High-Frequency Oscillator in the Neonate
High-Frequency Oscillator in the Neonate
Introduction
Greater than 75% of infants born less than 27 weeks gestation require some form of mechanical ventilation to survive.[1] While survival rates for extremely premature (less than 28 weeks) or very low-birth-weight (VLBW) infants have increased, the incidence of chronic lung disease (CLD) from ventilator-induced lung injury remains high.[2] For this reason, effective lung ventilation and oxygenation are key in preventing CLD.
The major risk factors for the development of CLD include immature lungs, oxygen toxicity, and ventilator-induced lung trauma.[2][3] The term bronchopulmonary dysplasia (BPD) was introduced in 1967 by Northway et al,[4] and is still used to describe infants who are dependent on oxygen beyond 28 days after birth.[5] While the pathogenesis of BPD has many factors, invasive mechanical ventilation is a primary risk factor. The most common cause of morbidity of prematurity is BPD. For this reason, research has focused on lung protective strategies to limit the development of CLD.
Developed in the 1970s, high-frequency oscillatory ventilation (HFOV) is a form of lung protective ventilation that may be used as a primary mode of ventilation in neonates or for those who fail conventional ventilation. HFOV functions by producing small tidal volumes generated by an oscillatory piston to minimize volutrauma.[6][3] While using small tidal volumes, HFOV can also prevent atelectasis, another major component of ventilator-induced lung injury.[3] Similar to conventional ventilation, oxygenation is controlled by the mean airway pressure (MAP) and the fraction of inspired oxygen (FiO2), while ventilation is controlled by the amplitude and frequency of oscillations. Unlike conventional ventilation, which is more likely to induce lung injury through shearing forces, high-frequency oscillation maintains alveolar volume and, therefore, prevents this shearing force.[4] HFOV strategies focus on limiting tidal volume to prevent volutrauma for extremely premature infants.
Indications
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Indications
There are many indications for the use of HFOV in the neonatal population, with the most common being failure of ventilation and oxygenation while on conventional ventilation. Although some centers use HFOV as a primary mode of ventilation for extremely preterm infants, more frequently, the oscillator is used as a form of rescue ventilation after the failure of conventional ventilation when high ventilator settings are required.[3]
Other indications for HFOV in neonates include the following:
- Meconium aspiration syndrome
- Pulmonary hypoplasia
- Persistent pulmonary hypertension of the newborn (PPHN)
- Respiratory distress syndrome (RDS)
- Airleak syndromes
Infants with congenital diaphragmatic hernia often have complex physiology due to pulmonary hypoplasia. HFOV has been used in this population to improve survival by limiting overdistension of hypoplastic lungs.[7] It should be noted that a randomized clinical trial showed potentially worse outcomes when patients were initially treated with HFOV than with conventional ventilation.[8]
HFOV is less effective in diseases such as BPD, where air trapping is often present due to increased airway resistance.[9]
Technique or Treatment
The premature infant’s lung is unique in that surfactant deficiency, along with high chest wall compliance and a varying functional residual capacity, facilitates lung injury through the cycle of atelectasis and reinflation.[7] For this reason, the neonatal lungs are well-suited for an open lung strategy to prevent atelectasis and overdistension.[4]
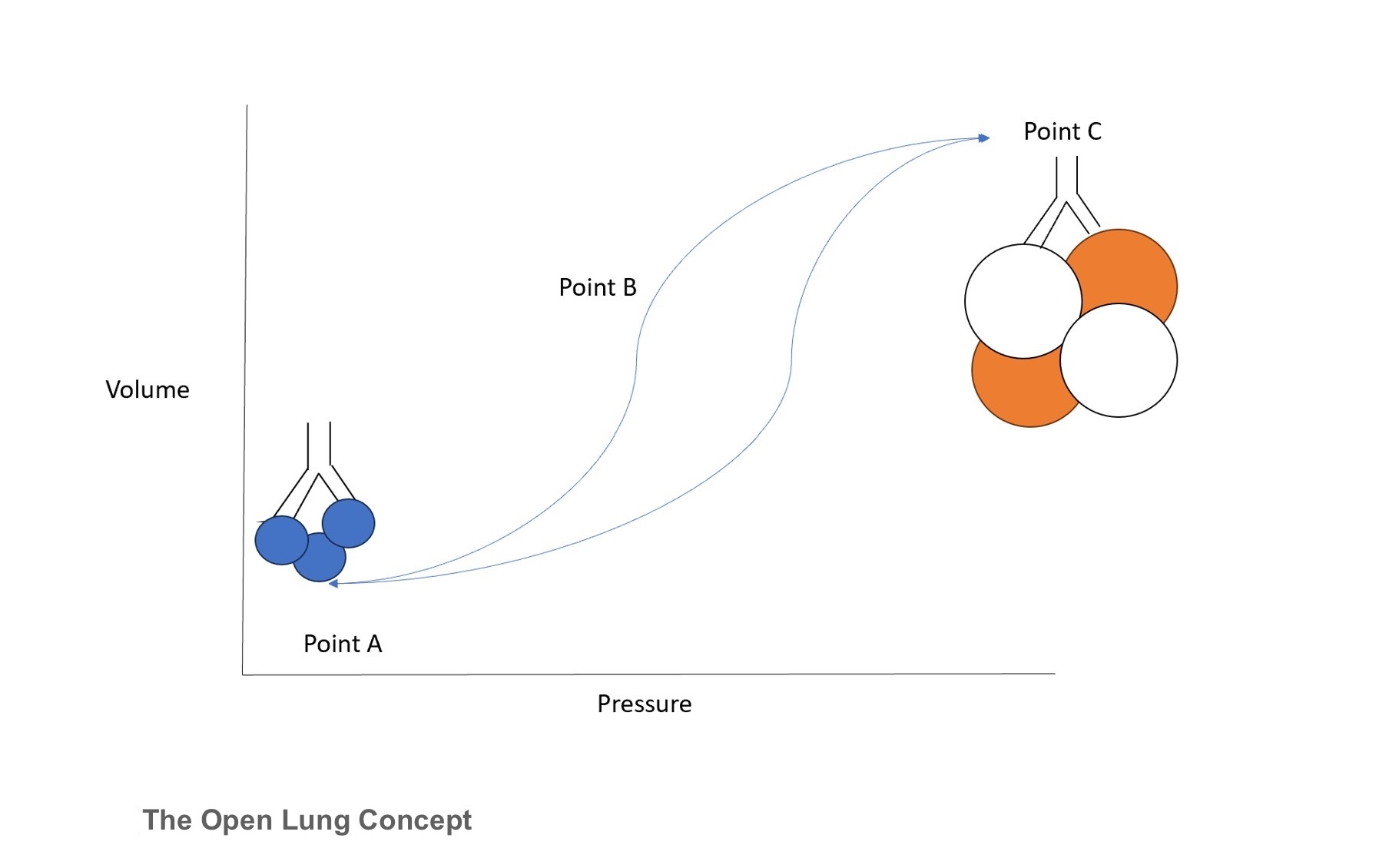
The open lung concept represents the relationship between pressure and lung volume throughout varying zones of lung recruitment (see Image. The Open Lung Concept). Point A depicts the zone of atelectasis, which results in high pulmonary vascular resistance. Clinically, this would present as an infant with a high oxygen requirement and limited chest shake on HFOV. Conversely, point C depicts the zone of over-distension, at which point high intrathoracic pressure can compromise systemic circulation. The best way to identify this point is through diaphragm flattening with a smaller-than-normal cardiac silhouette on chest radiographs. Finally, point B represents the “safe window” of optimal lung recruitment, at which point there is appropriate chest vibration, appropriate lung expansion, and a decreased oxygen requirement. Importantly, HFOV operates in this window throughout the entire respiratory cycle, again promoting the open lung concept.[10]
HFOV delivers small tidal volumes of less than dead space at supraphysiologic frequencies of 5 to 15 Hz or 300 to 900 breaths per minute. The tidal volumes are generated by an oscillatory piston while using a constant distension pressure to maintain adequate lung volumes.[3][6] By using constant pressure, HFOV results in more uniform lung inflation, thereby reducing the damage induced by conventional ventilation. The oscillator has 3 main settings: mean airway pressure (MAP), amplitude, and frequency.
Oxygenation is controlled by the MAP and FiO2. Lung recruitment is maintained by the MAP to maintain alveolar stability and minimize oxygen toxicity.[7] For rescue HFOV, the starting pressure is typically 2 cm H20 above the MAP used during conventional ventilation. The MAP can then be increased to improve lung expansion or if an infant requires a high FiO2. Alternatively, the MAP can be weaned if evidence of over-inflation or as FiO2 decreases over time.[7] Following chest radiographs while on HFOV is crucial to monitor appropriate lung inflation and prevent over-distension and under-distention. An increase in MAP leads to increased lung volume and downward displacement of the diaphragm.[9] If the MAP is set too high, blunting of the costodiaphragmatic angle can often be seen, and high airway pressures cause increased intrathoracic pressure that may interfere with venous return, leading to hypotension.[3] If hypotension develops, the MAP should be lowered to allow for adequate cardiac filling and ventricular output.
The amplitude is the primary component used to control tidal volume and occurs due to the voltage across the piston. A higher amplitude increases piston movement and, as a result, leads to higher tidal volumes. When initiating HFOV for a neonate, the amplitude is adjusted until appropriate chest vibration is observed. The amplitude is then adjusted to achieve optimal pCO2. If there is evidence of hypocapnia or hyperventilation, the amplitude should be decreased to decrease the tidal volume. Similarly, if a patient is under-ventilated with evidence of hypercapnia, the amplitude could be increased to increase the tidal volume and eliminate more carbon dioxide.
Finally, the frequency (measured in Hertz) is the rate at which oscillation occurs and is used along with the amplitude to control ventilation. A hertz equals 60 oscillations per minute and can be set from 8 to 15 Hz, leading to respiratory rates up to 900 per minute.[2] The optimal frequency will be different based on the disease process and patient size. Infants with noncompliant lungs will benefit from a short time constant and higher frequencies, while infants with very compliant lungs do better with lower frequencies.[3] Lung diseases with increased airway resistance, such as meconium aspiration syndrome, will benefit from a low frequency as it will result in a higher time constant and allow for sufficient emptying. Low-resistance diseases such as pulmonary interstitial emphysema and air leak syndromes require a higher frequency, given poor compliance.
Changes in frequency will affect the tidal volume of each delivered breath and in contrast to the amplitude, frequency has an inverse relationship to tidal volume. For example, a lower frequency will increase the tidal volume by increasing the inspiratory time. Therefore, the frequency can be increased to decrease tidal volume and CO2 elimination in an over-ventilated patient and decreased to increase tidal volume and CO2 elimination in an under-ventilated patient. Of note, infants with RDS are best managed with a higher frequency (12 to 15 Hz) compared to term infants who need decreased frequencies of 8 to 10 Hz.
Unlike other forms of high-frequency ventilation, the movement of the piston and diaphragm in HFOV results in both active inhalation and exhalation. The ratio between inspiratory and expiratory time on HFOV is rarely manipulated during clinical management, and the I:E ratio is typically set at 1:2, with inspiration occupying 33% of each oscillation.
There are 2 main concepts of ventilation in HFOV: convection and diffusion. The oscillatory pressure is applied to the trachea and dampened by the flow-dependent resistance of the airway.[3] Atelectatic alveoli have higher peripheral resistance and, therefore, are exposed to higher oscillatory pressure. This pressure is transmitted to adjacent alveoli, while alveoli distal to this high-pressure region have decreased flow due to lower pressures.[3] In other words, HFOV promotes gas exchange by direct ventilation of neighboring alveoli from high to low pressure, known as bulk convection.[3]
Clinical Significance
HFOV has been used in neonatal intensive care units (NICUs) for patients who fail conventional ventilation. Numerous studies have been conducted on HFOV and its use in NICUs. The first large, randomized control trial (RCT), the HIFI Study Group, was established in 1989 and compared HFOV to conventional ventilation.[7] While the study was not able to show a significant decrease in CLD or mortality with the use of HFOV, they did find a significant increase in severe intraventricular hemorrhage (IVH). Interestingly, recent studies have not shown this increase in IVH risk when using HFOV.[3]
In 2015, Cools et al. reviewed 19 RCTs that compared HFOV to conventional ventilation in preterm and VLBW infants and found no differences in IVH between the groups.[11] While the group identified a decreased risk of BPD in using the HFOV, this effect was not seen in all studies.
Another meta-analysis evaluated 20 RCTs that included approximately 2800 patients and found that HFOV was associated with decreased mortality from RDS compared to conventional ventilation.[12] An RCT conducted by Courtney et al determined that when compared to conventional ventilation, VLBW infants assigned to HFOV were extubated earlier and had lower rates of BPD.[6]
In a large multicenter trial conducted by Kinsella et al, 200 neonates with severe hypoxic respiratory failure and PPHN were found to have improvements in oxygenation with HFOV and inhaled nitric oxide compared to conventional ventilation.[13] Yang et al evaluated over 1000 preterm infants with severe RDS and discovered that most patients were weaned within 3 days after initiation of HFOV.[14] They also found decreased mortality in preterm infants treated with the oscillator.[15]
Studies have shown that HFOV and surfactant administration reduces oxygen and volutrauma-induced lung injury. Yoder et al compared the effect of prolonged HFOV with low tidal volume positive pressure ventilation in immature baboons to model neonatal CLD. The study found improved lung function and less inflammation in the HFOV group.[16]
There are limitations when using HFOV. One such limitation is the derecruitment caused by suctioning. Any time off the oscillator can lead to significant alveolar collapse and take a long time to return to baseline. Yet another limitation is the amount of sedation required for neonates, especially older infants, as the oscillator is unlike normal ventilation. As previously alluded, high MAPs can lead to hypotension due to decreased venous return. Finally, depending on each clinician’s experience, there may be provider discomfort in using this mode of ventilation.
Overall, HFOV has been used in the NICU to prevent CLD by limiting volutrauma and oxygen toxicity. Despite several experimental and clinical studies showing decreased lung injury with HFOV, there remains no definitive guideline for ventilation as each case is different and requires a patient-specific approach.
Enhancing Healthcare Team Outcomes
Implementation of HFOV in the NICU underscores the role of the interprofessional team in improving ventilatory management in neonates with lung disease. This form of ventilation highlights the coordination required between physicians, advanced care practitioners, pharmacists, nurses, and respiratory therapists to provide optimal care to each patient. Prioritizing patient-centered care necessitates tailoring HFOV strategies to the individual needs of each neonate. Engaging with the family, providing education and support, and involving them in care decisions are essential components of a holistic approach.
High-frequency ventilation continues to be used as a rescue strategy in neonates who fail conventional ventilatory management. As discussed, this mode of ventilation may also be used as a lung-protective strategy to prevent CLD. Coordinated care involves aligning the efforts of all team members, from initial assessment to ongoing monitoring and adjustments in HFOV settings. This ensures that the care plan is consistently applied and adapted as needed based on the neonate's response.
Regular review of outcomes data and continuous quality improvement initiatives help in refining HFOV practices. Prioritizing patient safety involves rigorous monitoring for potential complications, ensuring all equipment is functioning correctly, and adhering to protocols designed to minimize risks associated with HFOV.
Enhanced team performance is achieved through ongoing training, simulation exercises, and debriefings to ensure all interprofessional team members are proficient in HFOV techniques and can respond effectively to any situation. Continuous professional development and feedback loops contribute to a high-performing team dedicated to the best possible neonatal care.
Media
References
Elgin TG, Berger JN, Thomas BA, Colaizy TT, Klein JM. Ventilator Management in Extremely Preterm Infants. NeoReviews. 2022 Oct 1:23(10):e661-e676. doi: 10.1542/neo.23-10-e661. Epub [PubMed PMID: 36180732]
Johnson AH, Peacock JL, Greenough A, Marlow N, Limb ES, Marston L, Calvert SA, United Kingdom Oscillation Study Group. High-frequency oscillatory ventilation for the prevention of chronic lung disease of prematurity. The New England journal of medicine. 2002 Aug 29:347(9):633-42 [PubMed PMID: 12200550]
Ackermann BW, Klotz D, Hentschel R, Thome UH, van Kaam AH. High-frequency ventilation in preterm infants and neonates. Pediatric research. 2023 Jun:93(7):1810-1818. doi: 10.1038/s41390-021-01639-8. Epub 2022 Feb 8 [PubMed PMID: 35136198]
Berger TM, Fontana M, Stocker M. The journey towards lung protective respiratory support in preterm neonates. Neonatology. 2013:104(4):265-74. doi: 10.1159/000354419. Epub 2013 Oct 1 [PubMed PMID: 24107385]
Shetty S, Greenough A. Neonatal ventilation strategies and long-term respiratory outcomes. Early human development. 2014 Nov:90(11):735-9. doi: 10.1016/j.earlhumdev.2014.08.020. Epub 2014 Sep 16 [PubMed PMID: 25233800]
Courtney SE, Durand DJ, Asselin JM, Hudak ML, Aschner JL, Shoemaker CT, Neonatal Ventilation Study Group. High-frequency oscillatory ventilation versus conventional mechanical ventilation for very-low-birth-weight infants. The New England journal of medicine. 2002 Aug 29:347(9):643-52 [PubMed PMID: 12200551]
Ventre KM, Arnold JH. High frequency oscillatory ventilation in acute respiratory failure. Paediatric respiratory reviews. 2004 Dec:5(4):323-32 [PubMed PMID: 15531258]
Snoek KG, Capolupo I, van Rosmalen J, Hout Lde J, Vijfhuize S, Greenough A, Wijnen RM, Tibboel D, Reiss IK, CDH EURO Consortium. Conventional Mechanical Ventilation Versus High-frequency Oscillatory Ventilation for Congenital Diaphragmatic Hernia: A Randomized Clinical Trial (The VICI-trial). Annals of surgery. 2016 May:263(5):867-74. doi: 10.1097/SLA.0000000000001533. Epub [PubMed PMID: 26692079]
Level 1 (high-level) evidenceMeyers M, Rodrigues N, Ari A. High-frequency oscillatory ventilation: A narrative review. Canadian journal of respiratory therapy : CJRT = Revue canadienne de la therapie respiratoire : RCTR. 2019:55():40-46. doi: 10.29390/cjrt-2019-004. Epub 2019 May 2 [PubMed PMID: 31297448]
Level 3 (low-level) evidenceKrebs J, Pelosi P, Tsagogiorgas C, Zoeller L, Rocco PR, Yard B, Luecke T. Open lung approach associated with high-frequency oscillatory or low tidal volume mechanical ventilation improves respiratory function and minimizes lung injury in healthy and injured rats. Critical care (London, England). 2010:14(5):R183. doi: 10.1186/cc9291. Epub 2010 Oct 14 [PubMed PMID: 20946631]
Cools F, Offringa M, Askie LM. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. The Cochrane database of systematic reviews. 2015 Mar 19:2015(3):CD000104. doi: 10.1002/14651858.CD000104.pub4. Epub 2015 Mar 19 [PubMed PMID: 25785789]
Level 1 (high-level) evidenceWang C, Guo L, Chi C, Wang X, Guo L, Wang W, Zhao N, Wang Y, Zhang Z, Li E. Mechanical ventilation modes for respiratory distress syndrome in infants: a systematic review and network meta-analysis. Critical care (London, England). 2015 Mar 20:19(1):108. doi: 10.1186/s13054-015-0843-7. Epub 2015 Mar 20 [PubMed PMID: 25881121]
Level 1 (high-level) evidenceKinsella JP, Truog WE, Walsh WF, Goldberg RN, Bancalari E, Mayock DE, Redding GJ, deLemos RA, Sardesai S, McCurnin DC, Moreland SG, Cutter GR, Abman SH. Randomized, multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe, persistent pulmonary hypertension of the newborn. The Journal of pediatrics. 1997 Jul:131(1 Pt 1):55-62 [PubMed PMID: 9255192]
Level 1 (high-level) evidenceHsu JF, Yang MC, Chu SM, Yang LY, Chiang MC, Lai MY, Huang HR, Pan YB, Fu RH, Tsai MH. Therapeutic effects and outcomes of rescue high-frequency oscillatory ventilation for premature infants with severe refractory respiratory failure. Scientific reports. 2021 Apr 19:11(1):8471. doi: 10.1038/s41598-021-88231-6. Epub 2021 Apr 19 [PubMed PMID: 33875758]
Yang MC, Hsu JF, Hsiao HF, Yang LY, Pan YB, Lai MY, Chu SM, Huang HR, Chiang MC, Fu RH, Tsai MH. Use of high frequency oscillatory ventilator in neonates with respiratory failure: the clinical practice in Taiwan and early multimodal outcome prediction. Scientific reports. 2020 Apr 20:10(1):6603. doi: 10.1038/s41598-020-63655-8. Epub 2020 Apr 20 [PubMed PMID: 32313052]
Yoder BA, Siler-Khodr T, Winter VT, Coalson JJ. High-frequency oscillatory ventilation: effects on lung function, mechanics, and airway cytokines in the immature baboon model for neonatal chronic lung disease. American journal of respiratory and critical care medicine. 2000 Nov:162(5):1867-76 [PubMed PMID: 11069828]