Introduction
Dermatopathologists are frequently required to evaluate cysts, which are enclosed tissue sacs containing fluid or semisolid matter. An epithelium lines true cysts, and that epithelium may be native, originate from embryologic remnant tissue, or come from another part of the body entirely. Pseudocysts are the name for cystic structures that lack such an epithelium.[1] While numerous subtypes exist, cutaneous cysts can be simplified into 3 groups:
- Appendageal cysts or folliculosebaceous unit cysts, which are derived from dermal appendages (eg, hair follicles) as retention cysts
- Developmental cysts, which are derived from vestigial structures (eg, embryonic remnants)
- Miscellaneous cysts (some of which may be classified as pseudocysts) may be due to ectopic glandular tissue, lymphatic origin, infection, or deposition.
Cysts can also be histopathologically grouped with sinuses and pits, but these are not elucidated here. Occasionally, hybrid cysts with features suggestive of multiple lineages are encountered, such as an epidermal inclusion cyst that transitions to a trichilemmal cyst. Malignant transformation rarely occurs in otherwise typical cysts. A structured approach to the histopathologic diagnosis of cutaneous cysts involves the assessment of the following features:
- Epithelial lining
- Cyst contents
- Ancillary histopathologic features
- Clinical setting or clinical picture
Additional nuances include recognizing microscopic features or clinical settings where cysts may serve as cutaneous markers for an underlying genodermatosis or inflammatory disease. A practical approach to diagnosing true cysts involving the skin is provided.
Anatomical Pathology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomical Pathology
Appendageal Cysts or Folliculosebaceous Unit Cysts
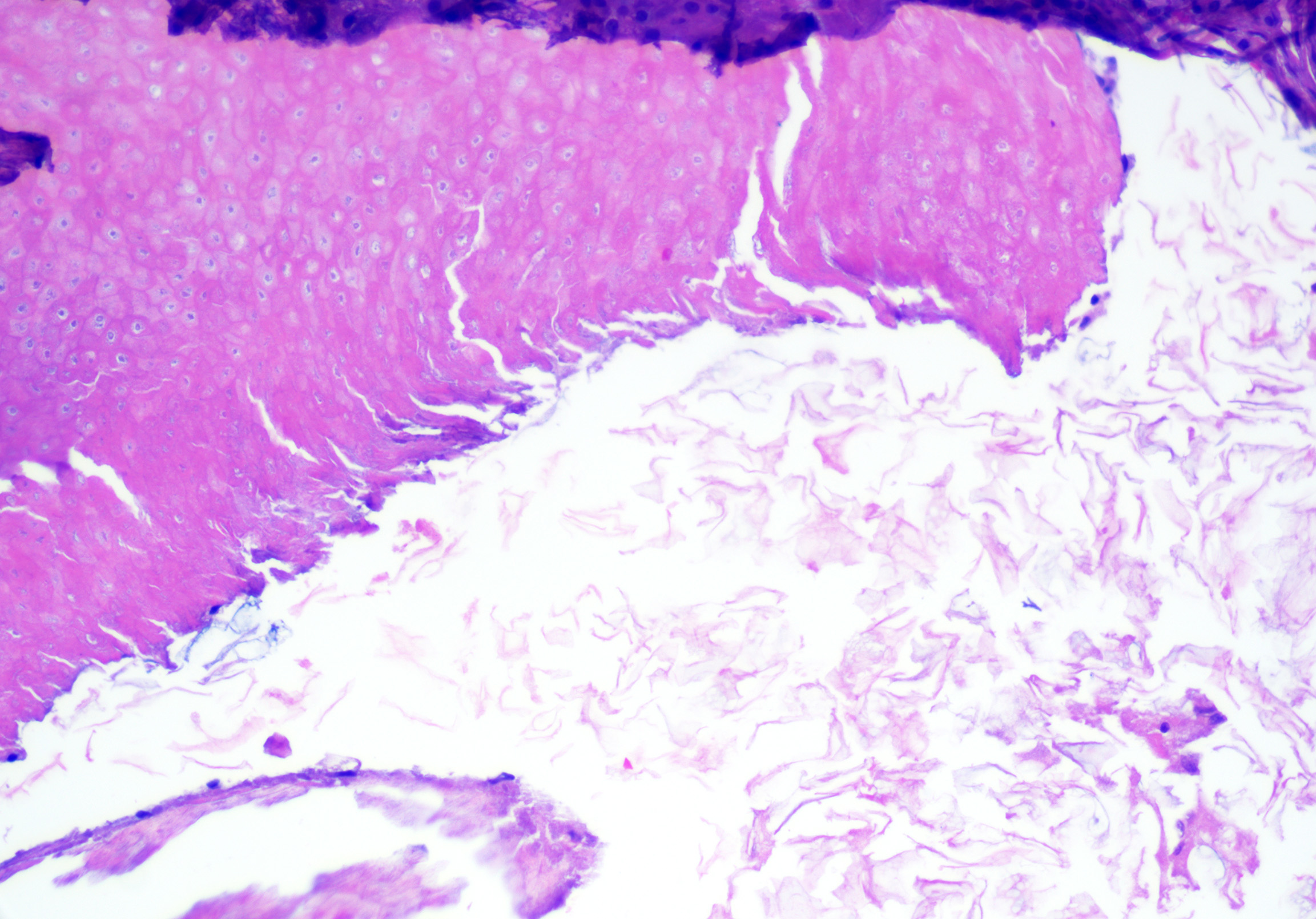
Epidermal inclusion cysts (ie, epidermoid cysts, infundibular cysts)
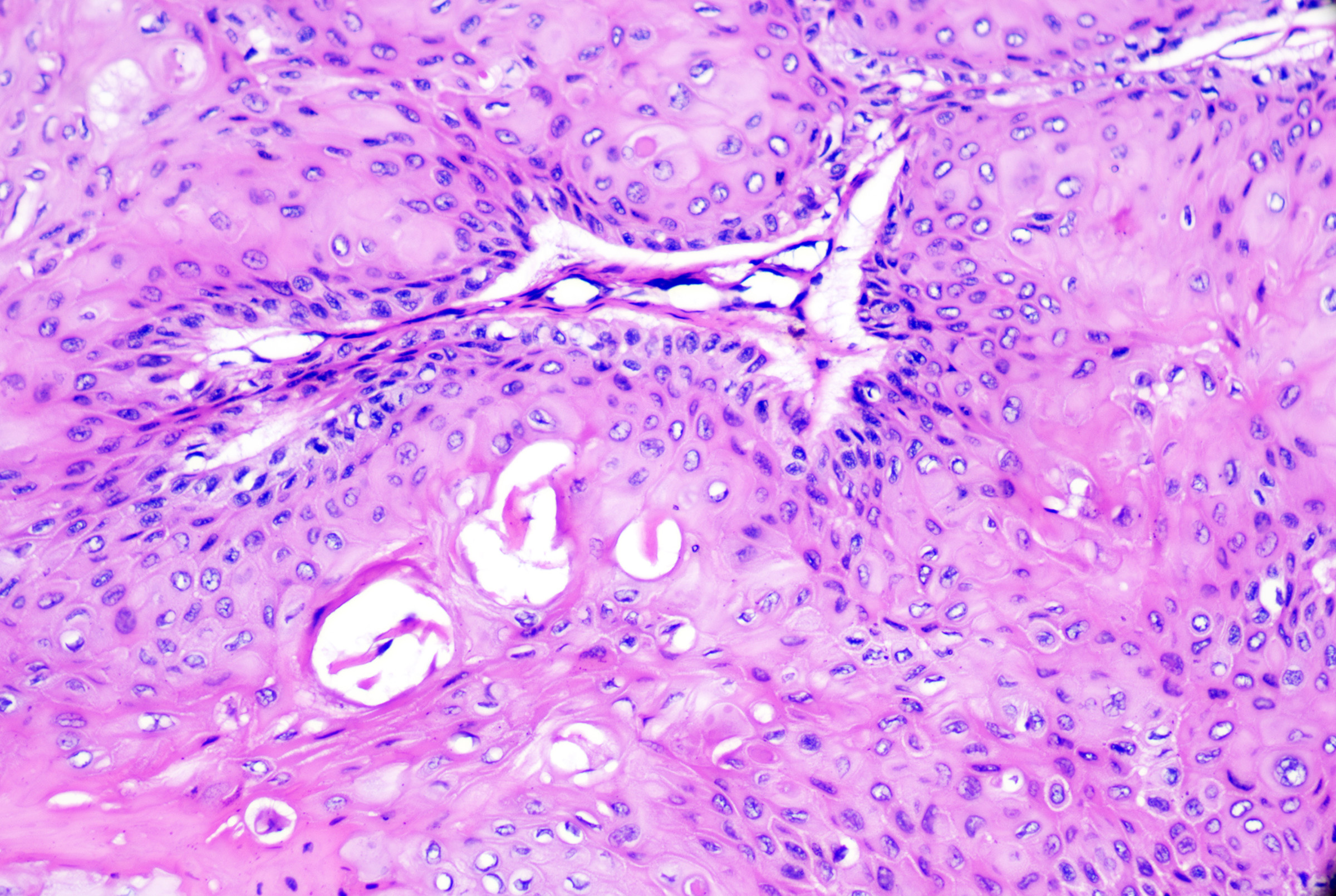
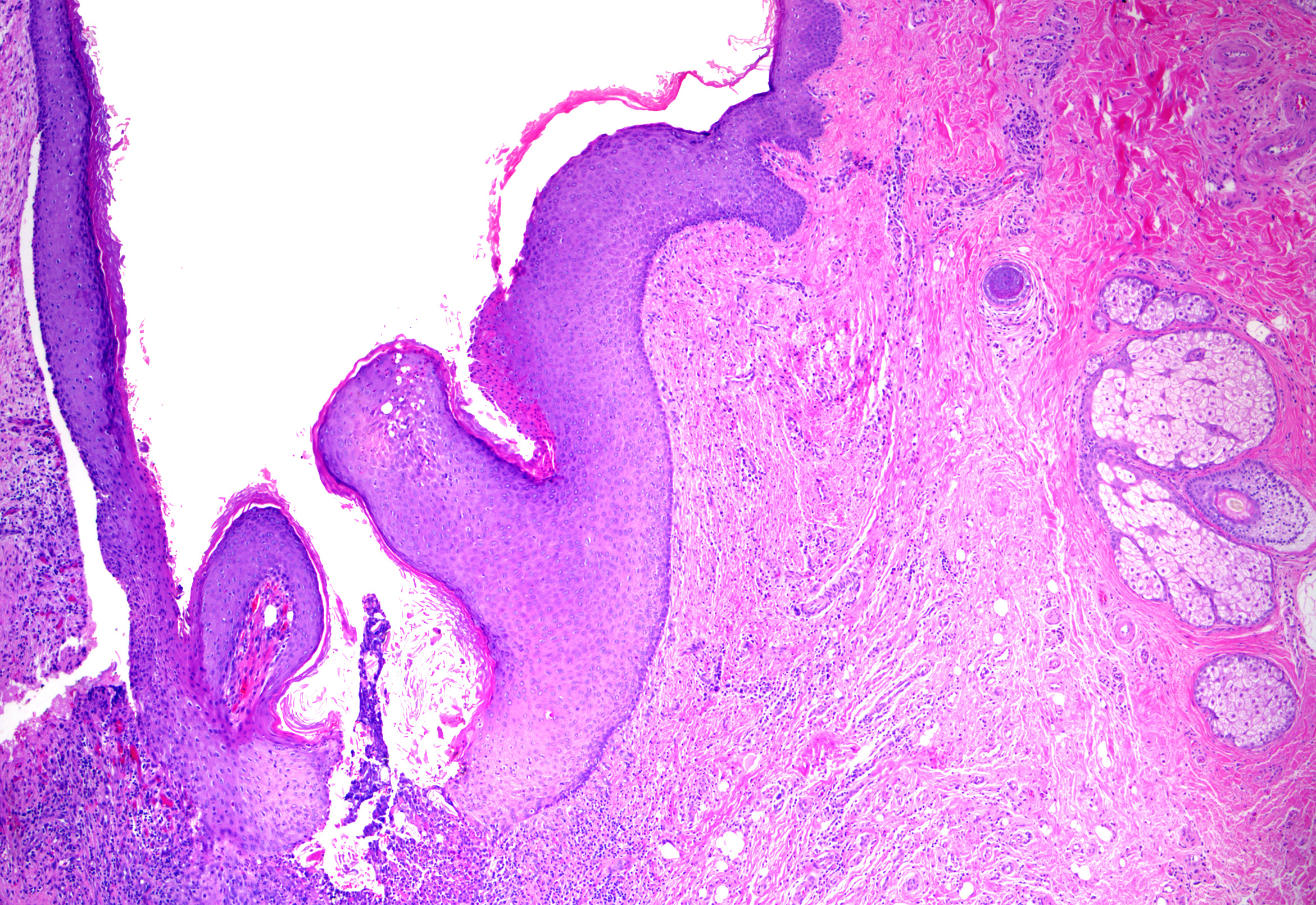
Epidermal inclusion cysts originate from and mimic the follicular infundibulum or traumatic dermal implantation of the epidermis. They are more common in males and present as solitary, slow-growing, 1 to 4-cm dermal nodules with a central punctum open to the overlying epidermis on the head and neck, trunk, upper extremities, and genitalia.[2] Palmoplantar lesions exist but may result from traumatic implantation of epidermal skin, human papillomavirus infection (most commonly HPV-60 infection), or dilatation of eccrine glands rather than follicular derivation.[3][4] Rupture, infection (usually due to Staphylococcus aureus overall or anaerobes in the genital area), or traumatization may result in rapid enlargement, erythema, pain, and drainage of purulent material. Epidermal inclusion cysts can be seen in many genodermatoses and inflammatory dermatoses, including Gardner syndrome (multiple epidermal inclusion cysts with biometrical differentiation), basal cell nevus syndrome (epidermal inclusion cysts, milia), and pachyonychia congenital.[5] See Image. Epidermoid Cyst With Pilomatrical Differentiation.
Microscopic features of epidermal inclusion cysts include:
- Open unilocular cyst (rarely multilocular)
- Stratified squamous epithelium with epidermal keratinization (ie, keratohyaline granules and flat surface epithelium) with retained granular layer (see Image. Epidermoid Cyst)
- Lamellated or shredded-appearing keratin without hair shafts (see Image. Epidermoid Cyst Lining)
- Keratin fragments (if ruptured), which may be the only clue
- Focal inflammation with features of abscess formation, granulation tissue, foreign body granulomatous reaction, or dermal fibrosis
Multiple variants of epidermal inclusion cysts exist in the literature, which include:
- Verrucous cyst: Similar to conventional epidermal inclusion cyst, however, the lining demonstrates papillomatosis, peaks of parakeratosis, coarse keratohyalin granules, and sometimes HPV-related koilocytosis.[6]
- Epidermal inclusion cyst with pilomatrical differentiation: Epidermal inclusion cysts with typical lining and foci of basaloid matrical cells and ghost cell or shadow cell keratinization (see Image. Epidermoid Cyst With Pilomatrical Differentiation). While sporadic lesions exist, these lesions have been reported in association with Gardner syndrome (a variant of familial adenomatous polyposis syndrome).[7]
- Proliferating epidermal inclusion cyst: Epidermal inclusion cysts with marked hyperplasia of lining epithelium, sometimes with features of verrucous epidermal inclusion cyst, squamous eddies, and outward extension into the surrounding dermis. This variant may have foci of carcinomatous change.[8]
- Hair matrix cyst: Epidermal inclusion cyst of basaloid cells of the normal hair matrix and amorphous keratinous content.
Milia
Milia are dermal cysts that originate from a pilosebaceous or eccrine sweat duct.[9] They may be physiologic in newborns, where they present on the head and neck, or appear later in life due to dermabrasion, topical corticosteroid use, radiotherapy, or secondary to mechanobullous diseases (eg, porphyria cutanea tarda, epidermolysis bullosa) or inflammatory diseases (eg, discoid lupus erythematosus, pseudoxanthoma elasticum).[5] Mycosis fungoides may present as milia with dense surrounding lymphocytic infiltration.[10] Histopathology is similar to a small epidermal inclusion cyst, with stratified squamous epithelium and a central keratinous area, which can be connected to vellus hairs or eccrine sweat ducts. Milia may be associated with Brooke-Spiegler syndrome, Rombo syndrome, Bazex-Dupre-Christol syndrome (possibly from UBE2A gene mutation), atrichia with papular lesions, porphyrias (eg, porphyria cutanea tarda), epidermolysis bullosa, generalized basaloid follicular hamartoma syndrome, and familial milia and absent dermatoglyphics.[5]
Comedones (ie, comedonal cysts)
Comedones are often confused with milia, though the former are keratinous plus in dilated pilosebaceous units. Closed comedones (ie, whitehead) and open comedones (ie, blackheads) are cystic dilatations of the sebaceous follicle with a darker appearance due to the oxidation of melanin when open. Histopathology reveals dilated hair follicles with significant keratinous material. Multiple closed comedones may be seen in Favre-Racouchot syndrome, chloracne, or familial dyskeratoses.[11]
Pigmented follicular cysts
Pigmented follicular cysts are rare lesions originating from the follicular infundibulum. They are pigmented papules most commonly reported in adult males on the head or neck. Vulvar lesions have also been reported.[12][13] On histopathology, pigmented follicular cysts are typically unilocular and may connect to the overlying epidermis. The lining and contents are identical to epidermal inclusion cysts but contain pigmented terminal-sized hairs (see Image. Follicular Pigmented Cyst). Unlike pigmented follicular cysts, pigmented epidermal cysts have melanin in the wall of the cyst or melanophages with surrounding stroma. In contrast, pigmented follicular cysts have rete ridges and dermal papillae in the lining, which is not usually seen in epidermal inclusion cysts. Rarely the cysts are hybrid, with concomitant trichilemmal differentiation and sebaceous glands in the wall.[12]
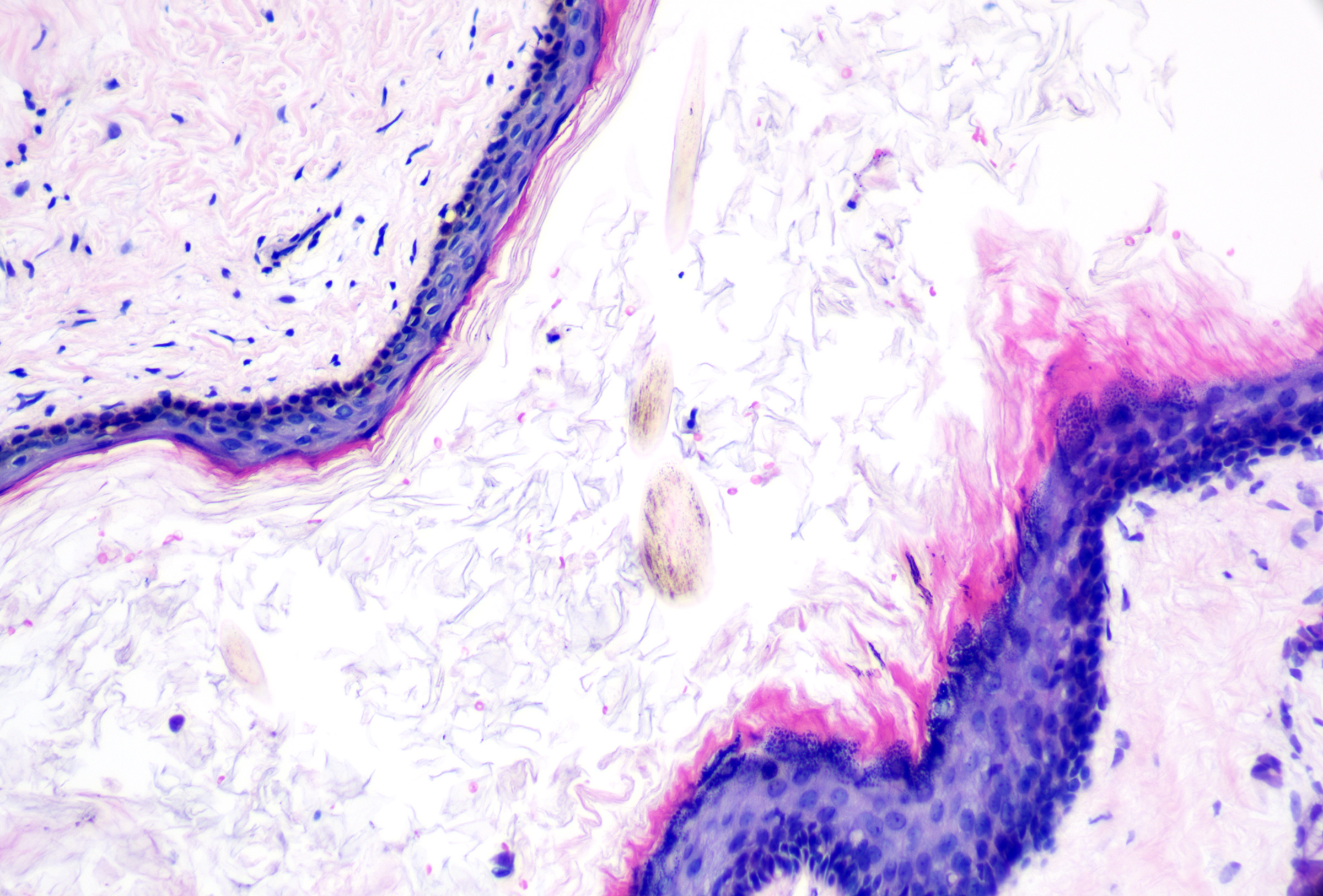
Vellus hair cysts
Vellus hair cysts originate from the infundibulum of vellus hairs. They present as small, often multiple, eruptive skin-colored or hyperpigmented papules. Isolated lesions and familial variants (usually with autosomal dominant inheritance) have also been reported.[14] The trunk and limbs are most commonly involved, often seen on the chest or axillae of young adults and children. Vellus hair cysts may coexist with steatocystomas, milia, or trichostasis spinulosa; while vellus hair cysts express keratin 17, steatocystomas typically express keratin 17 and keratin 10.[15][16] Syndromic associations with vellus hair cysts can be ectodermal dysplasia, Lowe syndrome (ie, oculocerebrorenal syndrome), familial eruptive vellus hair cysts, and pachyonychia congenita.[16][17][18]
Microscopic features of vellus hair cysts can include:
- Thin-walled unilocular cyst with epidermoid-type (ie, stratified squamous) epithelium
- Retained but subtle or attenuated granular layer (see Image. Vellus Hair Cyst 40x)
- Lamellated keratin and small, lightly pigmented vellus-sized hairs (see Image. Vellus Hair Cyst 100x)
- Possible attachment to overlying epidermis
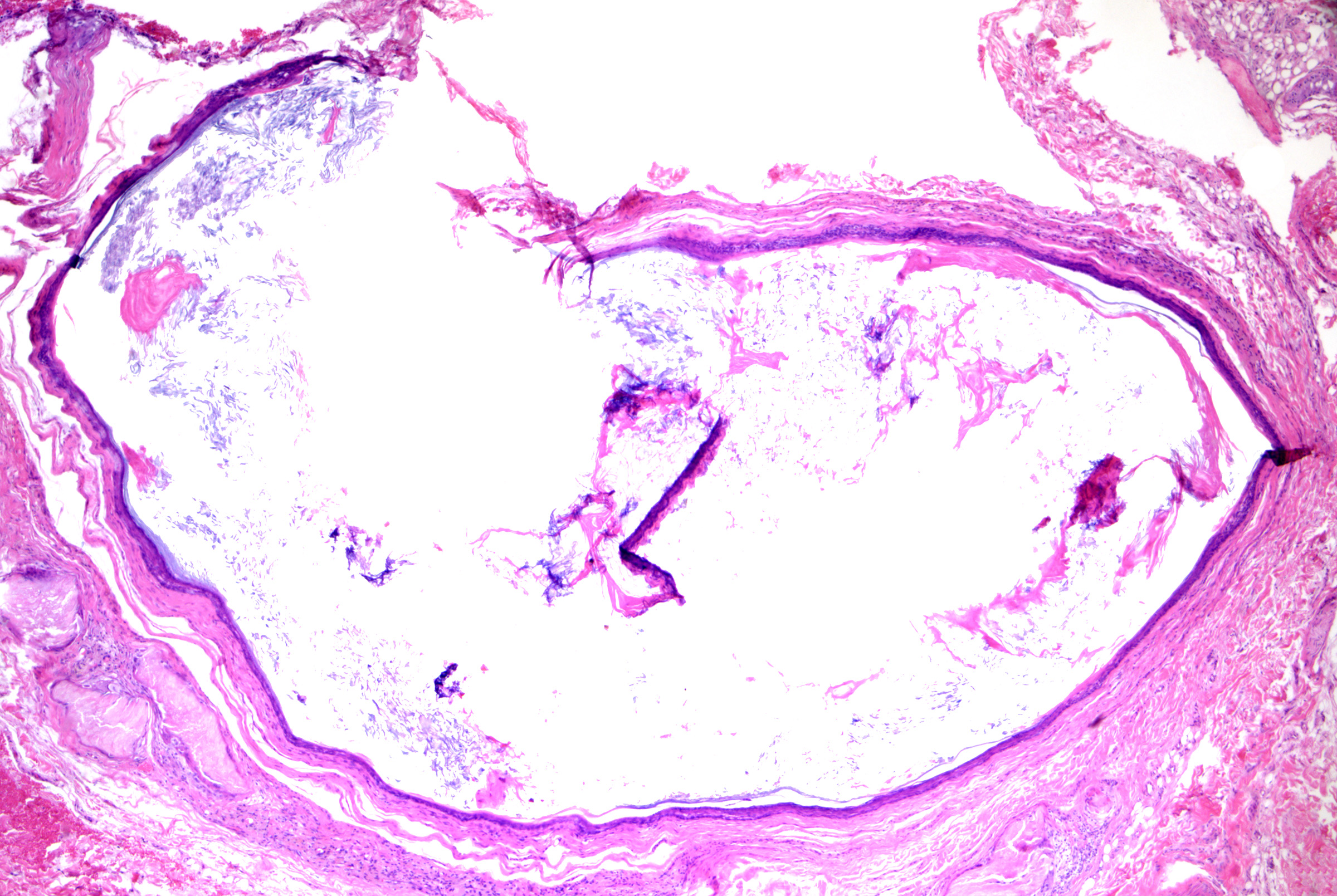
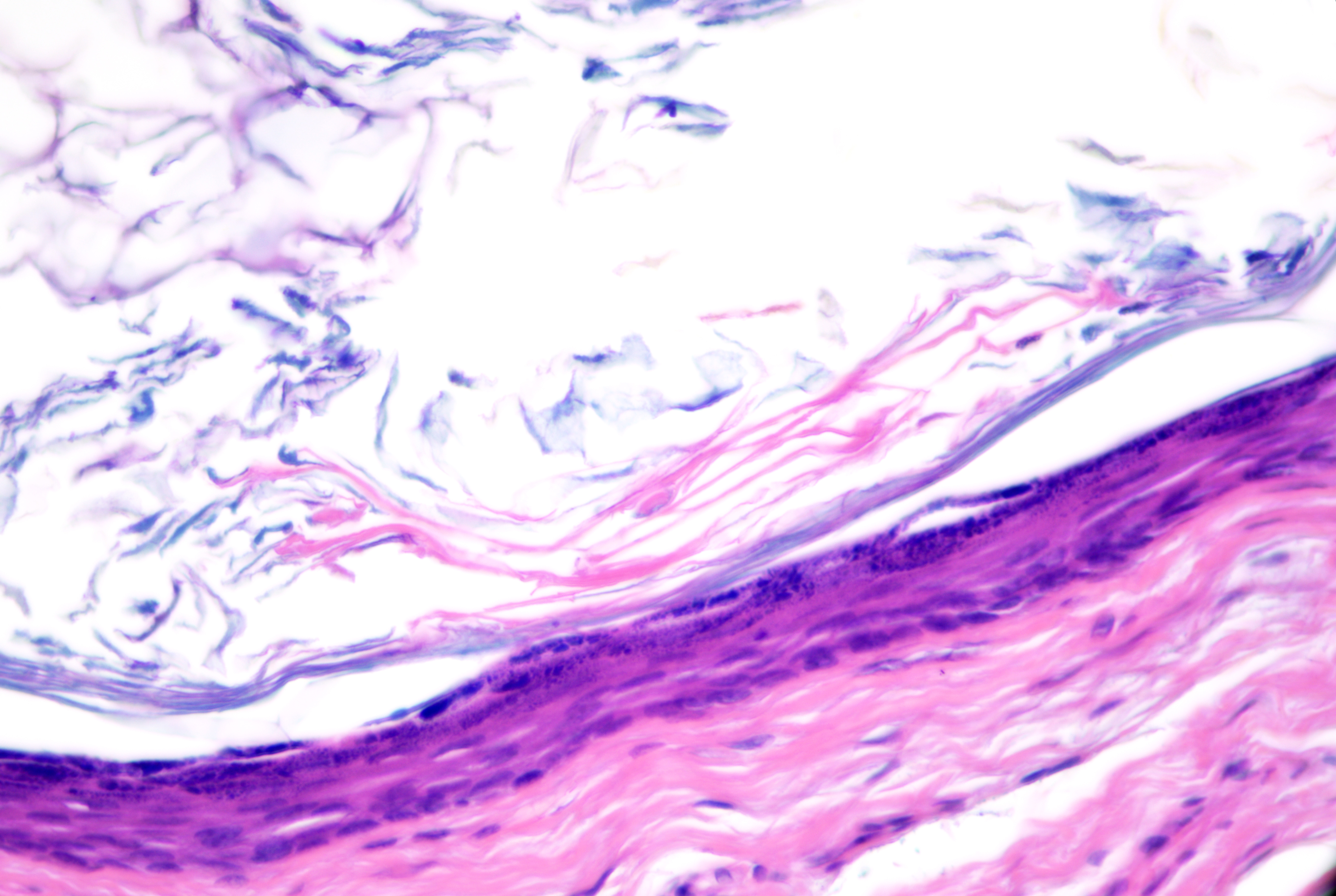
Pilar cysts (ie, trichilemmal cysts, isthmus-catagen cysts, sebaceous cysts)
Pilar cysts originate from the external (outer) root sheath at the level of the follicular isthmus. The cysts usually present as a solitary nodule most commonly located on the scalp of a female adult.[19] During treatment, cysts can be easily removed with the cyst lining intact, giving a shelled-out appearance. Cystic trichilemmal carcinoma is an in-situ carcinoma that may be found within a pilar cyst (see Image. Trichilemmal Carcinoma).[20][21]
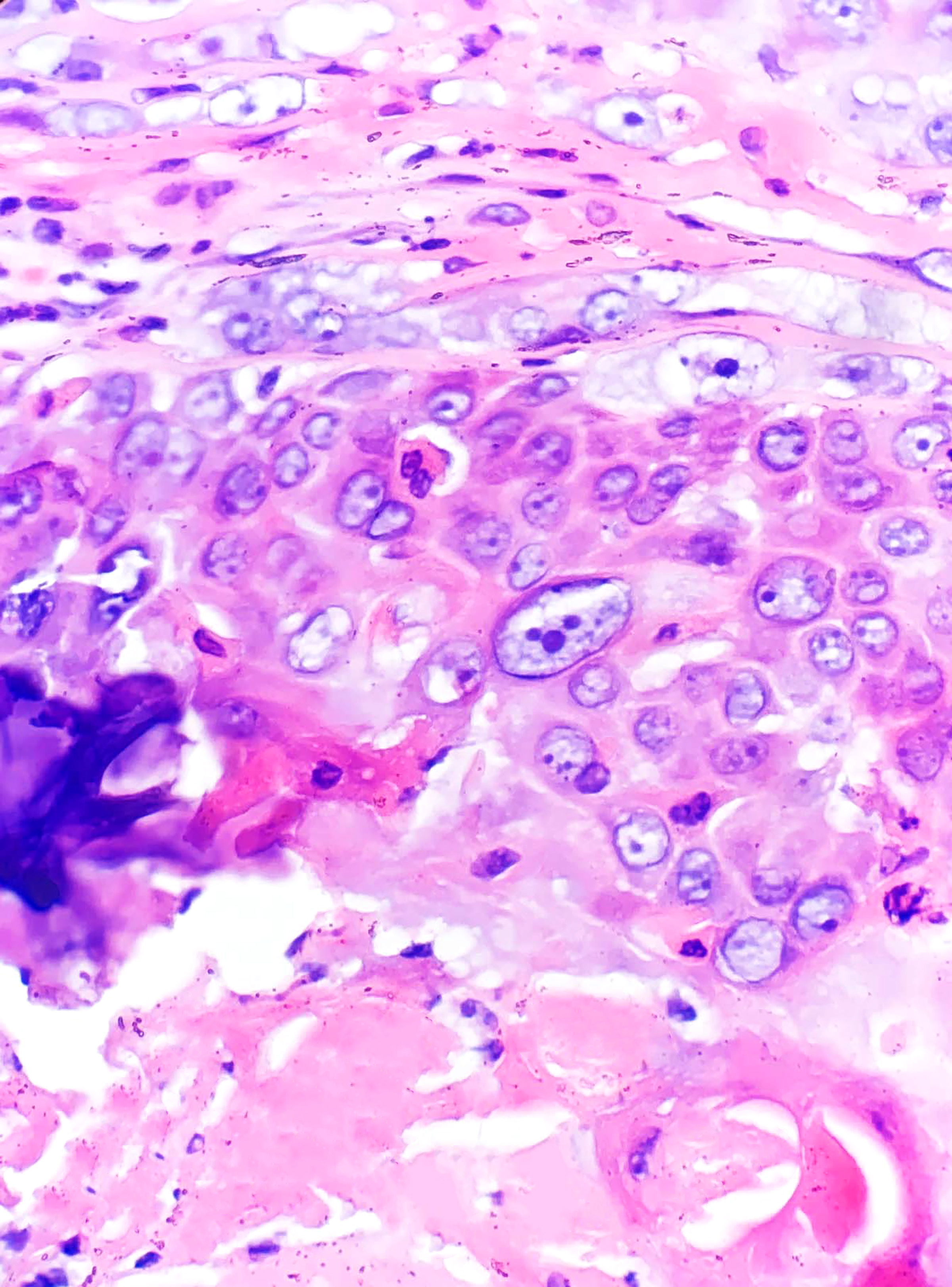
Proliferating pilar cysts (ie, proliferating pilar tumors) are variants usually found on the scalps of older women thought to be related to squamous cell carcinoma. Chromosomal alterations (15q, 6q, and 6p22.2) have been described in these lesions.[22] Lesions (particularly with atypical histopathologic features) may exhibit aggressive behavior.[23] Multiple lesions have been described.[24] Histopathology usually reveals a multinodular dermal-based tumor with inward lobular squamous cell proliferation (as opposed to outward squamous cell nest proliferation in proliferating epidermal cysts) and peripheral palisading. More benign variants have a pushing/rounded border (see Image. Proliferating Pilar Tumor) and no/mild cytologic atypia (see Image. Pilar Tumor Epithelium). Atypical lesions have more deeply infiltrating borders but lack anaplasia. Tumors with marked cytologic atypia, atypical mitoses, an invasive growth pattern, or geographic tumor necrosis carry a risk of metastatic disease. They are best classified as malignant proliferating trichilemmal tumors (see Image. Proliferating Trichilemmal Tumor).[25]
Microscopic features of pilar cysts can include:
- Unilocular (occasionally multilocular), without connection to the epidermis (ie, no punctum)
- Stratified squamous epithelium lining with trichilemmal keratinization (with keratin 10/17 expression)
- Cells with basaloid nuclei that become larger with abundant pale cytoplasm towards the cyst lumen and keratinize to eosinophilic compact keratin without an intervening granular layer (see Image. Pilar Cyst)
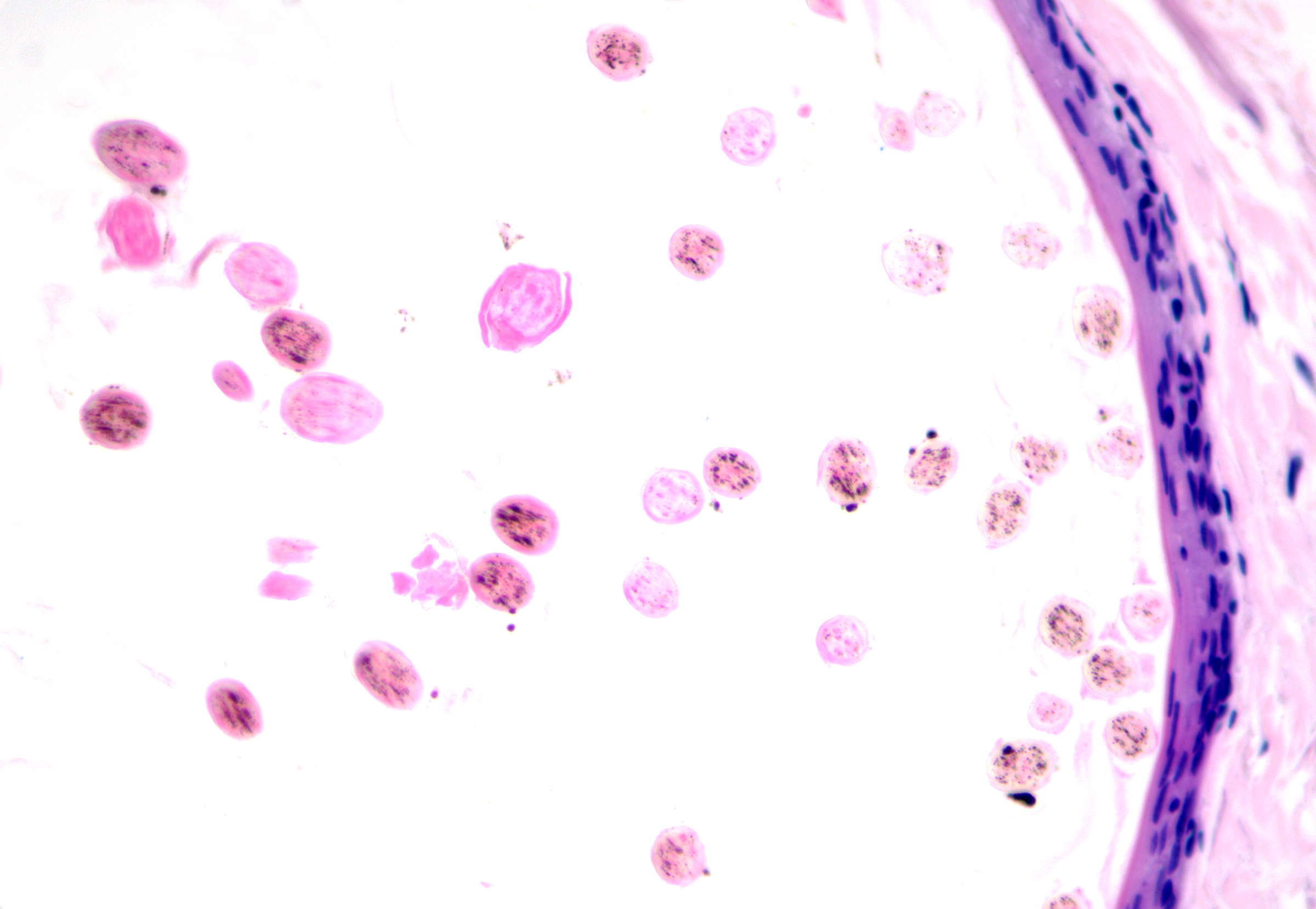
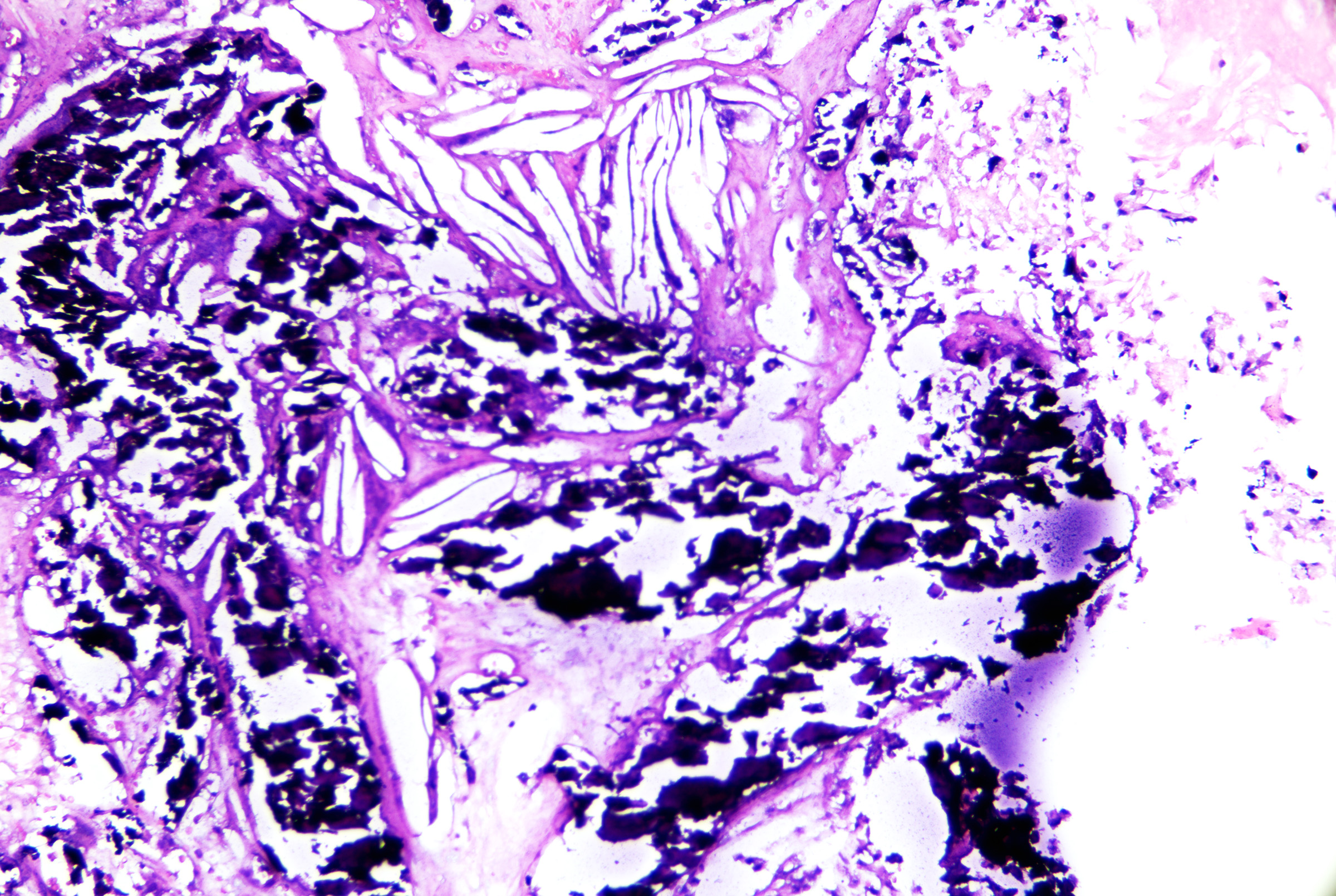
- Calcification and cholesterol clefts in the cyst contents (see Image. Pilar Cyst Contents).
Onycholemmal cysts
Onycholemmal cysts are rare subungual dermal cysts that can present with nail changes (eg, onychodystrophy). Histopathology reveals a free-lying cyst without a granular layer in the nail bed with onycholemmal keratin and calcification. Malignant lesions, which will show nests and strands of atypical keratinocytes, have been reported.[26]
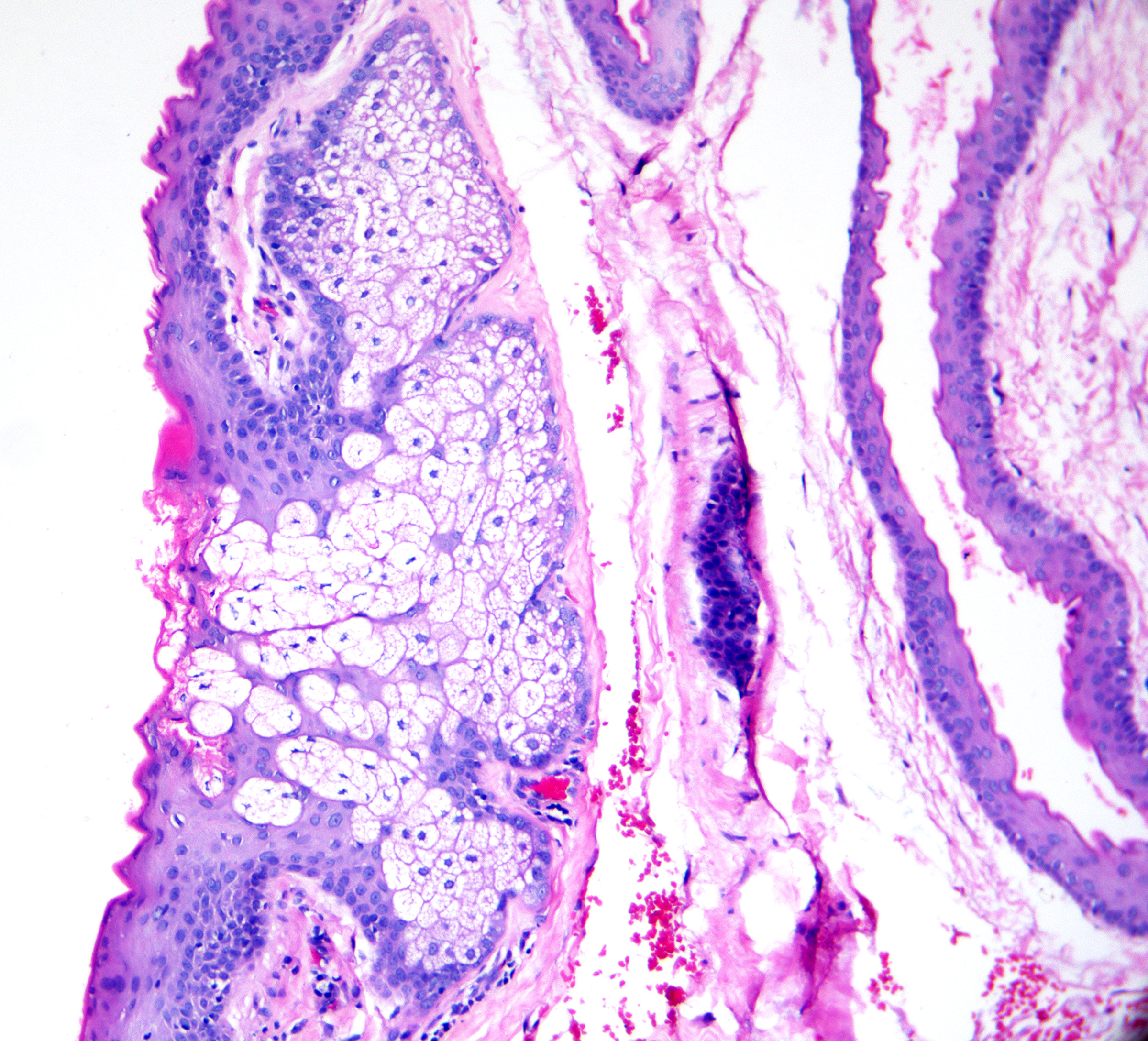
Steatocystoma
Steatocystomas are cysts that originate from the sebaceous duct. They may be solitary (ie, steatocystoma simplex) or multiple (steatocystoma multiplex) skin-colored or yellow papules or nodules involving hair-bearing areas (eg, chest, trunk, axilla, genitalia, face).[27] Rare acral cysts have been described.[28] Lesions lack a punctum and drain an oily fluid if ruptured; ruptured lesions can also become infected, called steatocystoma multiplex suppurativum. Steatocystoma may coexist with a vellus hair cyst, but steatocystoma with trichoblastoma or trichoepithelioma is thought to portend an unfavorable prognosis.[29]
Heritable and syndromic forms of steatocystoma exist, many of which are autosomal dominant.[30][31] Many other syndromes are associated with steatocystoma, including familial steatocystoma, pachyonychia congenita, and Alagille syndrome; the coexistence of Alagille syndrome and steatocystomas is thought to portend a favorable prognosis.[30][31]
Microscopic features of steatocystoma can include:
- Dermal-based cyst, often exhibiting a collapsed or folded architecture
- Stratified squamous epithelium with an eosinophilic, corrugated cuticle (recapitulating the sebaceous duct)
- Undulating cuticle that resembles shark fins (see Image. Steatocystoma)
- Sebaceous glands that are present in or adjacent to the cyst wall (see Image. Steatocystoma Cuticle)
- Foci of flaky keratin
Lesions without associated sebaceous glands are termed cutaneous keratocysts. They may be seen in association with basal cell nevus syndrome, usually as epidermal cysts with thick brown fluid rather than keratocysts of the jaw. Cutaneous keratocysts have a festooned configuration with squamous epithelium and no granular layer. Expression of D2-40 in the basal layer of cutaneous keratocysts has been described in basal cell nevus syndrome–associated cutaneous keratocysts.[32]
Follicular hybrid cysts
Follicular hybrid cysts originate from multiple concomitant regions of the folliculosebaceous apparatus. Their clinical features are variable but usually present as solitary, nonspecific cystic lesions. Histopathology usually reveals cysts with architectural and keratinization patterns reflecting multiple zones of origin. Combinations of infundibular, isthmic, sebaceous duct, apocrine, and matrical differentiation have been reported.[33][34]
Cystic panfolliculoma
Cystic panfolliculoma is an uncommon, benign tumor on the head in older adults that recapitulates multiple parts of the hair follicle (eg, infundibulum, isthmus, matrix).[35][36] Histopathology reveals a well-circumscribed cyst with foci of differentiation from multiple parts of the hair follicle (eg, thin infundibular epithelium with foci of matrical or outer root sheath differentiation); BerEP4 can be used to label the follicular germinative differentiation peripherally, while Cytokeratin 903 and Cytokeratin 5/6 can stain the tumor cells.[37]
Hidrocystomas
Hidrocystomas originate from native apocrine and eccrine glands. Hidrocystomas are considered tumors rather than simple cysts, although eccrine hidrocystomas may represent marked dilatation of otherwise normal glands. By light microscopy, some eccrine-like tumors may exhibit immunohistochemical protein expression of apocrine tumors.[38]
Eccrine hidrocystomas present as solitary or multiple lesions on the cheeks or periorbital skin, and they may get more extensive in hot environments and with sweating. They may be associated with squamous cell carcinoma. True eccrine hidrocystomas are negative for HMFG-1; some lesions with histopathologic features of eccrine hidrocystomas express apocrine markers. Thus, accurate classification as eccrine may be impossible based solely on cytomorphologic features.[38] There is controversy about whether eccrine and apocrine hidrocystomas are unique entities, with many sources favoring that eccrine hidrocystomas are apocrine hidrocystomas.
Apocrine hidrocystomas (ie, apocrine gland cysts, apocrine cystadenomas) are proliferations of apocrine glands that present as solitary translucent, skin-colored to dark blue papules with a preference for the eyelid margin.[39] Tumors with solid and prominent papillary areas are best termed apocrine papillary cystadenomas. Apocrine hidrocystomas express human milk fat globulin-1 (HMFG-1); the myoepithelial layer expresses p63 and SMA.[38] Apocrine hidrocystomas may be associated with Schopf-Schulz-Passarge syndrome.[40]
Microscopic features of eccrine hidrocystomas can include:[41]
- Unilocular cyst near eccrine glands
- One to 2 layers of cuboidal epithelium without decapitation secretion
- Lack of myoepithelial layer (see Image. Eccrine Hidrocystoma)
Microscopic features of apocrine hidrocystomas can include:
- Unilocular or multilocular cyst
- One to 2 layers of cuboidal or columnar epithelium with decapitation secretion
- A flat layer of myoepithelial cells (see Image. Apocrine Hidrocystoma)
- Presence of papillae
Developmental Cysts
Dermoid cysts
Dermoid cysts are congenital cysts resulting from trapping of surface ectoderm along embryonic fusion lines.[42] They most commonly present as asymptomatic congenital cystic swelling of the lateral one-third of the eyebrow. Occasionally, they may be diagnosed in later childhood or adulthood. They can also be found in other locations, like the nasal root, glabella, and scalp, where they may be collocated with a tuft of hair.[43][44][45][46] Microscopic features of dermoid cysts reveal dermal-based cysts lined by nonkeratinizing stratified squamous epithelium with a retained granular layer. They contain epidermoid-type, lamellated keratin. Folliculosebaceous elements (eg, hair follicles, sebaceous glands), eccrine glands, apocrine glands, and arrector pili smooth muscle may be present (see Image. Dermoid Cyst).[47]
Lesions with true ectodermal, mesodermal, and endodermal differentiation are termed mature cystic teratomas; these rare tumors of the skin present at the glabella, back, or knee with multiple types of tissue (eg, gastrointestinal mucosa, thyroid, or respiratory epithelium).[48][49]
Bronchogenic cysts
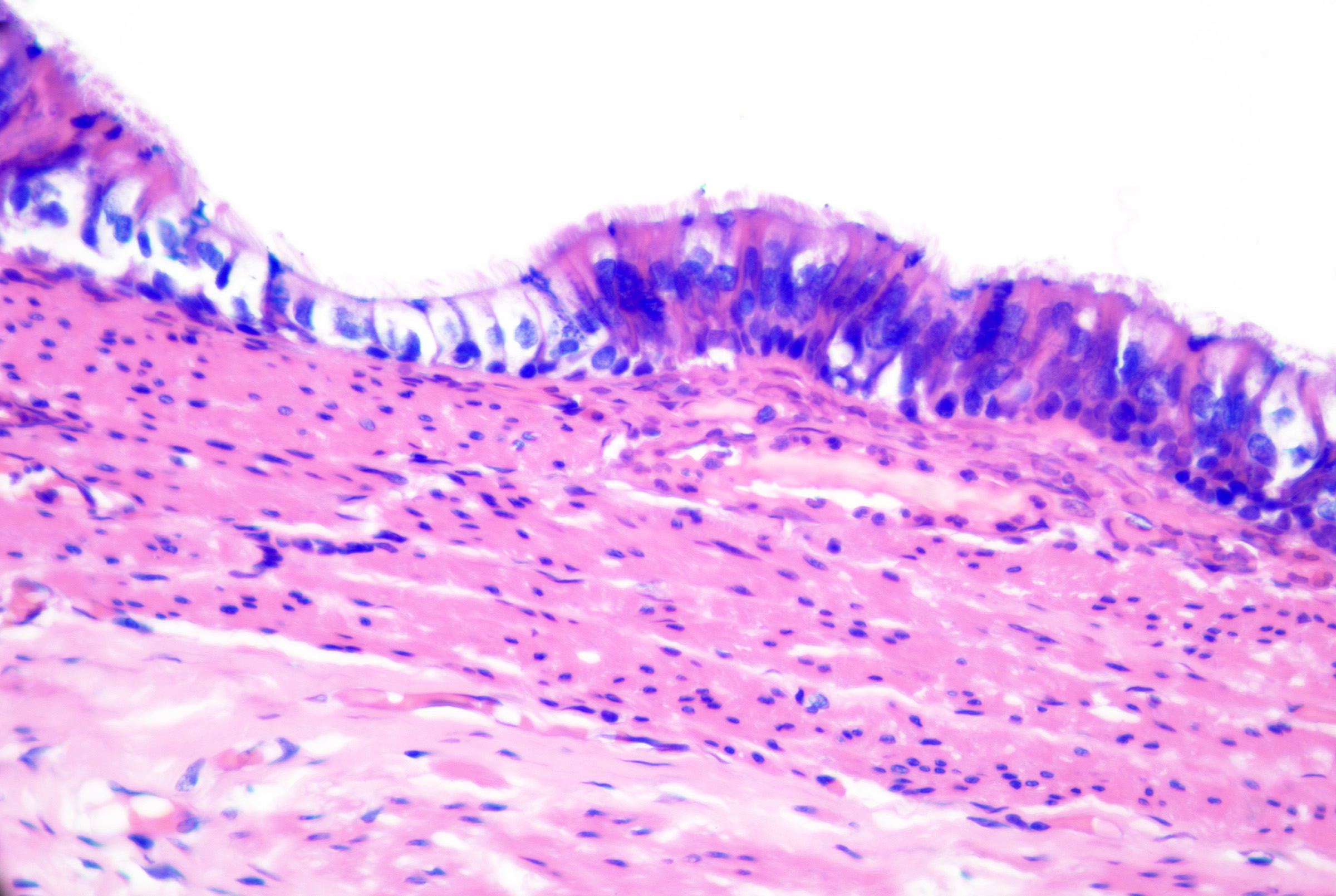
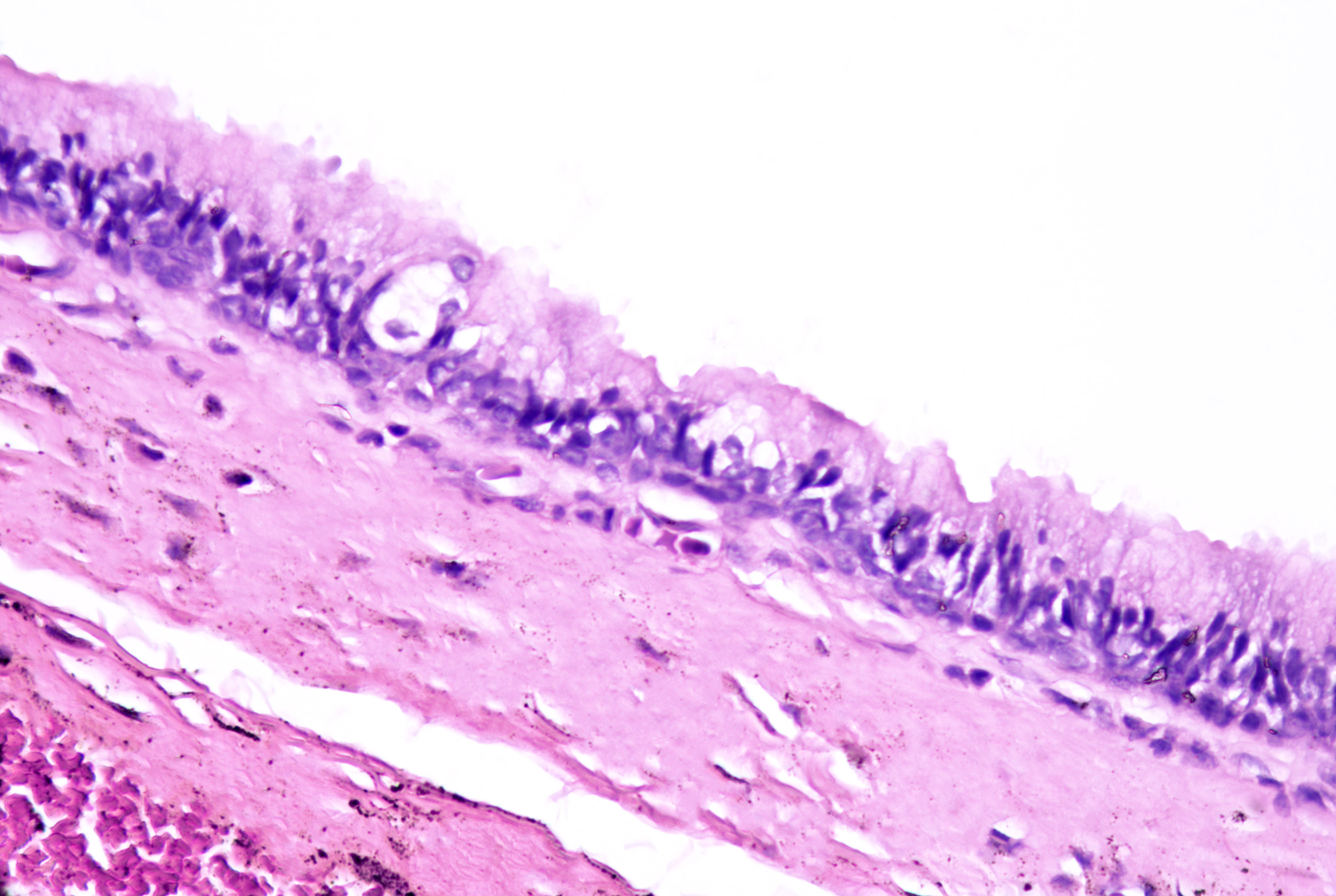
Bronchogenic cysts originate from sequestered respiratory epithelium from embryonic tracheobronchial development. They present soon after birth as midline cystic nodules in the suprasternal notch (most common), neck, back, or chin.[50] Males are affected more commonly than females.[51][50] Histopathology reveals a ciliated pseudostratified columnar or cuboidal respiratory-type epithelium (see Image. Bronchogenic Cysts). Stratified squamous epithelium may be focally present. Goblet cells are commonly identified, and surrounding smooth muscle (see Image. Bronchogenic Cysts With Muscle and Glands), glandular tissue, lymphoid follicles, and rarely cartilage may be observed.[47][52]
Branchial cleft cysts
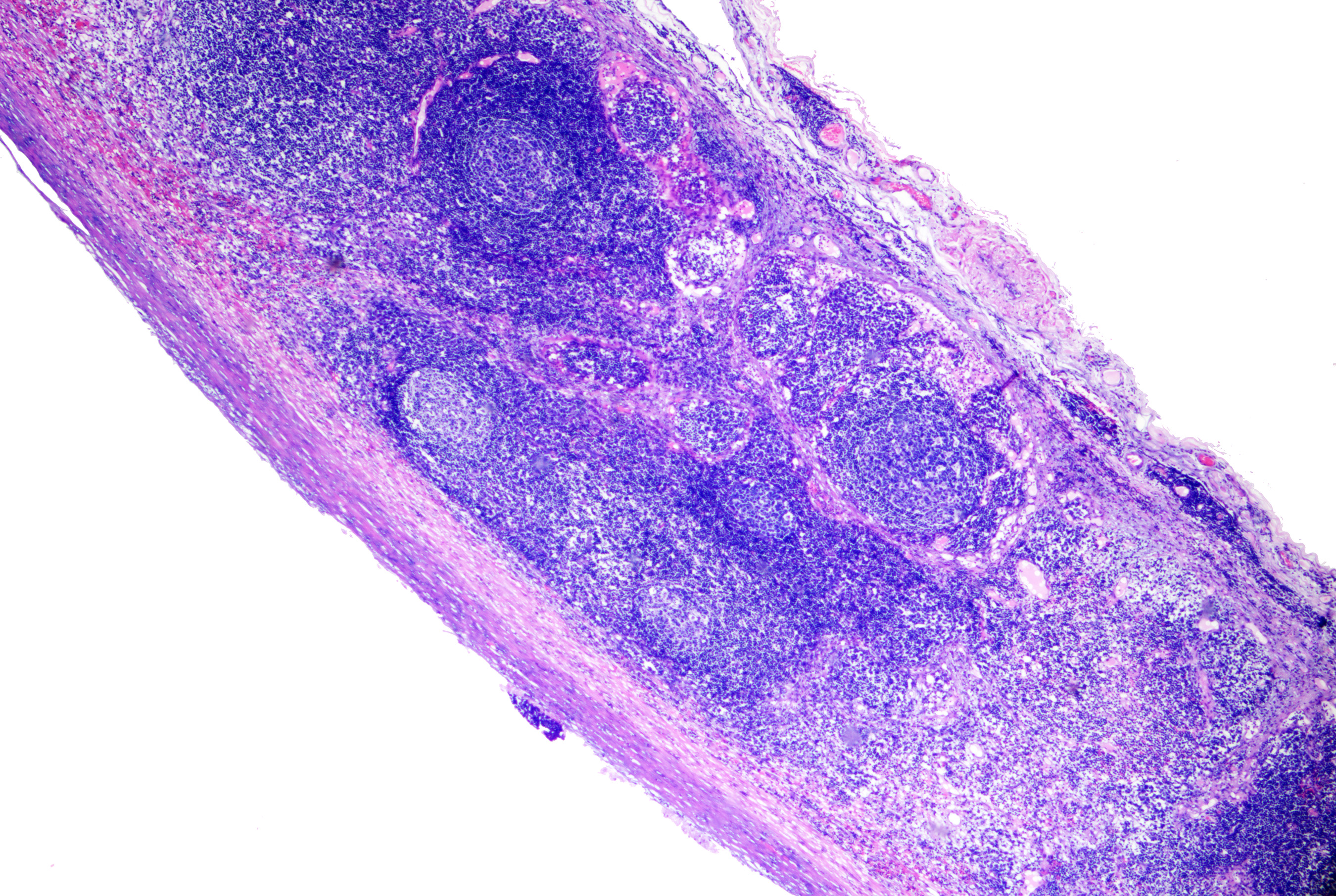
The origin of branchial cleft cysts is not precisely known, but they may be from branchial cleft mucosal remnants and cystic changes in cervical chain lymph nodes; it is thought that material from the second branchial pouch leads to the formation of cysts on the anterior sternocleidomastoid in young patients, while material from the first branchial pouch leads to the formation of cysts at the auricular or mandibular sites.[53] These cysts present laterally located cystic nodules in the preauricular area, along the mandible, or anywhere from the jaw's angle to the sternocleidomastoid muscle's anterior border.[54] Lesions are typically evident in childhood or early adulthood.[55] The microscopic features of branchial cleft cysts include a stratified squamous or pseudostratified columnar epithelium, which may be ciliated, striking lymphoid follicle formation, and turbid fluid rich in cholesterol (see Image. Branchial Cyst).
Thyroglossal duct cysts
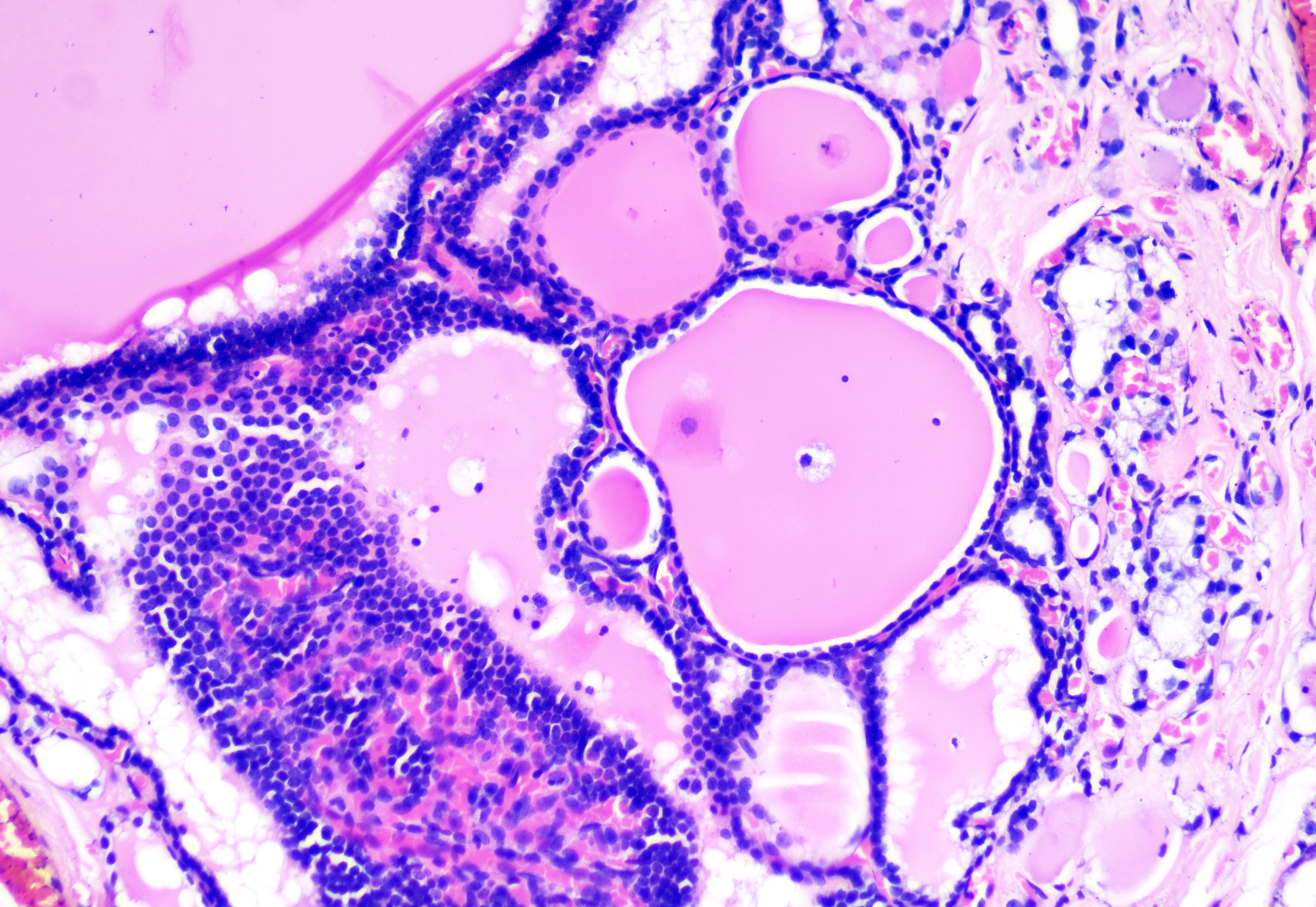
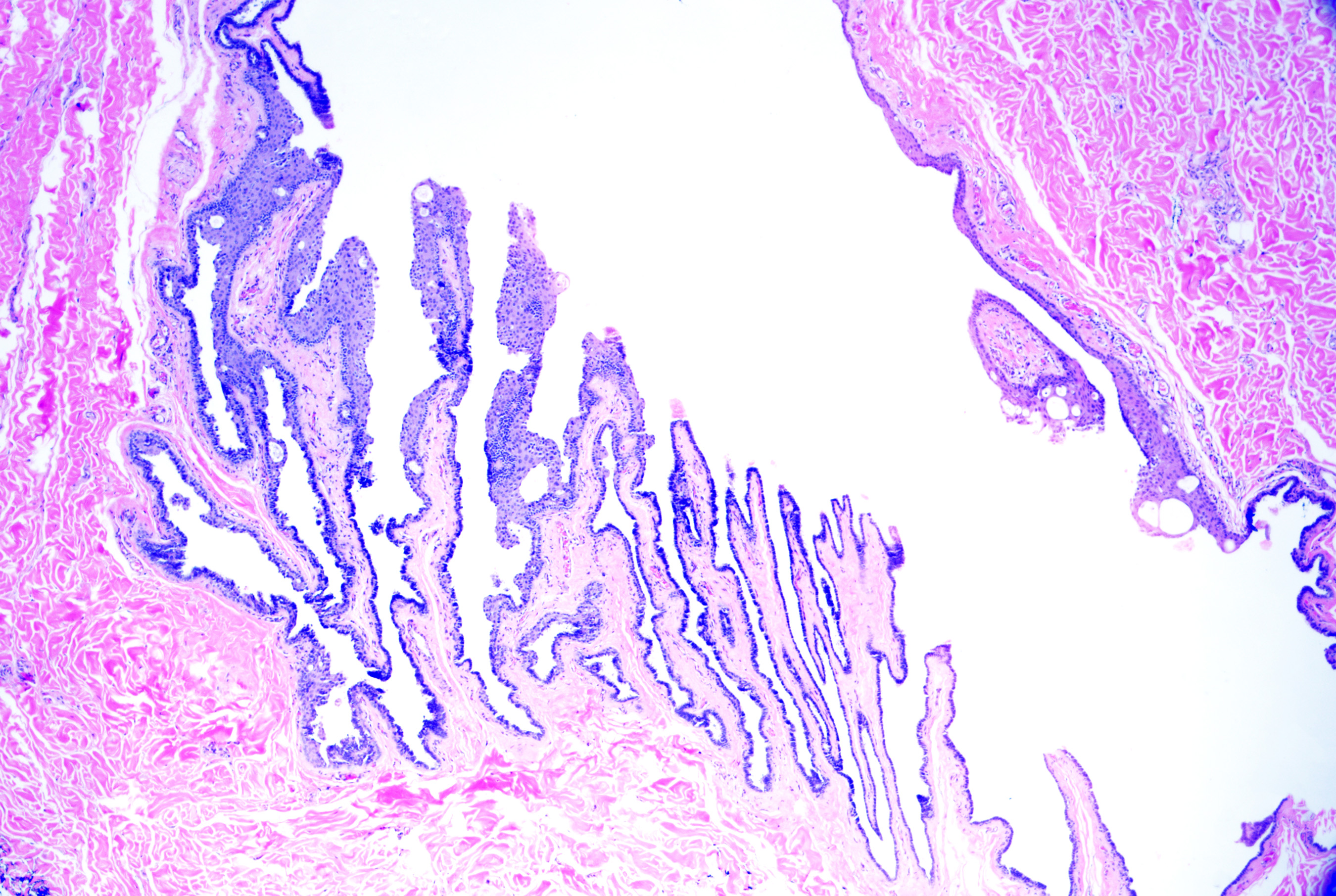
Thyroglossal duct cysts arise from remnants of the thyroglossal duct that form as the thyroid descends from the pharyngeal floor to the anterior neck. They present as a midline cystic nodule on the anterior neck that moves with swallowing and tongue protrusion; they can also present as lateral cystic nodules from foregut remnants. Children and young adults are most commonly affected.[56] The microscopic features include variable lining epithelium, which may be columnar (with or without cilia), cuboidal, or stratified squamous (see Image. Thyroglossal Duct Cyst). Thyroid follicles (ie, cuboidal cells surrounding amorphous pink material called thyroid colloid) are present in the surrounding tissue (see Image. Thyroglossal Cyst With Follicles).
Cervical thymic cysts
Cervical thymic cysts are thymopharyngeal duct remnants, mostly remnants of the thymopharyngeal duct, a derivative of the third pharyngeal pouch.[57] They present in the mediastinum or neck area as asymptomatic anterior masses, usually lateral to the left of the thyroid.[57] The microscopic features include variable lining, which may be stratified or simple, and squamous or columnar. Cilia may be present. Hassall's corpuscles are typically identified. Lymphoid follicle formation and cholesterol granulomas may also be seen.[47][51]
Cutaneous ciliated cysts
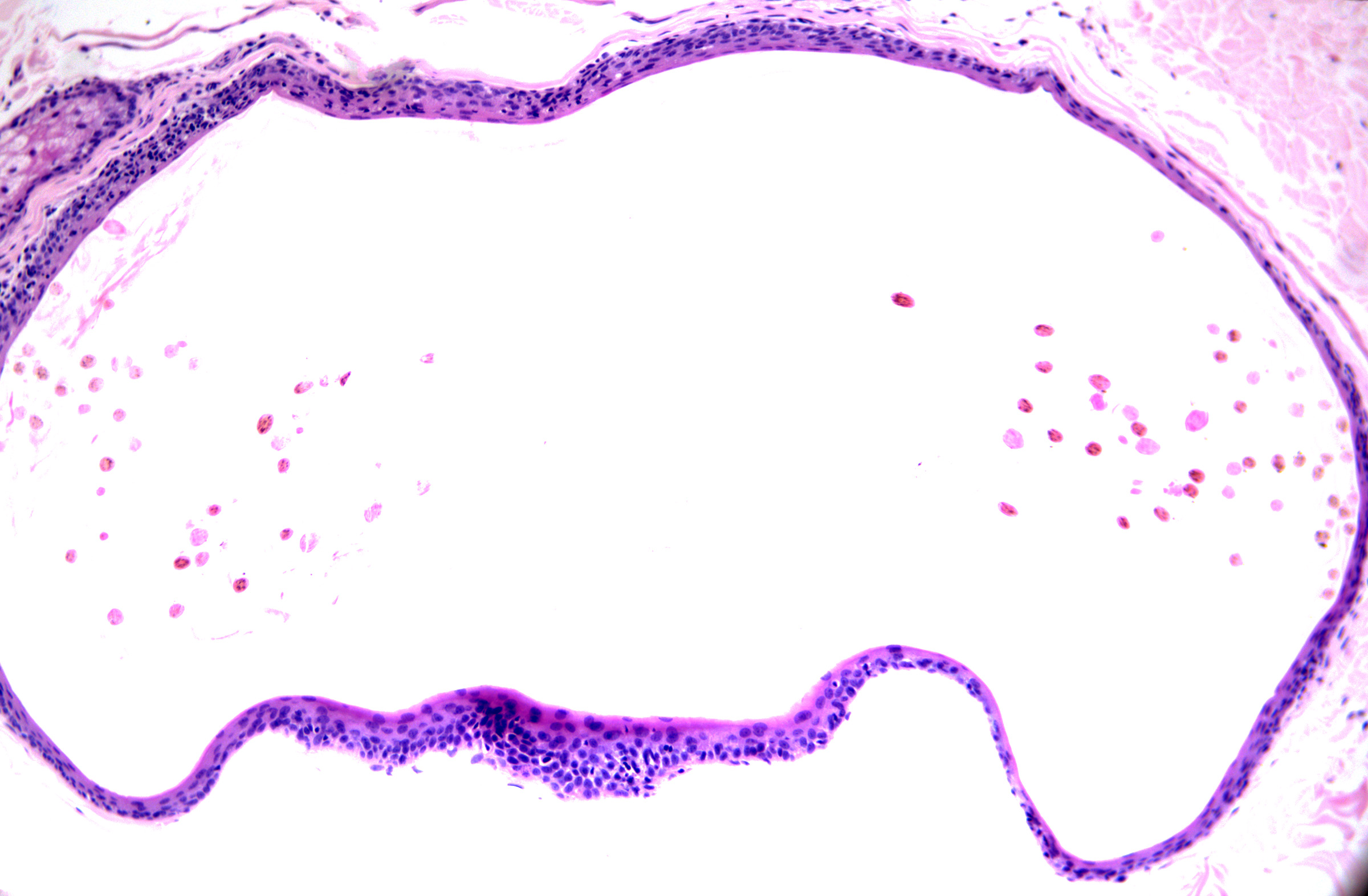
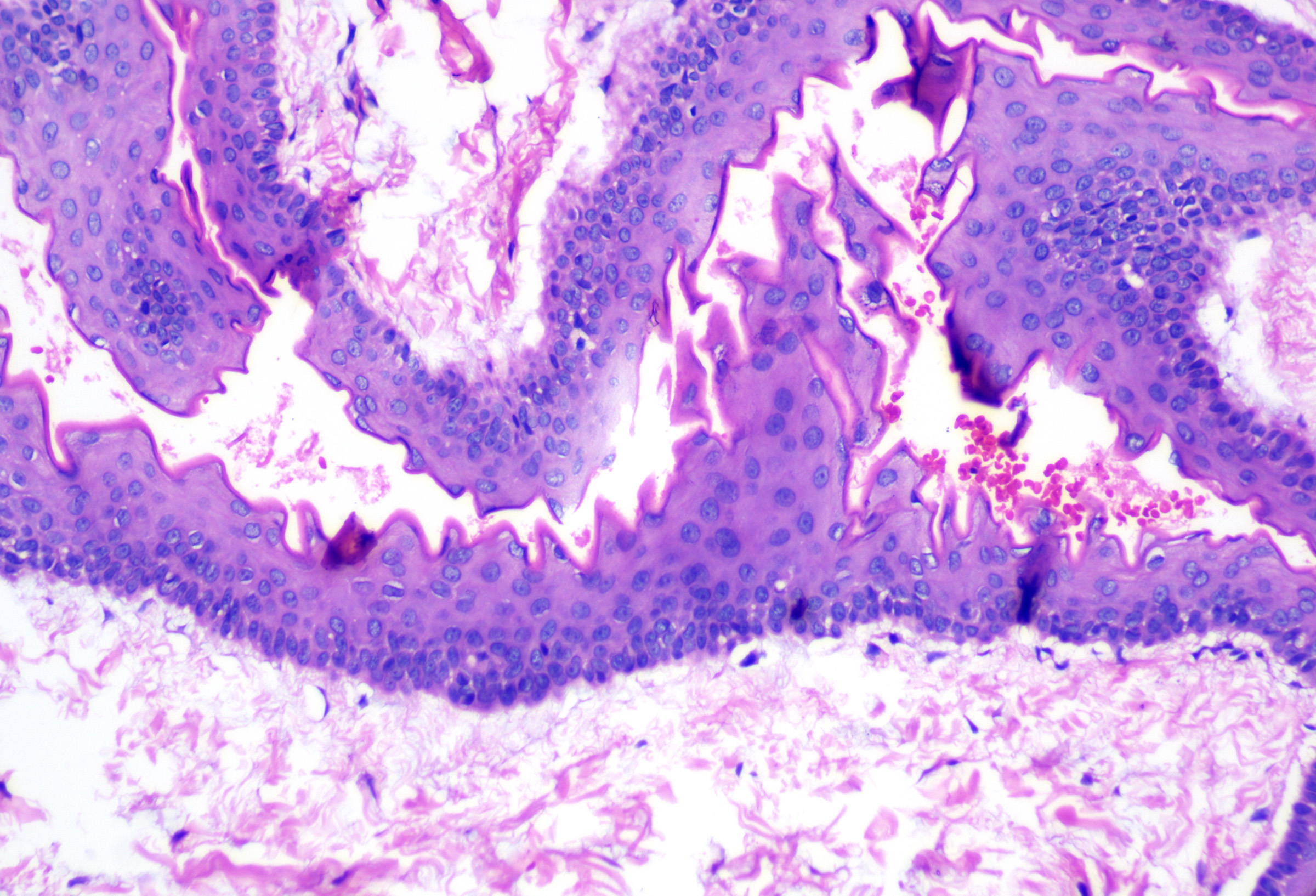
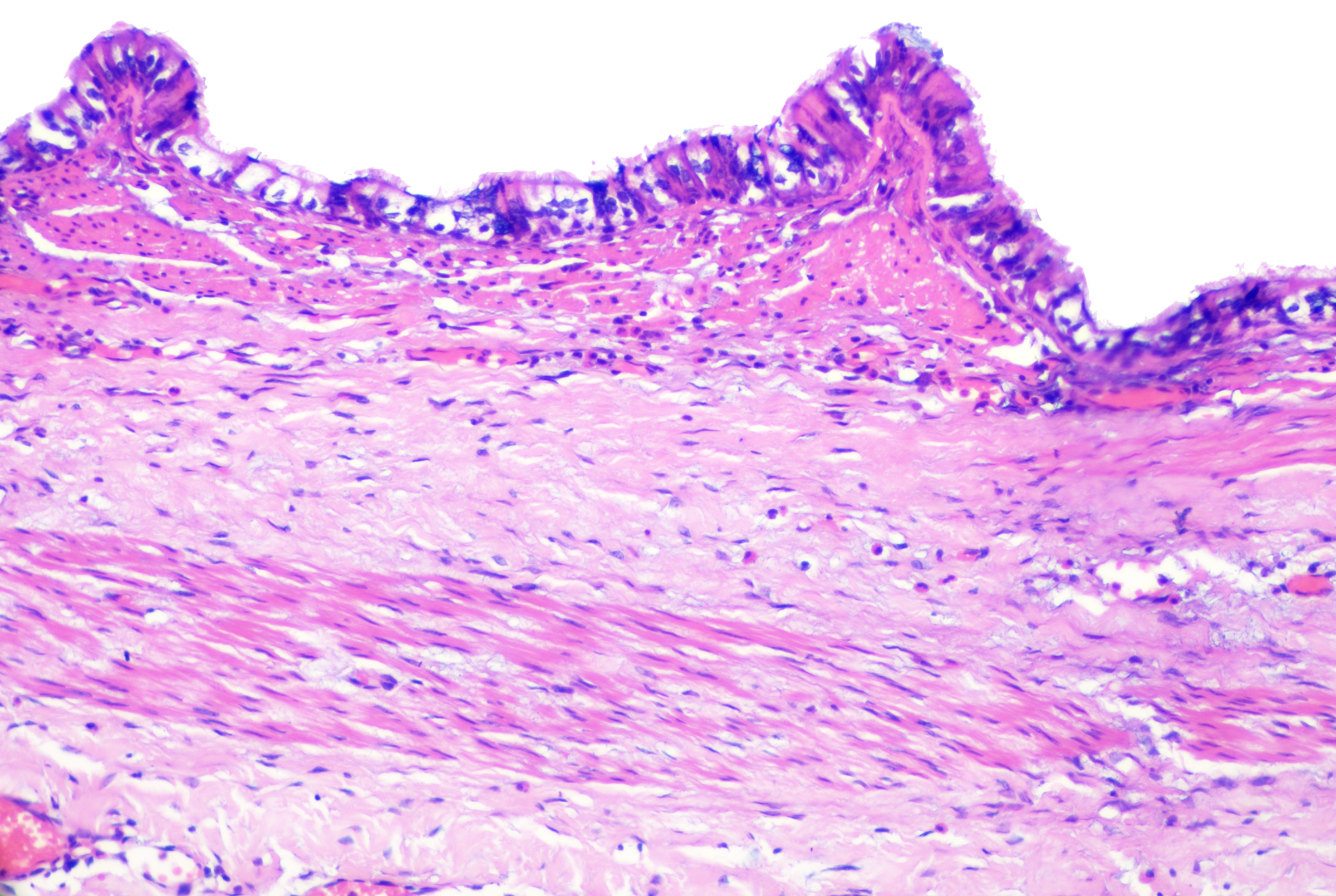
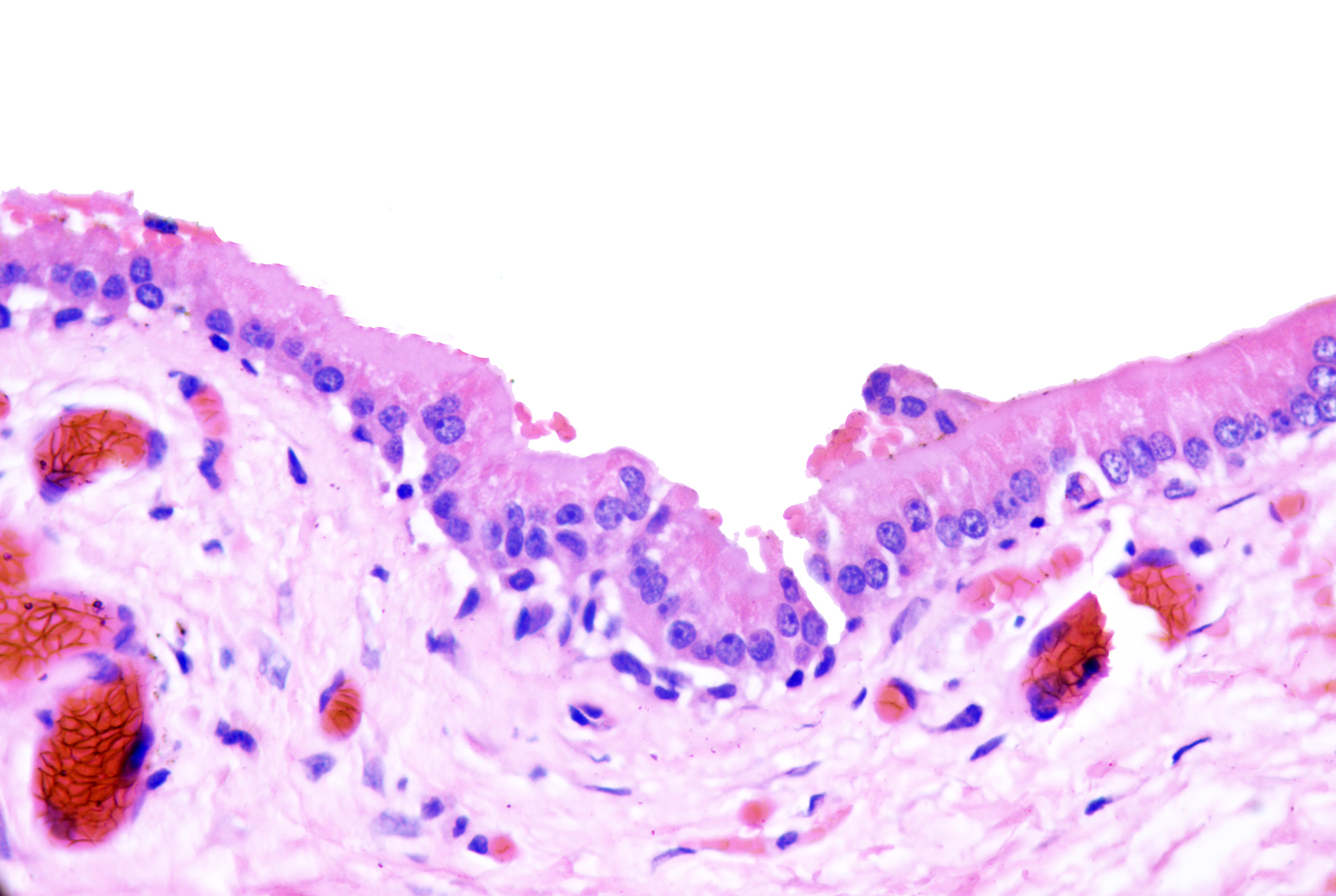
These cysts originate as Mullerian duct remnants (which usually form the uterus, cervix, fallopian tubes, and proximal vagina) in females and perhaps eccrine sweat glands in males.[47][58] They present as cystic swelling on females' lower limbs and perineum in the second to third decades of life.[47][59] Lesions in males have been reported to involve the inguinal area, leg, and back.[58][60][61] Histopathology reveals a cyst without an epidermal connection. It has infolded architecture with simple ciliated cuboidal or columnar epithelium (with foci of pseudostratification and squamatization that may occur). Intraluminal papillae are commonly observed (see Image. Cutaneous Ciliated Cyst).[47][59] Peg-like cells similar to those identified in the fallopian tube may also be identified (see Image. Cutaneous Cyst With Epithelium).[62][63] Lesions in females express progesterone and estrogen receptor WT1 and PAX-8, which are consistent with Mullerian origin.[59] In non-Mullerian cases, an eccrine origin has been suggested.[58][64]
Median raphe cysts
Median raphe cysts originate from embryonic ectodermal epithelial or endodermal urethral rests.[65][66] They present in the first 3 decades of life as midline cysts or canal-like plaques involving the ventral penile glans, shaft, scrotum, perineum, or perianal location due to defective closure of the median raphe or outgrowth of the entodermal urethral lining (called erythroid cyst). While present from birth, evaluation may be delayed until adulthood.[65][67] The microscopic features of median raphe cysts include a cystic cavity lined by either pseudostratified columnar epithelium or stratified squamous epithelium (see Image. Median Raphe Cyst). PAS-positive diastase-resistant mucin, Goblet cell, ciliated metaplasia, and apocrine secretion may be identified.[68][69][70] GATA3 and Cytokeratin 7 (CK7) expression support urothelial origin.[71] Rare pigmented variants with dendritic melanocytes, perhaps representing melanocytic colonization, have been reported.[72]
Vulval mucinous and ciliated cysts
These cysts are present in the vestibule of the vulva and show pseudostratified ciliated columnar epithelium with mucinous changes. They may demonstrate squamous metaplasia.[73]
Omphalomesenteric duct cysts
Omphalomesenteric duct cysts may be associated with Meckel diverticula. They are present in the periumbilical region, where histopathology shows gastrointestinal mucosa contiguous with the stratified squamous epithelium of skin; smooth muscle may be present.[74]
Miscellaneous Cysts
Miscellaneous cysts are not detailed as a part of this activity since their evaluation by dermatopathology is usually intimated by their existing condition. Miscellaneous cysts can include parasitic cysts (eg, Taenia solium, sparganosis), fungal cysts (eg, phaehyphomycosis), digital mucous cysts (ie, tense nail or joint nodules), myxoid cysts (ie, focal deposition of mucin), metaplastic synvovial cysts (ie, solitary cysts with partly hyalinated synvoium in areas of trauma), pseudocysts of the auricle (ie, inflammatory intracartilaginous cysts common in young Chinese men on the upper ear), cutaneous endometriosis cysts (ie, cysts of endometrial glands and stroma deposited in the skin), cutaneous endosalpingiosis cysts (ie, cysts of fallopian tube epithelium), or lymphatic cysts (eg, cystic hygroma).
Clinicopathologic Correlations
The pathologist may encounter a large number of true cysts involving the skin. In the vast majority, accurate diagnosis can be achieved by carefully assessing the cyst lining and contents using routine stains. Certain lesions may serve as markers for genetic or inflammatory dermatoses. While some cysts may exhibit variability/similarity in their lining epithelium, ancillary histopathologic findings, clinical features, anatomic location, and occasionally immunohistochemistry assist in correct classification. Rarely, an otherwise typical cyst may show malignant degeneration, and careful examination of the lesion is crucial. Overall, cutaneous cysts represent a group of lesions with relatively reproducible clinicopathologic features, which, once familiar, enable pathologists to render the correct diagnosis without significant difficulty.
Clinical Significance
Many cysts are isolated, clinical phenomena, only diagnosed and removed at the patient's request. Most are small and asymptomatic. Nevertheless, they may be clinically significant in certain circumstances.
Rupture, infection, and abscess formation
While most acquired and developmental cysts may become inflamed, secondarily infected, and rupture, leading to sterile or infectious abscess formation, epidermal inclusion cysts are the most frequent culprit.[75][76][77] The rupture of an epidermal inclusion cyst may lead to pain and swelling, requiring surgical intervention. Rarely, soft tissue extension, cellulitis, and even necrotizing fasciitis have been reported.[78] Dermoid cysts may have intracranial connections. Manipulation, surgical intervention, or spontaneous infection may have serious consequences, including meningitis.[79]
Disfigurement and compression of other structures
Large cysts of various types and locations may lead to marked disfigurement or compression of nearby structures, requiring surgical intervention.[80][81][82] Even small cysts, if multiple, are a potential source of significant cosmetic or psychological morbidity. Examples of these lesions include steatocystoma multiplex or multiple epidermal inclusion cysts of the scrotum.[83][84][83]
Malignant degeneration and spread to regional lymph nodes
Malignant degeneration has been reported in epidermal inclusion cysts, pilar cysts, follicular hybrid cysts, branchial cleft cysts, and apocrine hidrocystomas.[85][86][87][88] The spread to regional lymph nodes has been reported in proliferating pilar tumors with mostly benign-appearing epithelium.[89]
Association with heritable and inflammatory dermatoses or syndromes
Some cysts may serve as cutaneous markers for underlying diseases. Identifying the syndromes or dermatoses may be essential in the early identification of malignancy or other causes of morbidity or mortality.
- Multiple epidermal inclusion cysts (including those with pilomatrical differentiation) may be seen in Gardner syndrome, which may also show lipomas, colonic polyps, desmoid tumors, and other systemic manifestations.[90][91]
- Milia may be seen in porphyrias, epidermolysis bullosa acquisita, heritable epidermolysis bullosa, Rombo syndrome, Brooke-Spiegler syndrome, and atrichia with papular lesions.[5]
- Steatocystoma and vellus hair cysts can be seen in pachyonychia congenita type 2.[92]
- Cutaneous keratocysts may be seen in Gorlin syndrome.[32]
- Apocrine hidrocystomas are associated with Schopf-Schulz-Passarge syndrome.[40]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
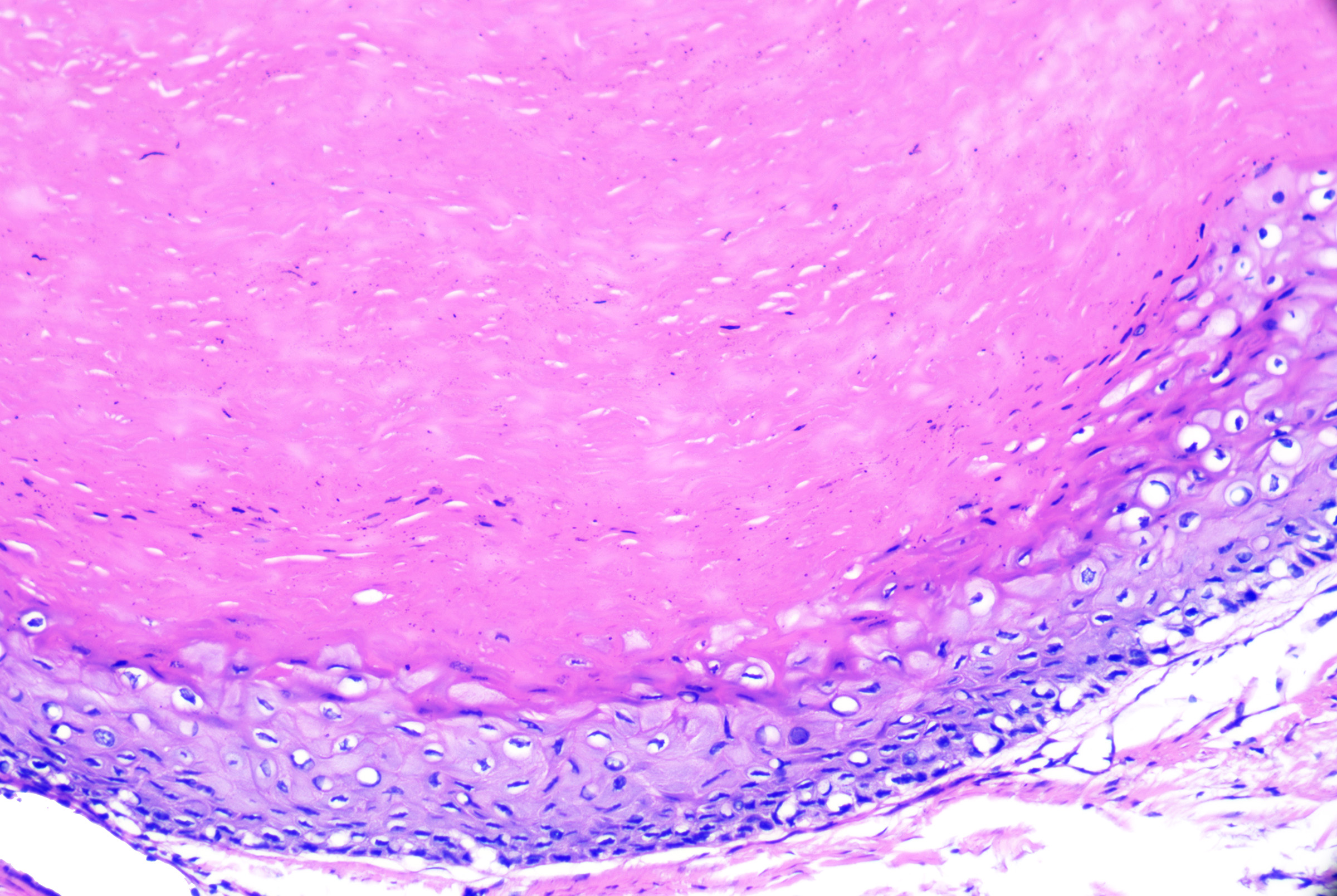
Pilar Cyst Outer Root Sheath. The pilar cyst demonstrating the outer root sheath differentiation at the level of the follicular isthmus. Keratinocytes develop abundant pale-staining cytoplasm and keratinize to compact, eosinophilic keratin without an intervening granular layer. H&E staining at 10x magnification.
Contributed by J Ho, MBBS DSc
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
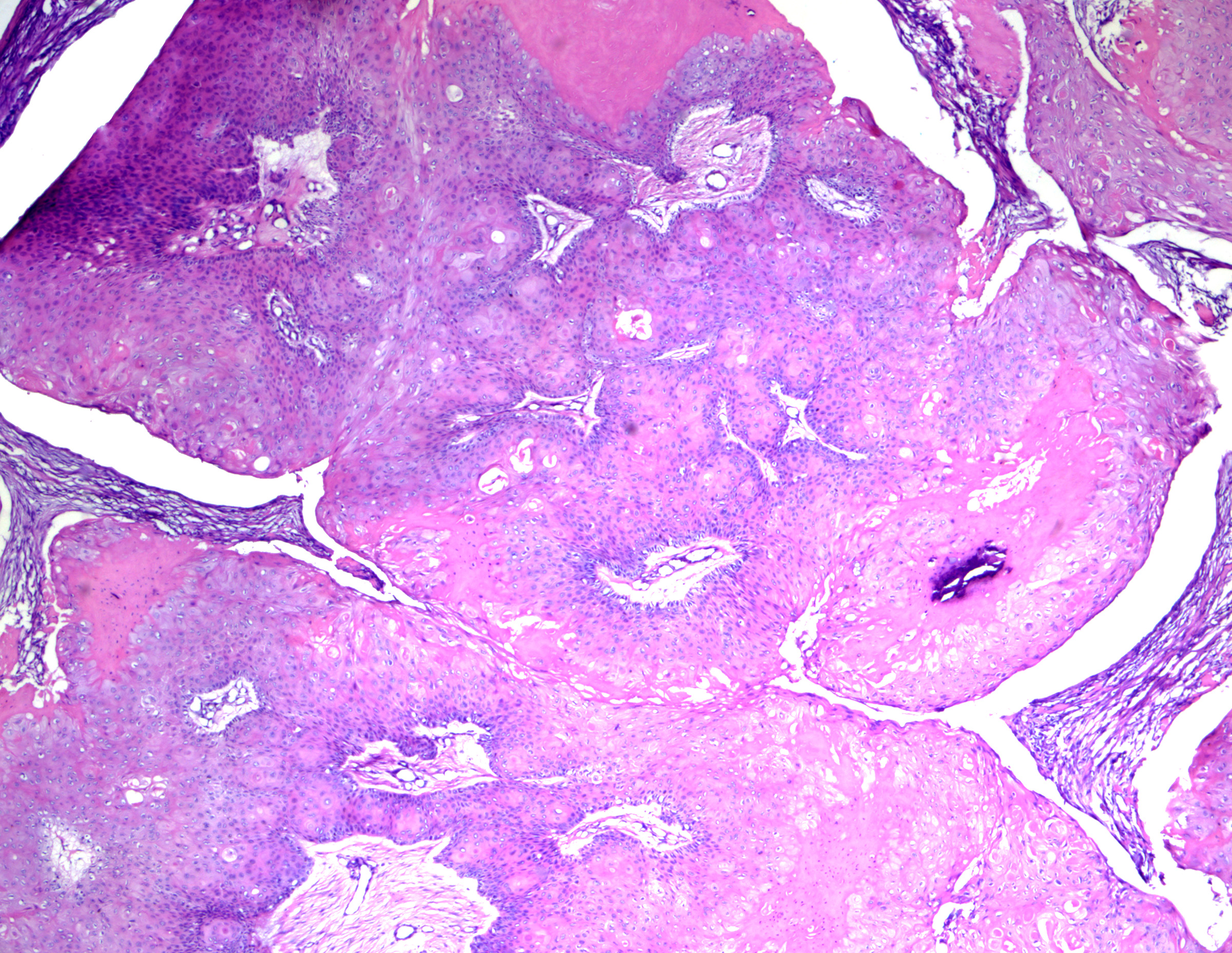
Proliferating Pilar Tumor. While often confused with a well-differentiated squamous cell carcinoma at low power, note the rounded borders. Identification of trichilemmal-type keratinization is critical for making the correct diagnosis. H&E staining at 40x magnification.
Contributed by J Ho, MBBS, DSc
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
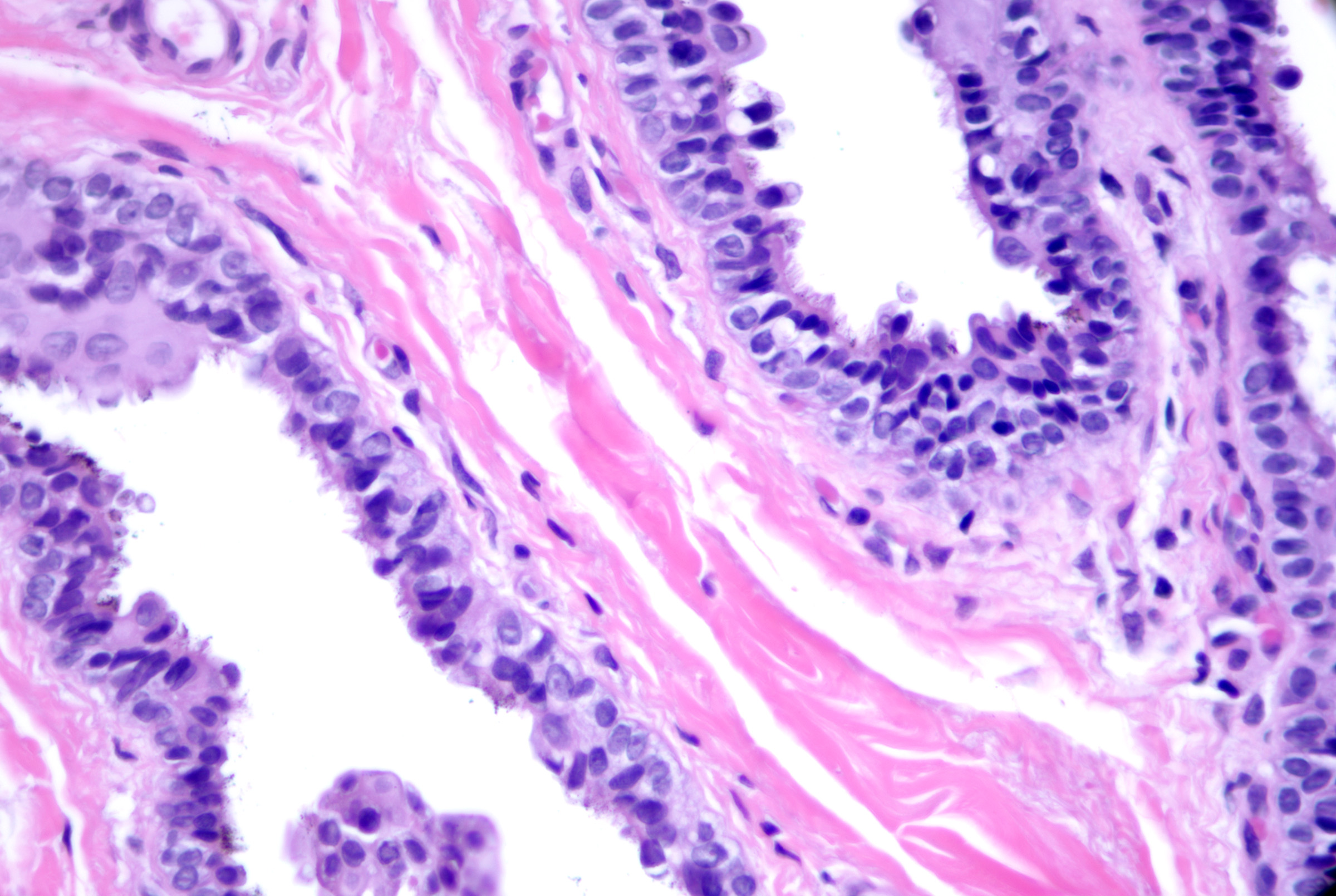
Cutaneous Cyst With Epithelium. A cutaneous ciliated cyst demonstrating variably ciliated columnar, cuboidal, and focally squamatized epithelium. Peg cells similar to that seen in the fallopian tubes are present lending support to Mullerian origin. H&E staining at 200x magnification.
Contributed by J Ho, MBBS, DSc
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
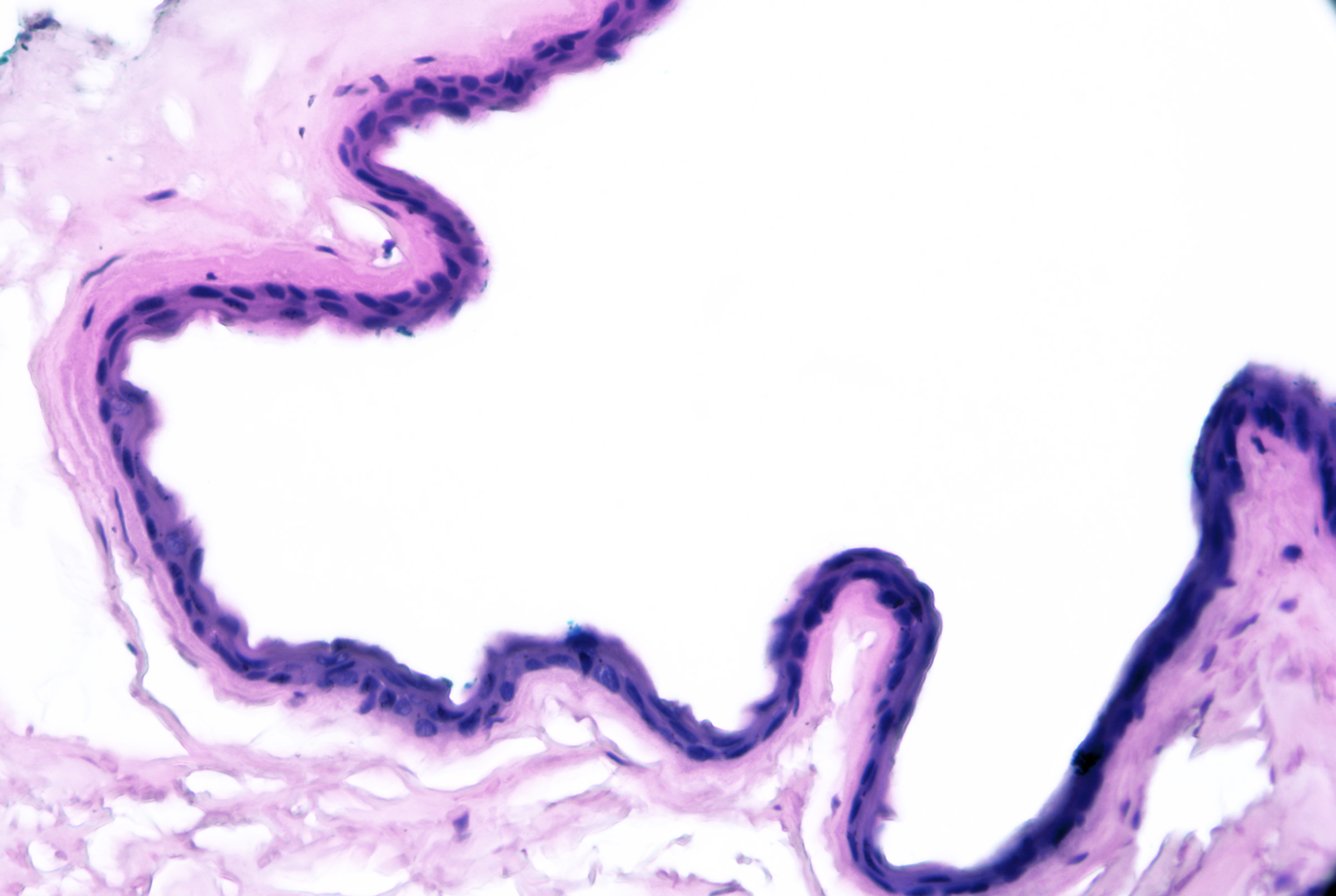
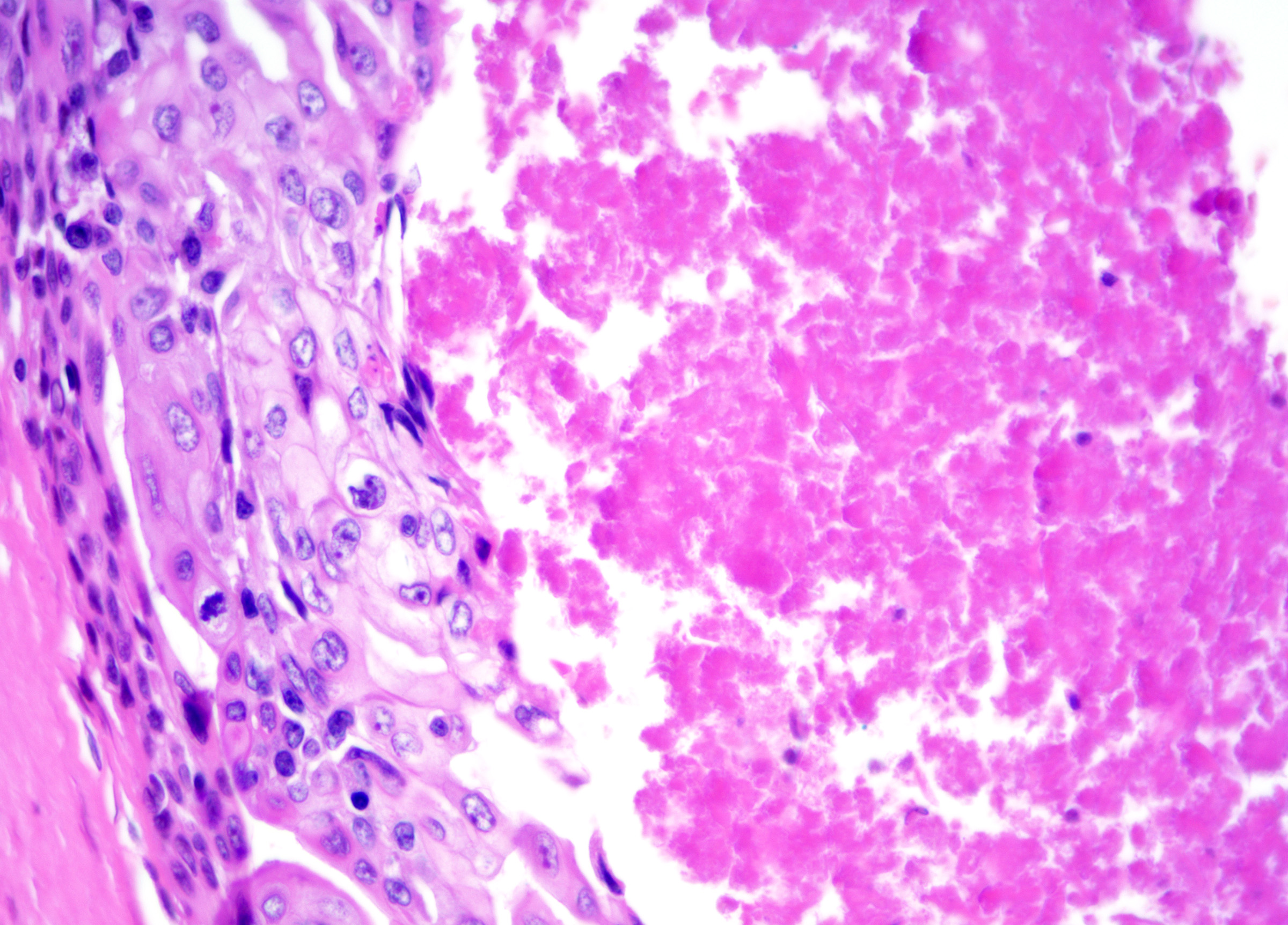
Eccrine Hidrocystoma. Note 1-2 layers of simple cuboidal epithelium without decapitation secretion. Some lesions that appear to be eccrine in origin stain positively for apocrine markers and it is impossible to classify lesions solely based on cytomorphology correctly. H&E x200.
Contributed by J Ho, MBBS, DSc
References
Lee SS, Kim SY, Im M, Lee Y, Seo YJ, Lee JH. Pseudocyst of the scalp. Annals of dermatology. 2011 Oct:23(Suppl 2):S267-9. doi: 10.5021/ad.2011.23.S2.S267. Epub 2011 Oct 31 [PubMed PMID: 22148068]
Hoang VT, Trinh CT, Nguyen CH, Chansomphou V, Chansomphou V, Tran TTT. Overview of epidermoid cyst. European journal of radiology open. 2019:6():291-301. doi: 10.1016/j.ejro.2019.08.003. Epub 2019 Sep 5 [PubMed PMID: 31516916]
Level 3 (low-level) evidenceEgawa K, Kitasato H, Honda Y, Kawai S, Mizushima Y, Ono T. Human papillomavirus 57 identified in a plantar epidermoid cyst. The British journal of dermatology. 1998 Mar:138(3):510-4 [PubMed PMID: 9580810]
Choi JE, Kwon IH, Seo SH, Kye YC, Ahn HH. Pathogenesis of Plantar Epidermal Cyst: Three-Dimensional Reconstruction Analysis. Annals of dermatology. 2016 Feb:28(1):133-5. doi: 10.5021/ad.2016.28.1.133. Epub 2016 Jan 28 [PubMed PMID: 26848239]
Berk DR, Bayliss SJ. Milia: a review and classification. Journal of the American Academy of Dermatology. 2008 Dec:59(6):1050-63. doi: 10.1016/j.jaad.2008.07.034. Epub 2008 Sep 25 [PubMed PMID: 18819726]
Reis MD, Tellechea O, Baptista AP. Verrucous cyst. European journal of dermatology : EJD. 1998 Apr-May:8(3):186-8 [PubMed PMID: 9649670]
Cooper PH, Fechner RE. Pilomatricoma-like changes in the epidermal cysts of Gardner's syndrome. Journal of the American Academy of Dermatology. 1983 May:8(5):639-44 [PubMed PMID: 6863619]
Sau P, Graham JH, Helwig EB. Proliferating epithelial cysts. Clinicopathological analysis of 96 cases. Journal of cutaneous pathology. 1995 Oct:22(5):394-406 [PubMed PMID: 8594071]
Level 3 (low-level) evidencePatsatsi A, Uy CDC, Murrell DF. Multiple milia formation in blistering diseases. International journal of women's dermatology. 2020 Jun:6(3):199-202. doi: 10.1016/j.ijwd.2020.03.045. Epub 2020 Apr 1 [PubMed PMID: 32637544]
Fraser-Andrews E, Ashton R, Russell-Jones R. Pilotropic mycosis fungoides presenting with multiple cysts, comedones and alopecia. The British journal of dermatology. 1999 Jan:140(1):141-4 [PubMed PMID: 10215785]
Level 3 (low-level) evidenceWei Z, Wang F, Zheng S. Favre-Racouchot Syndrome. JAMA dermatology. 2024 Jan 31:():. doi: 10.1001/jamadermatol.2023.5768. Epub 2024 Jan 31 [PubMed PMID: 38294808]
Salopek TG, Lee SK, Jimbow K. Multiple pigmented follicular cysts: a subtype of multiple pilosebaceous cysts. The British journal of dermatology. 1996 Apr:134(4):758-62 [PubMed PMID: 8733387]
Chuang YH, Hong HS, Kuo TT. Multiple pigmented follicular cysts of the vulva successfully treated with CO2 laser: case report and literature review. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2004 Sep:30(9):1261-4 [PubMed PMID: 15355374]
Level 3 (low-level) evidenceMayron R, Grimwood RE. Familial occurrence of eruptive vellus hair cysts. Pediatric dermatology. 1988 May:5(2):94-6 [PubMed PMID: 3412998]
Patokar AS, Holani AR, Khandait GH, Khatu SS. Eruptive Vellus Hair Cysts: An Underdiagnosed Entity. International journal of trichology. 2022 Jan-Feb:14(1):31-33. doi: 10.4103/ijt.ijt_100_20. Epub 2022 Feb 1 [PubMed PMID: 35300099]
Torchia D, Vega J, Schachner LA. Eruptive vellus hair cysts: a systematic review. American journal of clinical dermatology. 2012 Feb 1:13(1):19-28. doi: 10.2165/11589050-000000000-00000. Epub [PubMed PMID: 21958358]
Level 1 (high-level) evidenceRodgers SA, Kitagawa K, Selim MA, Bellet JS. Familial eruptive vellus hair cysts. Pediatric dermatology. 2012 May-Jun:29(3):367-9. doi: 10.1111/j.1525-1470.2011.01411.x. Epub 2011 Dec 9 [PubMed PMID: 22150961]
Takeshita T, Takeshita H, Irie K. Eruptive vellus hair cyst and epidermoid cyst in a patient with pachyonychia congenita. The Journal of dermatology. 2000 Oct:27(10):655-7 [PubMed PMID: 11092270]
Liu M, Han H, Zheng Y, Xiao S, Feng Y. Pilar cyst on the dorsum of hand: A case report and review of literature. Medicine. 2020 Jul 31:99(31):e21519. doi: 10.1097/MD.0000000000021519. Epub [PubMed PMID: 32756192]
Level 3 (low-level) evidenceLin SK, Cassarino DS. Cystic tricholemmal carcinoma in situ. The American Journal of dermatopathology. 2013 Aug:35(6):e99-e102. doi: 10.1097/DAD.0b013e31827401b5. Epub [PubMed PMID: 23759876]
Arsenovic N, Sen S, Naik V, Reed M, Moreira R. Trichilemmal cyst with carcinoma in situ within an atypical fibroxanthoma. The American Journal of dermatopathology. 2009 Aug:31(6):587-90. doi: 10.1097/DAD.0b013e3181a0d235. Epub [PubMed PMID: 19590414]
Fischer GM, Lindeman NI, Ligon AH, Russell-Goldman E. Proliferating Pilar Tumors Are Characterized by Recurrent 15q, 6q, and 6p22.2 Alterations. The American Journal of dermatopathology. 2023 Apr 1:45(4):217-226. doi: 10.1097/DAD.0000000000002308. Epub 2022 Nov 7 [PubMed PMID: 36346171]
Nemeh MN, Curtiss P, Nijhawan RI. Epidemiology, clinical characteristics, treatment, and outcomes of proliferating pilar tumors: A systematic review. Journal of the American Academy of Dermatology. 2024 Jan:90(1):122-124. doi: 10.1016/j.jaad.2023.05.097. Epub 2023 Jun 24 [PubMed PMID: 37364614]
Level 1 (high-level) evidenceKearns-Turcotte S, Thériault M, Blouin MM. Malignant proliferating trichilemmal tumors arising in patients with multiple trichilemmal cysts: A case series. JAAD case reports. 2022 Apr:22():42-46. doi: 10.1016/j.jdcr.2022.01.033. Epub 2022 Feb 17 [PubMed PMID: 35310136]
Level 2 (mid-level) evidenceYe J, Nappi O, Swanson PE, Patterson JW, Wick MR. Proliferating pilar tumors: a clinicopathologic study of 76 cases with a proposal for definition of benign and malignant variants. American journal of clinical pathology. 2004 Oct:122(4):566-74 [PubMed PMID: 15487455]
Level 3 (low-level) evidenceLydrup E, Pedersen Pilt A, Schmidt VJ, Trøstrup H. Subungual Onycholemmal Cysts: A Case Report. Case reports in dermatology. 2021 May-Aug:13(2):394-398. doi: 10.1159/000515248. Epub 2021 Jul 19 [PubMed PMID: 34413739]
Level 3 (low-level) evidenceDüzova AN, Sentürk GB. Suggestion for the treatment of steatocystoma multiplex located exclusively on the face. International journal of dermatology. 2004 Jan:43(1):60-2 [PubMed PMID: 14693025]
Rollins T, Levin RM, Heymann WR. Acral steatocystoma multiplex. Journal of the American Academy of Dermatology. 2000 Aug:43(2 Pt 2):396-9 [PubMed PMID: 10901733]
Cho S, Chang SE, Choi JH, Sung KJ, Moon KC, Koh JK. Clinical and histologic features of 64 cases of steatocystoma multiplex. The Journal of dermatology. 2002 Mar:29(3):152-6 [PubMed PMID: 11990250]
Level 3 (low-level) evidenceAhmed G, Prabha N, Ganguly S. Familial Steatocystoma Multiplex Generalisita Suppuritiva: Oral Rifampicin and Clindamycin Combination Worth a Trial. Indian journal of dermatology. 2021 Sep-Oct:66(5):553-555. doi: 10.4103/ijd.IJD_117_20. Epub [PubMed PMID: 35068515]
Smith FJ, Corden LD, Rugg EL, Ratnavel R, Leigh IM, Moss C, Tidman MJ, Hohl D, Huber M, Kunkeler L, Munro CS, Lane EB, McLean WH. Missense mutations in keratin 17 cause either pachyonychia congenita type 2 or a phenotype resembling steatocystoma multiplex. The Journal of investigative dermatology. 1997 Feb:108(2):220-3 [PubMed PMID: 9008238]
Goggins CA, Zerah ML, Anatelli F, Norton SA. Cutaneous Keratocyst With D2-40 Immunoreactivity in Basal Cell Nevus Syndrome. The American Journal of dermatopathology. 2021 Sep 1:43(9):659-661. doi: 10.1097/DAD.0000000000001638. Epub [PubMed PMID: 33606372]
May SA, Quirey R, Cockerell CJ. Follicular hybrid cysts with infundibular, isthmic-catagen, and pilomatrical differentiation: a report of 2 patients. Annals of diagnostic pathology. 2006 Apr:10(2):110-3 [PubMed PMID: 16546048]
Requena L, Sánchez Yus E. Follicular hybrid cysts. An expanded spectrum. The American Journal of dermatopathology. 1991 Jun:13(3):228-33 [PubMed PMID: 1714246]
Chakraborty D, Ahmed SKS, Das S. Cystic Panfolliculoma, an Uncommon Cutaneous Adnexal Tumour in an Atypical Age Group and an Unusual Site. Indian journal of dermatology. 2023 Nov-Dec:68(6):729. doi: 10.4103/ijd.ijd_451_23. Epub 2024 Jan 9 [PubMed PMID: 38371543]
Neill B, Bingham C, Braudis K, Zurowski S. A rare cutaneous adnexal neoplasm: cystic panfolliculoma. Journal of cutaneous pathology. 2016 Dec:43(12):1183-1185. doi: 10.1111/cup.12807. Epub 2016 Sep 26 [PubMed PMID: 27550230]
Juang SJ, Win KT, Lai FJ. Panfolliculoma: Report of the Youngest Case and Literature Review of Its Histopathologic Variants. Life (Basel, Switzerland). 2022 Jun 13:12(6):. doi: 10.3390/life12060881. Epub 2022 Jun 13 [PubMed PMID: 35743912]
Level 3 (low-level) evidencede Viragh PA, Szeimies RM, Eckert F. Apocrine cystadenoma, apocrine hidrocystoma, and eccrine hidrocystoma: three distinct tumors defined by expression of keratins and human milk fat globulin 1. Journal of cutaneous pathology. 1997 Apr:24(4):249-55 [PubMed PMID: 9138118]
Sarabi K, Khachemoune A. Hidrocystomas--a brief review. MedGenMed : Medscape general medicine. 2006 Sep 6:8(3):57 [PubMed PMID: 17406184]
Rambhia KD, Kharkar V, Mahajan S, Khopkar US. Schopf-Schulz-Passarge Syndrome. Indian dermatology online journal. 2018 Nov-Dec:9(6):448-451. doi: 10.4103/idoj.IDOJ_26_18. Epub [PubMed PMID: 30505790]
Eckert F, Betke M, Schmoeckel C, Neuweiler J, Schmid U. Myoepithelial differentiation in benign sweat gland tumors. Demonstrated by a monoclonal antibody to alpha-smooth muscle actin. Journal of cutaneous pathology. 1992 Aug:19(4):294-301 [PubMed PMID: 1331211]
Ren F, Bressler L, Pruitt L, Wang H, Liu L, Elston DM. Midline cutaneous anomalies of the craniospinal axis. Journal of the American Academy of Dermatology. 2023 Dec:89(6):1238-1244. doi: 10.1016/j.jaad.2023.06.062. Epub 2023 Aug 19 [PubMed PMID: 37598328]
Orozco-Covarrubias L, Lara-Carpio R, Saez-De-Ocariz M, Duran-McKinster C, Palacios-Lopez C, Ruiz-Maldonado R. Dermoid cysts: a report of 75 pediatric patients. Pediatric dermatology. 2013 Nov-Dec:30(6):706-11. doi: 10.1111/pde.12080. Epub 2013 Mar 14 [PubMed PMID: 23488469]
Purnell CA, Skladman R, Alden TD, Corcoran JF, Rastatter JC. Nasal dermoid cysts with intracranial extension: avoiding coronal incision through midline exposure and nasal bone osteotomy. Journal of neurosurgery. Pediatrics. 2019 Dec 6:25(3):298-304. doi: 10.3171/2019.9.PEDS19132. Epub 2019 Dec 6 [PubMed PMID: 31812133]
Partha Sri M, Prabhat AK, Dammalapati MR, Yirang K, Patibandla BMK, Kantamaneni K, Atluri LM, El Menawy Z, Uppalapati SK. Dermoid Cyst of the Penis: A Case Report of an Unusual Penile Mass. Cureus. 2022 Oct:14(10):e30227. doi: 10.7759/cureus.30227. Epub 2022 Oct 12 [PubMed PMID: 36381808]
Level 3 (low-level) evidenceDwivedi G, Saxena P, Patnaik U, Kumari A, Sood A. Dermoid Cyst Floor of Mouth: A Diagnostic Conundrum. Indian journal of otolaryngology and head and neck surgery : official publication of the Association of Otolaryngologists of India. 2022 Oct:74(Suppl 2):1961-1963. doi: 10.1007/s12070-020-01939-1. Epub 2020 Jul 3 [PubMed PMID: 36452799]
Pérez-Muñoz N, Llamas-Velasco M, Castillo-Capponi G, Morgado-Carrasco D, Iglesias-Sancho M, Carrasco-García MÁ, Fernández-Figueras MT. Dermatopathology of Cutaneous Cystic Lesions: A Practical Review With Diagnostic Clues and Pitfalls. The American Journal of dermatopathology. 2019 Nov:41(11):783-793. doi: 10.1097/DAD.0000000000001362. Epub [PubMed PMID: 31633550]
Tsai TF, Chuan MT, Hsiao CH. A cystic teratoma of the skin. Histopathology. 1996 Oct:29(4):384-6 [PubMed PMID: 8910050]
Camacho F. Benign cutaneous cystic teratoma. Journal of cutaneous pathology. 1982 Oct:9(5):345-51 [PubMed PMID: 7142521]
Zvulunov A, Amichai B, Grunwald MH, Avinoach I, Halevy S. Cutaneous bronchogenic cyst: delineation of a poorly recognized lesion. Pediatric dermatology. 1998 Jul-Aug:15(4):277-81 [PubMed PMID: 9720691]
Kurban RS, Bhawan J. Cutaneous cysts lined by nonsquamous epithelium. The American Journal of dermatopathology. 1991 Oct:13(5):509-17 [PubMed PMID: 1951989]
Kundal AK, Zargar NU, Krishna A. Scapular bronchogenic cyst. Journal of Indian Association of Pediatric Surgeons. 2008 Oct:13(4):147-8. doi: 10.4103/0971-9261.44768. Epub [PubMed PMID: 20011500]
Golledge J, Ellis H. The aetiology of lateral cervical (branchial) cysts: past and present theories. The Journal of laryngology and otology. 1994 Aug:108(8):653-9 [PubMed PMID: 7930913]
Najib Z, Berrada O, Lahjaouj M, Oukessou Y, Rouadi S, Abada RA, Roubal M, Mahtar M. Cervical lymphoepithelial cyst: Case report and literature review. Annals of medicine and surgery (2012). 2021 Jan:61():185-187. doi: 10.1016/j.amsu.2020.12.041. Epub 2020 Dec 28 [PubMed PMID: 33489106]
Level 3 (low-level) evidenceGlosser JW, Pires CA, Feinberg SE. Branchial cleft or cervical lymphoepithelial cysts: etiology and management. Journal of the American Dental Association (1939). 2003 Jan:134(1):81-6 [PubMed PMID: 12555960]
Taha A, Enodien B, Frey DM, Taha-Mehlitz S. Thyroglossal Duct Cyst, a Case Report and Literature Review. Diseases (Basel, Switzerland). 2022 Jan 25:10(1):. doi: 10.3390/diseases10010007. Epub 2022 Jan 25 [PubMed PMID: 35225860]
Level 3 (low-level) evidenceCigliano B, Baltogiannis N, De Marco M, Faviou E, Antoniou D, De Luca U, Soutis M, Settimi A. Cervical thymic cysts. Pediatric surgery international. 2007 Dec:23(12):1219-25 [PubMed PMID: 17938938]
Kim Y, Kim H. The Cutaneous Ciliated Cyst in Young Male: The Possibility of Ciliated Cutaneous Eccrine Cyst. Case reports in medicine. 2015:2015():589831. doi: 10.1155/2015/589831. Epub 2015 Sep 30 [PubMed PMID: 26491452]
Level 3 (low-level) evidenceFabien-Dupuis C, Cooper B, Upperman J, Zhou S, Shillingford N. Mullerian-Type Ciliated Cyst of the Thigh with PAX-8 and WT1 Positivity: A Case Report and Review of the Literature. Case reports in medicine. 2016:2016():2487820. doi: 10.1155/2016/2487820. Epub 2016 Dec 14 [PubMed PMID: 28070193]
Level 3 (low-level) evidenceLee JS, Kim YC, Lee ES. Cutaneous ciliated cyst of the inguinal area in a man. The Journal of dermatology. 2006 Feb:33(2):146-9 [PubMed PMID: 16556287]
Ashton MA. Cutaneous ciliated cyst of the lower limb in a male. Histopathology. 1995 May:26(5):467-9 [PubMed PMID: 7544765]
Joehlin-Price AS, Huang JH, Brooks JS, Scharschmidt TJ, Iwenofu OH. PAX-8 expression in cutaneous ciliated cysts: evidence for Müllerian origin. The American Journal of dermatopathology. 2014 Feb:36(2):167-70. doi: 10.1097/DAD.0b013e31829e7a41. Epub [PubMed PMID: 23907320]
Paik DY, Janzen DM, Schafenacker AM, Velasco VS, Shung MS, Cheng D, Huang J, Witte ON, Memarzadeh S. Stem-like epithelial cells are concentrated in the distal end of the fallopian tube: a site for injury and serous cancer initiation. Stem cells (Dayton, Ohio). 2012 Nov:30(11):2487-97. doi: 10.1002/stem.1207. Epub [PubMed PMID: 22911892]
Tran TAN. Ciliated and Mucinous Adenomatous Syringometaplasia: The Missing Link of the "Ciliated Eccrine Metaplasia" Theory in the Histogenesis of Cutaneous Eccrine Ciliated Cyst? The American Journal of dermatopathology. 2021 Nov 1:43(11):827-830. doi: 10.1097/DAD.0000000000001924. Epub [PubMed PMID: 33606368]
Park CO, Chun EY, Lee JH. Median raphe cyst on the scrotum and perineum. Journal of the American Academy of Dermatology. 2006 Nov:55(5 Suppl):S114-5 [PubMed PMID: 17052527]
Shao IH, Chen TD, Shao HT, Chen HW. Male median raphe cysts: serial retrospective analysis and histopathological classification. Diagnostic pathology. 2012 Sep 14:7():121. doi: 10.1186/1746-1596-7-121. Epub 2012 Sep 14 [PubMed PMID: 22978603]
Level 2 (mid-level) evidenceSyed MMA, Amatya B, Sitaula S. Median raphe cyst of the penis: a case report and review of the literature. Journal of medical case reports. 2019 Jul 14:13(1):214. doi: 10.1186/s13256-019-2133-5. Epub 2019 Jul 14 [PubMed PMID: 31301740]
Level 3 (low-level) evidenceRomaní J, Barnadas MA, Miralles J, Curell R, de Moragas JM. Median raphe cyst of the penis with ciliated cells. Journal of cutaneous pathology. 1995 Aug:22(4):378-81 [PubMed PMID: 7499581]
Verma SB. Canal-like median raphe cysts: an unusual presentation of an unusual condition. Clinical and experimental dermatology. 2009 Dec:34(8):e857-8. doi: 10.1111/j.1365-2230.2009.03604.x. Epub 2009 Oct 10 [PubMed PMID: 19817756]
Ünal B, Başsorgun Cİ, Eren Karanis Mİ, Elpek GÖ. Perianal median raphe cyst: a rare lesion with unusual histology and localization. Case reports in dermatological medicine. 2015:2015():487814. doi: 10.1155/2015/487814. Epub 2015 Feb 22 [PubMed PMID: 25793130]
Level 3 (low-level) evidenceCarrasco L, Torre-Castro J, Ortiz S, Cuevas Santos J, Jo M, Rodríguez Peralto JL, Beer T, Requena L. Median raphe cysts: A clinico-pathologic and immunohistochemical study of 52 cases. Journal of cutaneous pathology. 2023 Jun:50(6):536-543. doi: 10.1111/cup.14367. Epub 2023 Jan 23 [PubMed PMID: 36442871]
Level 3 (low-level) evidenceIshida M, Iwai M, Yoshida K, Kagotani A, Okabe H. Pigmented median raphe cyst of the penis with consideration of the possible mechanism of melanocytic colonization: A case report. Oncology letters. 2014 Feb:7(2):342-344 [PubMed PMID: 24396444]
Level 3 (low-level) evidenceRobboy SJ, Ross JS, Prat J, Keh PC, Welch WR. Urogenital sinus origin of mucinous and ciliated cysts of the vulva. Obstetrics and gynecology. 1978 Mar:51(3):347-51 [PubMed PMID: 628540]
Singaravel S, Yadav PC. Histomorphology of the lesions of the umbilicus: Are we naïve about the navel? Indian journal of pathology & microbiology. 2021 Jan-Mar:64(1):91-95. doi: 10.4103/IJPM.IJPM_146_20. Epub [PubMed PMID: 33433415]
Gupta R, Verma P, Bansal N, Semwal T. A Case of Ruptured Perineal Epidermal Cyst. Cureus. 2020 Oct 22:12(10):e11099. doi: 10.7759/cureus.11099. Epub 2020 Oct 22 [PubMed PMID: 33240695]
Level 3 (low-level) evidenceAbreu Velez AM, Brown VM, Howard MS. An inflamed trichilemmal (pilar) cyst: Not so simple? North American journal of medical sciences. 2011 Sep:3(9):431-4. doi: 10.4297/najms.2011.3431. Epub [PubMed PMID: 22362454]
Level 3 (low-level) evidenceTaskin OC, Gucer H, Winer D, Mete O. Thyroglossal Duct Cyst Associated with Xanthogranulomatous Inflammation. Head and neck pathology. 2015 Dec:9(4):530-3. doi: 10.1007/s12105-015-0628-y. Epub 2015 Apr 21 [PubMed PMID: 25896144]
Bosman WM, Brekelmans W, Verduijn PS, Borger van der Burg BL, Ritchie ED. Necrotising fasciitis due to an infected sebaceous cyst. BMJ case reports. 2014 Apr 30:2014():. doi: 10.1136/bcr-2013-201905. Epub 2014 Apr 30 [PubMed PMID: 24789153]
Level 3 (low-level) evidenceRafiei Tabatabei S, Azma R, Kahbazi M, Farzan A, Kazemi Aghdam M, Seifi K, Nahanmoghaddam N. Enterobacter Meningitis Due To Dermoid Cyst Manipulation. Iranian journal of child neurology. 2018 Fall:12(4):169-177 [PubMed PMID: 30279720]
de Mendonça JCG, Jardim ECG, Dos Santos CM, Masocatto DC, de Quadros DC, Oliveira MM, Macena JA, Teixeira FR. Epidermoid Cyst: Clinical and Surgical Case Report. Annals of maxillofacial surgery. 2017 Jan-Jun:7(1):151-154. doi: 10.4103/ams.ams_68_16. Epub [PubMed PMID: 28713757]
Level 3 (low-level) evidenceSsi-Yan-Kai IC, Pearson AR. Recurrent giant orbital apocrine hidrocystoma. Eye (London, England). 2012 Jun:26(6):895-6. doi: 10.1038/eye.2012.57. Epub 2012 Apr 13 [PubMed PMID: 22498797]
Level 3 (low-level) evidenceChen W, Xu M, Wang Q, Xu H, Chen J, Li X. Pediatric bronchogenic cysts in the head and neck region: A study of 10 surgical cases and a review of the literature. Frontiers in pediatrics. 2022:10():1030692. doi: 10.3389/fped.2022.1030692. Epub 2022 Nov 3 [PubMed PMID: 36405846]
Level 3 (low-level) evidenceGeorgakopoulos JR, Ighani A, Yeung J. Numerous asymptomatic dermal cysts: Diagnosis and treatment of steatocystoma multiplex. Canadian family physician Medecin de famille canadien. 2018 Dec:64(12):892-899 [PubMed PMID: 30541803]
Kshirsagar VV, Modi V. Multiple Sebaceous Cysts on the Scrotum: A Rare Surgical Occurrence. Cureus. 2023 May:15(5):e39607. doi: 10.7759/cureus.39607. Epub 2023 May 28 [PubMed PMID: 37384083]
Faltaous AA, Leigh EC, Ray P, Wolbert TT. A Rare Transformation of Epidermoid Cyst into Squamous Cell Carcinoma: A Case Report with Literature Review. The American journal of case reports. 2019 Aug 3:20():1141-1143. doi: 10.12659/AJCR.912828. Epub 2019 Aug 3 [PubMed PMID: 31375657]
Level 3 (low-level) evidenceBaşak K, Başak PY. In situ carcinoma in a hybrid cyst: a case report. Journal of cutaneous pathology. 2017 Feb:44(2):189-192. doi: 10.1111/cup.12852. Epub 2016 Dec 1 [PubMed PMID: 27792259]
Level 3 (low-level) evidenceBanikas V, Kyrgidis A, Koloutsos G, Sakkas L, Antoniades K. Branchial cyst carcinoma revisited: stem cells, dormancy and malignant transformation. The Journal of craniofacial surgery. 2011 May:22(3):918-21. doi: 10.1097/SCS.0b013e31820fe217. Epub [PubMed PMID: 21558916]
Toyoda Y, Franck P, Brownstone ND, Lieberman M, Magro CM, Otterburn DM. Apocrine adenocarcinoma in the setting of apocrine hidrocystoma of the leg. Dermatology online journal. 2019 Jun 15:25(6):. pii: 13030/qt1jn1n606. Epub 2019 Jun 15 [PubMed PMID: 31329389]
Malhotra KP, Shukla S, Singhal A, Husain N. Proliferating trichilemmal cyst with nodal enlargement mimicking metastatic squamous cell carcinoma. Indian journal of dermatology, venereology and leprology. 2015 Jul-Aug:81(4):418-20. doi: 10.4103/0378-6323.157461. Epub [PubMed PMID: 25994896]
García de la Filia Molina I, Crespo Pérez L, Ríos León R, Barbado Cano A, Moreno García Del Real C, Aburto Bernardo A, Figueroa Tubio A, González Olivares C, Sánchez Aldehuelo R, Albillos Martínez A. Shadow cells in a cutaneous epidermoid cyst: searching for a polyposis syndrome. Gastroenterologia y hepatologia. 2019 Jun-Jul:42(6):386-387. doi: 10.1016/j.gastrohep.2018.06.004. Epub 2018 Jul 17 [PubMed PMID: 30029926]
Perniciaro C. Gardner's syndrome. Dermatologic clinics. 1995 Jan:13(1):51-6 [PubMed PMID: 7712650]
Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, Smith FJD, Hansen CD, Hull PR, Kaspar RL, McLean WHI, O’Toole E, Sprecher E. Pachyonychia Congenita. GeneReviews(®). 1993:(): [PubMed PMID: 20301457]