findings |
| 0 | Normal imaging findings on radiograph, MRI, and bone scan |
| I | Normal radiographs, abnormal bone scans, abnormal MRI |
| II | Abnormal radiograph with cystic and sclerotic changes in the femoral head |
| III | Subchondral collapse producing crescent sign |
| IV | Flattening of the femoral head |
| V | Joint space narrowing with and without acetabular involvement |
| VI | Advanced secondary degenerative changes |
* Stages 1 through 6 are further categorized into 3 subsets—mild (<15% articular surface), moderate (15% to 30%), and severe (>30%).[17][18]
Table 4. ARCO 2019 Revised Staging System
| Stage | Imaging Findings |
| I | Normal radiographs, MRI with T1 dark band-like signal along the necrotic area |
| II | Abnormal radiograph (subtle sclerosis, osteoporosis, or cystic change without subchondral fracture) and abnormal MRI |
|
III IIIA IIIB |
Subchondral fracture Femoral head depression <2 mm Femoral head depression >2 mm |
| IV | Secondary signs of osteoarthritis on plain film |
References for the table.[19][20]
When osteonecrosis occurs in the epiphyses of the femoral head and other long bones, the report should include specific items to communicate prognostic findings to the clinician as follows:
- Location of osteonecrosis
- Estimated volume of the femoral head and the percentage of weight-bearing surface
- Presence of coexisting degenerative changes
- Presence of joint effusion
- Presence of unstable osteochondral fragments or subchondral fracture [5]
The extent of the femoral head involved in osteonecrosis is a crucial factor in predicting femoral head collapse and is best evaluated using an MRI in the sagittal plane.[21] Osteonecrosis affecting >50% of the femoral head is much more likely to progress to articular collapse. Osteonecrosis affecting <30% of the femoral head is unlikely to lead to articular collapse. Additional factors associated with an increased risk of femoral head collapse include an increased thickness of the relative zone, joint effusion, surrounding edema, older age, and increased body mass index.[22]
Kienbock Disease
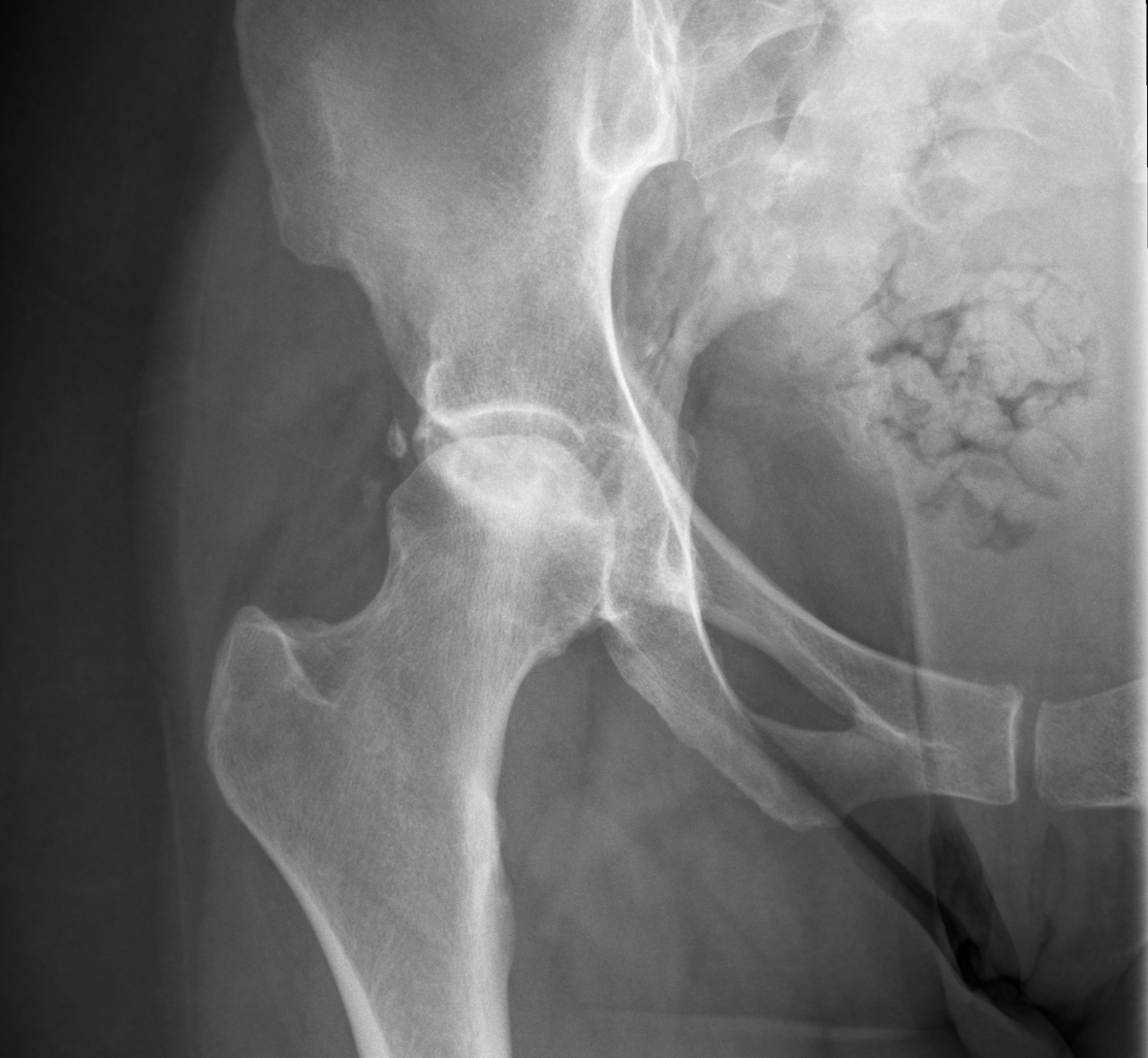
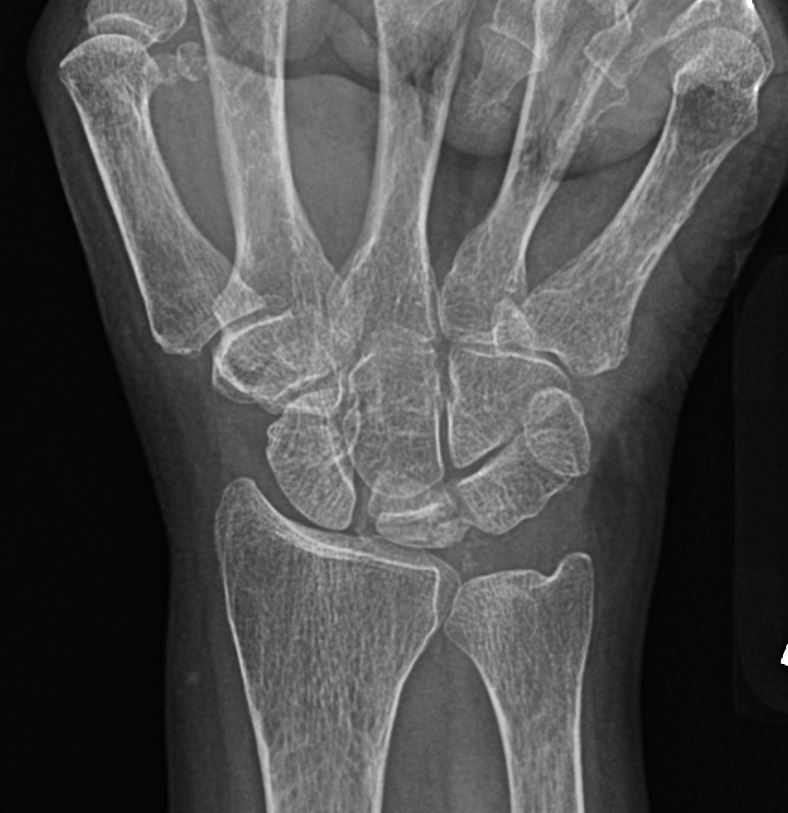
Osteonecrosis affecting the lunate is referred to as Kienbock disease or lunatomalacia. Although its etiology is often unknown, most patients have an antecedent history of trauma that disrupts the blood supply and ligaments surrounding the lunate.[23] Due to its variable blood supply, the lunate bone is at an increased risk of osteonecrosis. Radiographs are the initial imaging modality used to diagnose Kienbock disease, which often demonstrates an area of increased sclerosis or flattening with negative ulnar variance (see Image. Plain Film of Kienbock Disease). On MRI, the condition is diagnosed with near certainty when there is a diffuse T1 dark signal in the lunate, particularly in the setting of ulnar variance and normal appearing surrounding bones with variable T2 signal (see Image. MRI findings of Kienbock Disease).
Delayed diagnosis can lead to lunate collapse and subsequent alternation of the surrounding capital architecture. Once recognized, surgery is often required to prevent wrist degenerative changes.
A reproducible osseous classification system called the Lichtman classification combines radiologic and clinical findings to help surgeons make the appropriate treatment decision preoperatively to prevent resulting carpal pathology (see Table 5. Lichtman Classification).[24] When radiographic findings are inconclusive, MRI can aid in identifying radiographically occult disease.
Table 5. Lichtman Osseous Classification
| Stage | Imaging Findings |
| I | Normal radiograph |
| II | Visible lunate sclerosis on radiographs with preserved anatomy |
|
III IIIA IIIB IIIC |
Lunate collapse or fracture Lunate collapse with preserved carpal alignment and height Lunate collapse with fixed carpal instability Lunate fracture |
| IV | Advanced carpal degenerative changes |
References for the table.[24][25]
Malignant Transformation
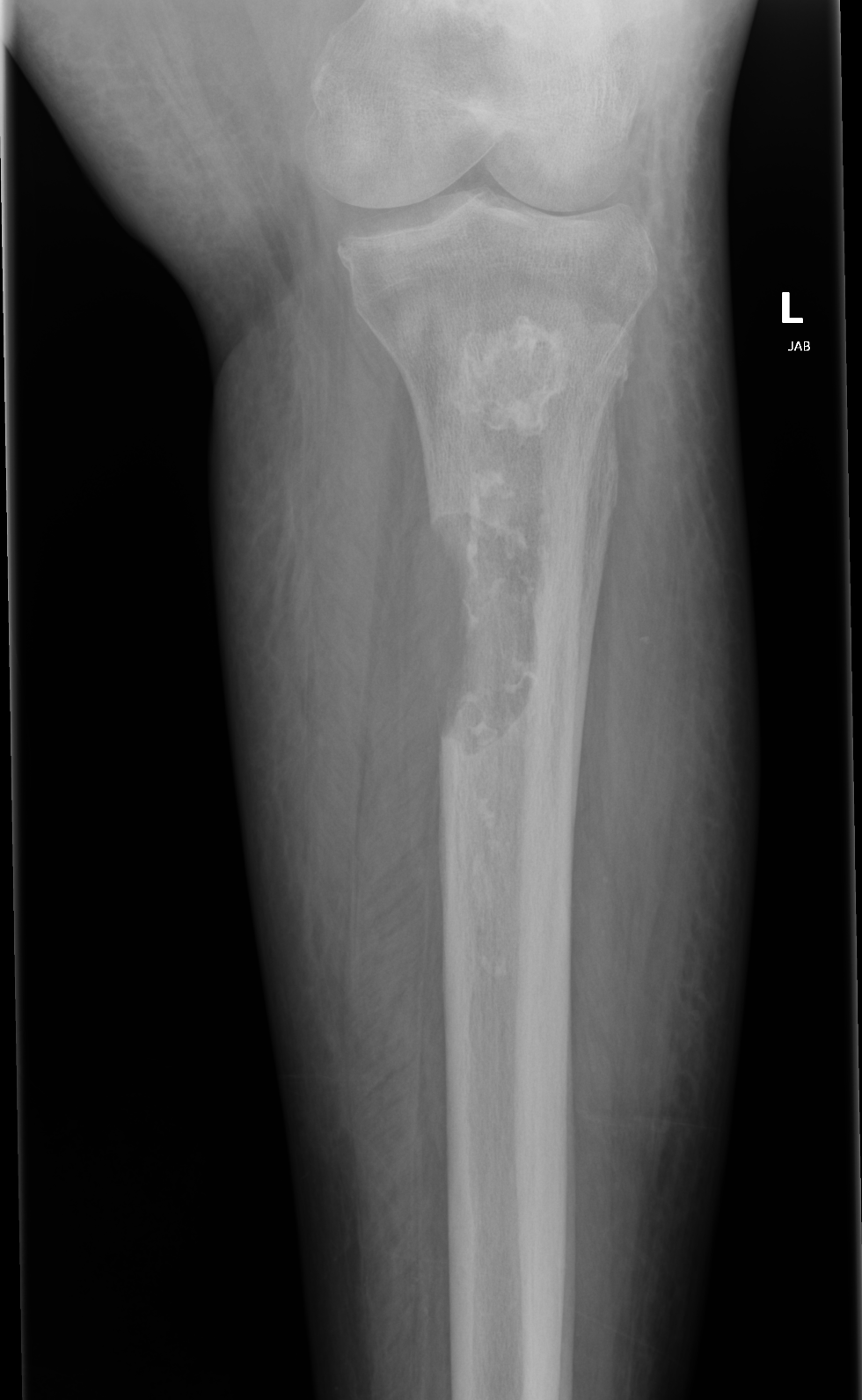
Another important but rare complication of osteonecrosis is the malignant degeneration of extensive bone infarcts into secondary osteosarcoma, also known as bone infarct-associated sarcoma. This entity is extremely rare, however well documented, and important to recognize and consider in patients with osteonecrosis affecting metadiaphyseal locations of long bones.
This condition is believed to arise from the reactive interface at the periphery of a lesion where excessive proliferative activity occurs.[26] Ostenecrosis-associated sarcoma occurs in the fifth to sixth decade of life and typically has a poor prognosis.[27]
Radiographs demonstrate previous characteristics of osteonecrosis with new focally aggressive osteolytic lesions extending through the cortex and involving the reactive interface of cancellous bone (see Image. Osteolytic Lesion). MRI shows a new mass-like replacement of the bone marrow in the area of osteonecrosis with associated cortical destruction and new soft tissue components (see Image. MRI of Malignant Transformation). Lesions are hypermetabolic on PET/CT and markedly hot on bone scintigraphy.[28] New bone pain in patients with known osteonecrosis affecting the metaphysis of long bones should be vigilantly monitored.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
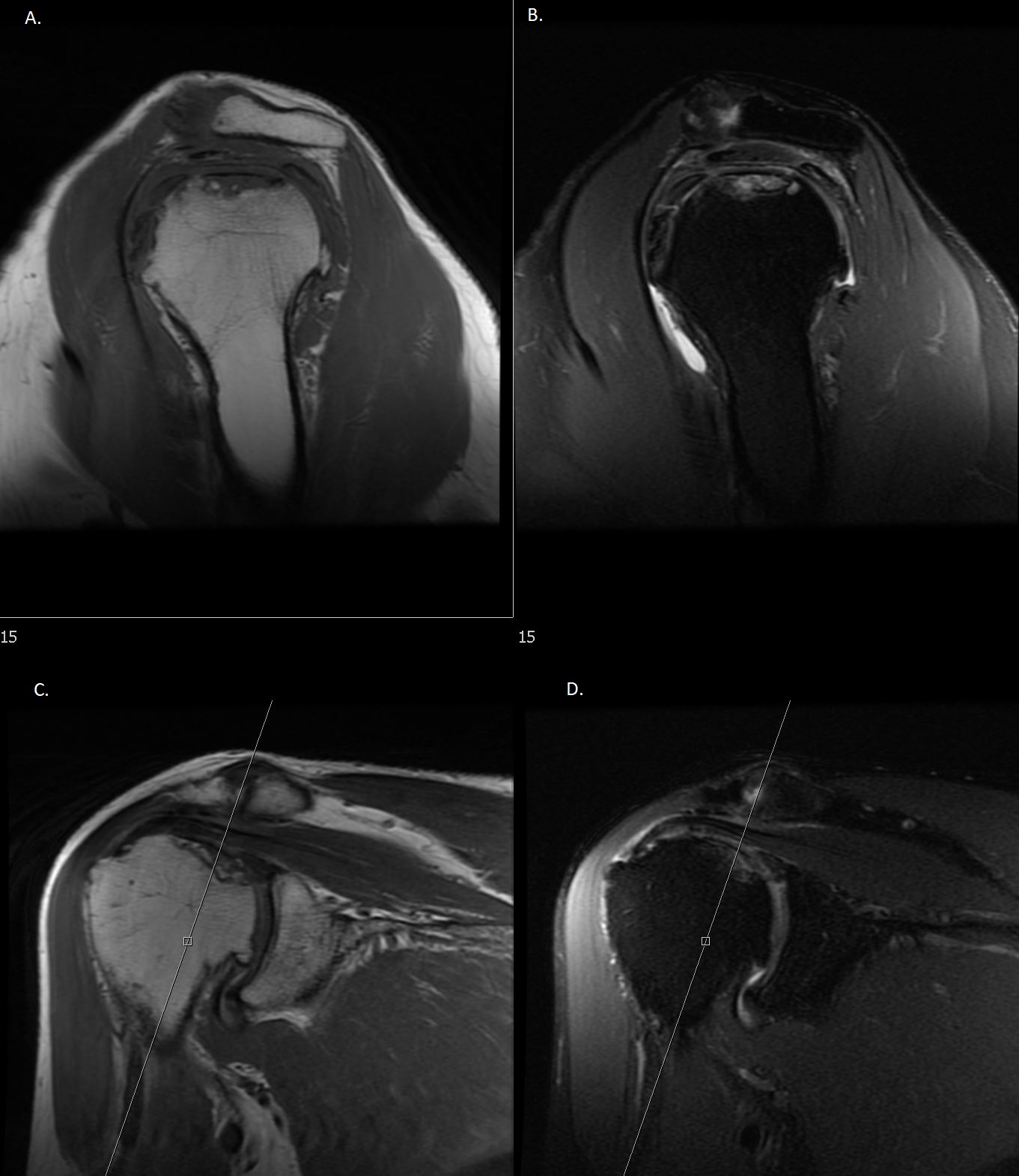
MRI findings of Double-Line Sign with Subchondral Collapse. Osteonecrosis affecting the superior humeral head with findings of subchondral collapse on MRI. Sag T1 (A) and coronal T1 (C) demonstrate a dark rim of sclerosis. Sag PD FS (B) and Cor PD FS (D) show high signal intensity of crescentic cortical fragments with flattening of the femoral head. Sag, sagittal; Cor, coronal; PD, proton density; FS, fat-suppressed.
Contributed by O Stroie, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
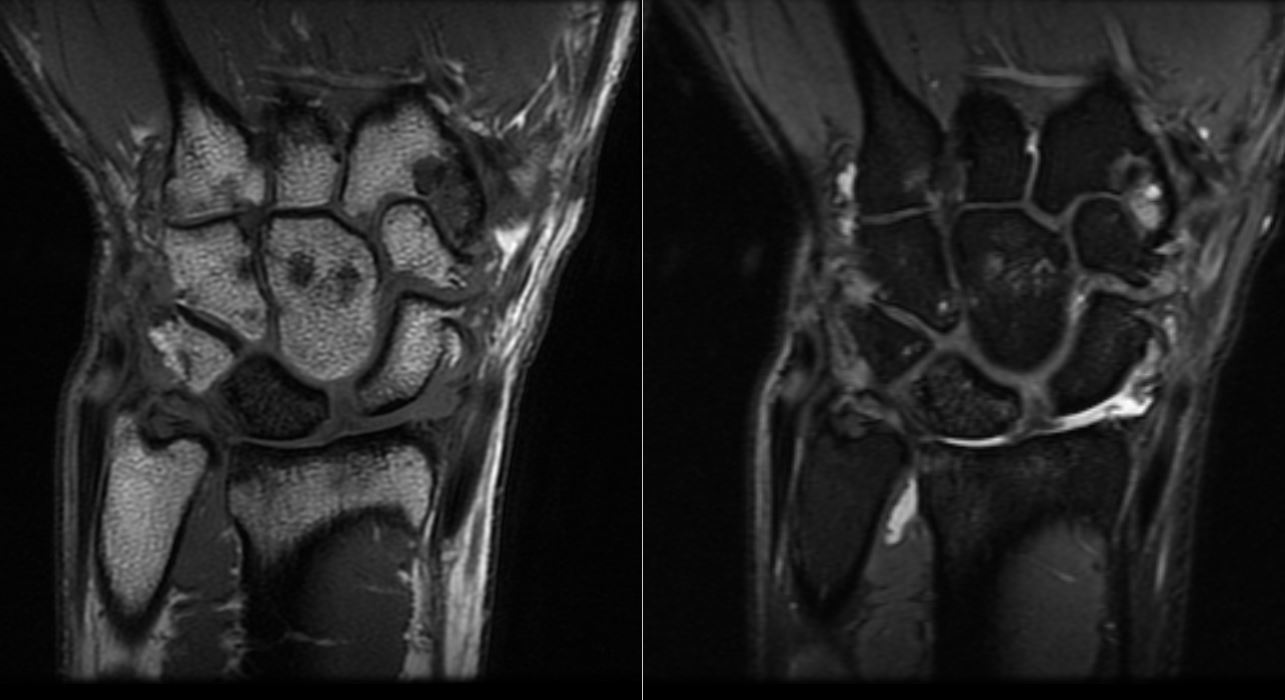
MRI findings of Kienbock Disease. MRI Cor T1 and Cor PD FS of the wrist shows a dark T1 signal in the lunate with diffuse marrow replacement. No evidence of collapse exists. There is a widening of the scapholunate space and volar tilting of the lunate. Cor, coronal; PD, proton density; FS, fat-suppressed.
Contributed by O Stroie, MD
(Click Image to Enlarge)
(Click Image to Enlarge)
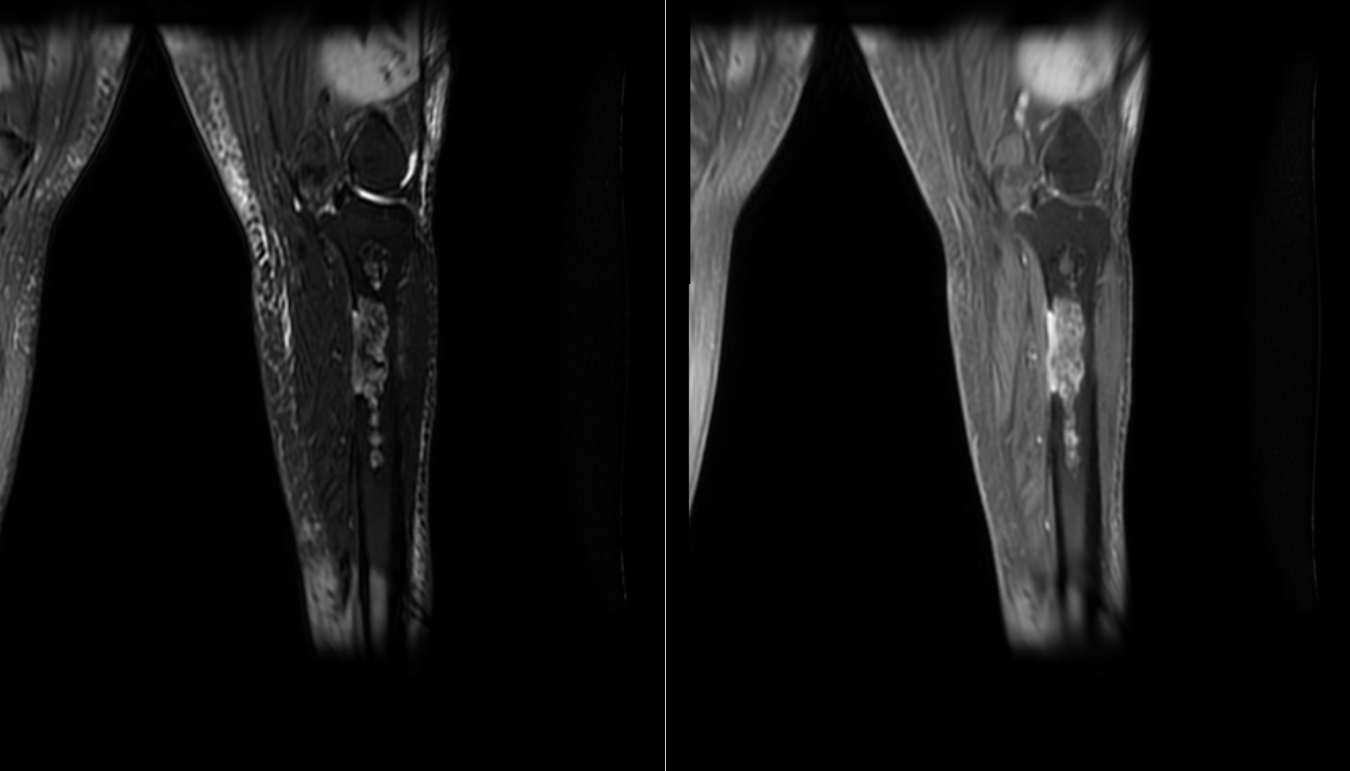
MRI of Malignant Transformation. Coronal T2 FS and T1 post-contrast images reveal multifocal areas of osteonecrosis with malignant sarcomatous transformation of the proximal tibial metadiaphysis. The images show diffuse enhancement of an eccentric medullary lesion extending through the cortex with associated small soft tissue mass. FS, fat-suppressed.
Contributed by O Stroie, MD
References
Murphey MD, Foreman KL, Klassen-Fischer MK, Fox MG, Chung EM, Kransdorf MJ. From the radiologic pathology archives imaging of osteonecrosis: radiologic-pathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc. 2014 Jul-Aug:34(4):1003-28. doi: 10.1148/rg.344140019. Epub [PubMed PMID: 25019438]
George G, Lane JM. Osteonecrosis of the Femoral Head. Journal of the American Academy of Orthopaedic Surgeons. Global research & reviews. 2022 May 1:6(5): [PubMed PMID: 35511598]
Shah KN, Racine J, Jones LC, Aaron RK. Pathophysiology and risk factors for osteonecrosis. Current reviews in musculoskeletal medicine. 2015 Sep:8(3):201-9. doi: 10.1007/s12178-015-9277-8. Epub [PubMed PMID: 26142896]
Morag Y, Morag-Hezroni M, Jamadar DA, Ward BB, Jacobson JA, Zwetchkenbaum SR, Helman J. Bisphosphonate-related osteonecrosis of the jaw: a pictorial review. Radiographics : a review publication of the Radiological Society of North America, Inc. 2009 Nov:29(7):1971-84. doi: 10.1148/rg.297095050. Epub [PubMed PMID: 19926757]
Tasu JP, Duboe PO, Florez N, Herpe G. Avascular osteonecrosis of the hip: The vision of the radiologist (radiology, MRI, CT and scintigraphy). Morphologie : bulletin de l'Association des anatomistes. 2021 Jun:105(349):85-93. doi: 10.1016/j.morpho.2020.12.001. Epub 2021 Jan 5 [PubMed PMID: 33419657]
Expert Panel on Musculoskeletal Imaging, Ha AS, Chang EY, Bartolotta RJ, Bucknor MD, Chen KC, Ellis HB Jr, Flug J, Leschied JR, Ross AB, Sharma A, Thomas JM, Beaman FD. ACR Appropriateness Criteria® Osteonecrosis: 2022 Update. Journal of the American College of Radiology : JACR. 2022 Nov:19(11S):S409-S416. doi: 10.1016/j.jacr.2022.09.009. Epub [PubMed PMID: 36436966]
Lee B, Lim JY, Lee DM, Park JW, Lee YK, Ha YC, Koo KH. Computed Tomography Staging of Osteonecrosis of the Femoral Head. Surgical technology international. 2019 Nov 10:35():417-421 [PubMed PMID: 31571188]
Geith T, Niethammer T, Milz S, Dietrich O, Reiser M, Baur-Melnyk A. Transient Bone Marrow Edema Syndrome versus Osteonecrosis: Perfusion Patterns at Dynamic Contrast-enhanced MR Imaging with High Temporal Resolution Can Allow Differentiation. Radiology. 2017 May:283(2):478-485. doi: 10.1148/radiol.2016152665. Epub 2016 Dec 1 [PubMed PMID: 27905865]
Mueller D, Schaeffeler C, Baum T, Walter F, Rechl H, Rummeny EJ, Woertler K. Magnetic resonance perfusion and diffusion imaging characteristics of transient bone marrow edema, avascular necrosis and subchondral insufficiency fractures of the proximal femur. European journal of radiology. 2014 Oct:83(10):1862-9. doi: 10.1016/j.ejrad.2014.07.017. Epub 2014 Jul 30 [PubMed PMID: 25129825]
Stevens K, Tao C, Lee SU, Salem N, Vandevenne J, Cheng C, Neumann G, Valentin-Opran A, Lang P. Subchondral fractures in osteonecrosis of the femoral head: comparison of radiography, CT, and MR imaging. AJR. American journal of roentgenology. 2003 Feb:180(2):363-8 [PubMed PMID: 12540435]
Agrawal K, Tripathy SK, Sen RK, Santhosh S, Bhattacharya A. Nuclear medicine imaging in osteonecrosis of hip: Old and current concepts. World journal of orthopedics. 2017 Oct 18:8(10):747-753. doi: 10.5312/wjo.v8.i10.747. Epub 2017 Oct 18 [PubMed PMID: 29094004]
Gayana S, Bhattacharya A, Kashyap R, Sen RK, Mittal BR. (18)F-fluoride PET/CT in avascular necrosis of the femoral head. Clinical nuclear medicine. 2013 Jun:38(6):e265-6. doi: 10.1097/RLU.0b013e318266d036. Epub [PubMed PMID: 23143050]
Kubota S, Inaba Y, Kobayashi N, Tateishi U, Ike H, Inoue T, Saito T. Prediction of femoral head collapse in osteonecrosis using 18F-fluoride positron emission tomography. Nuclear medicine communications. 2015 Jun:36(6):596-603. doi: 10.1097/MNM.0000000000000284. Epub [PubMed PMID: 25714808]
Moya-Angeler J, Gianakos AL, Villa JC, Ni A, Lane JM. Current concepts on osteonecrosis of the femoral head. World journal of orthopedics. 2015 Sep 18:6(8):590-601. doi: 10.5312/wjo.v6.i8.590. Epub 2015 Sep 18 [PubMed PMID: 26396935]
Mont MA, Salem HS, Piuzzi NS, Goodman SB, Jones LC. Nontraumatic Osteonecrosis of the Femoral Head: Where Do We Stand Today?: A 5-Year Update. The Journal of bone and joint surgery. American volume. 2020 Jun 17:102(12):1084-1099. doi: 10.2106/JBJS.19.01271. Epub [PubMed PMID: 32282421]
Jawad MU, Haleem AA, Scully SP. In brief: Ficat classification: avascular necrosis of the femoral head. Clinical orthopaedics and related research. 2012 Sep:470(9):2636-9. doi: 10.1007/s11999-012-2416-2. Epub [PubMed PMID: 22760600]
Steinberg ME, Hayken GD, Steinberg DR. A quantitative system for staging avascular necrosis. The Journal of bone and joint surgery. British volume. 1995 Jan:77(1):34-41 [PubMed PMID: 7822393]
Choi HR, Steinberg ME, Y Cheng E. Osteonecrosis of the femoral head: diagnosis and classification systems. Current reviews in musculoskeletal medicine. 2015 Sep:8(3):210-20. doi: 10.1007/s12178-015-9278-7. Epub [PubMed PMID: 26088795]
Hines JT, Jo WL, Cui Q, Mont MA, Koo KH, Cheng EY, Goodman SB, Ha YC, Hernigou P, Jones LC, Kim SY, Sakai T, Sugano N, Yamamoto T, Lee MS, Zhao D, Drescher W, Kim TY, Lee YK, Yoon BH, Baek SH, Ando W, Kim HS, Park JW. Osteonecrosis of the Femoral Head: an Updated Review of ARCO on Pathogenesis, Staging and Treatment. Journal of Korean medical science. 2021 Jun 21:36(24):e177. doi: 10.3346/jkms.2021.36.e177. Epub 2021 Jun 21 [PubMed PMID: 34155839]
Yoon BH, Mont MA, Koo KH, Chen CH, Cheng EY, Cui Q, Drescher W, Gangji V, Goodman SB, Ha YC, Hernigou P, Hungerford MW, Iorio R, Jo WL, Jones LC, Khanduja V, Kim HKW, Kim SY, Kim TY, Lee HY, Lee MS, Lee YK, Lee YJ, Nakamura J, Parvizi J, Sakai T, Sugano N, Takao M, Yamamoto T, Zhao DW. The 2019 Revised Version of Association Research Circulation Osseous Staging System of Osteonecrosis of the Femoral Head. The Journal of arthroplasty. 2020 Apr:35(4):933-940. doi: 10.1016/j.arth.2019.11.029. Epub 2019 Nov 27 [PubMed PMID: 31866252]
Ha AS, Wells L, Jaramillo D. Importance of sagittal MR imaging in nontraumatic femoral head osteonecrosis in children. Pediatric radiology. 2008 Nov:38(11):1195-200. doi: 10.1007/s00247-008-0979-6. Epub 2008 Aug 19 [PubMed PMID: 18712375]
Nam KW, Kim YL, Yoo JJ, Koo KH, Yoon KS, Kim HJ. Fate of untreated asymptomatic osteonecrosis of the femoral head. The Journal of bone and joint surgery. American volume. 2008 Mar:90(3):477-84. doi: 10.2106/JBJS.F.01582. Epub [PubMed PMID: 18310696]
Kennedy C, Abrams R. In Brief: The Lichtman Classification for Kienböck Disease. Clinical orthopaedics and related research. 2019 Jun:477(6):1516-1520. doi: 10.1097/CORR.0000000000000595. Epub [PubMed PMID: 30507834]
Lichtman DM, Lesley NE, Simmons SP. The classification and treatment of Kienbock's disease: the state of the art and a look at the future. The Journal of hand surgery, European volume. 2010 Sep:35(7):549-54. doi: 10.1177/1753193410374690. Epub 2010 Jul 9 [PubMed PMID: 20621943]
Lichtman DM, Pientka WF 2nd, MacLean S, Bain G. Precision Medicine for Kienböck Disease in the 21st Century. The Journal of hand surgery. 2022 Jul:47(7):677-684. doi: 10.1016/j.jhsa.2022.03.014. Epub [PubMed PMID: 35809999]
Laranga R, Focaccia M, Evangelista A, Lucarelli E, Donati DM, Spazzoli B. Bone Infarct-Associated Osteosarcoma: Epidemiologic and Survival Trends. Oncology research and treatment. 2022:45(6):326-335. doi: 10.1159/000521986. Epub 2022 Jan 14 [PubMed PMID: 35034028]
Domson GF, Shahlaee A, Reith JD, Bush CH, Gibbs CP. Infarct-associated bone sarcomas. Clinical orthopaedics and related research. 2009 Jul:467(7):1820-5. doi: 10.1007/s11999-009-0744-7. Epub 2009 Feb 20 [PubMed PMID: 19229663]
Konarski W, Poboży T, Hordowicz M, Śliwczyński A, Kotela I, Krakowiak J, Kotela A. Bone Infarcts and Tumorigenesis-Is There a Connection? A Mini-Mapping Review. International journal of environmental research and public health. 2022 Jul 29:19(15):. doi: 10.3390/ijerph19159282. Epub 2022 Jul 29 [PubMed PMID: 35954639]