Catheter Management of Aortic Valve Disorders
Catheter Management of Aortic Valve Disorders
Introduction
Aortic valve disease, specifically aortic valve stenosis, is one of the most common valvular disorders in the United States and worldwide. While traditional aortic valve replacement via open heart surgery was historically the preferred--and only--definitive treatment option, transcatheter therapy now plays a significant role. Since the first reported case of transcather aortic valve replacement (TAVR) in a human in 2002, TAVR has evolved rapidly and now has become the preferred first-line treatment option for many patients with aortic stenosis.[1] While the earliest TAVR patients were those deemed either prohibitive or very high risk for surgical aortic valve replacement (SAVR), the use of TAVR has expanded to include those who are intermediate and low risk. The evolution has TAVR has encountered numerous pitfalls and technical modifications that have improved safety and efficacy.[2] Landmark trials including the PARTNER 1 and PARTNER 2 studies have demonstrated the benefits of TAVR compared to medical management and SAVR. We describe here an overview of TAVR for aortic valve stenosis.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
The aortic valve separates the left ventricular outflow tract from the aortic root. It is generally a trileaflet valve composed of three distinct leaflets: the left coronary, right coronary, and non coronary leaflets. The aortic valve annulus is interconnected with the fibrous skeleton of the heart and is in continuity with the aorto-mitral curtain. The conduction system resides usually underneath the right coronary cusp extending towards the right-non commissure. In a small proportion of patients, the aortic valve is composed of two leaflets, leading to a bicuspid aortic valve.
Indications
TAVR Indications:
Current guidelines for TAVR include:
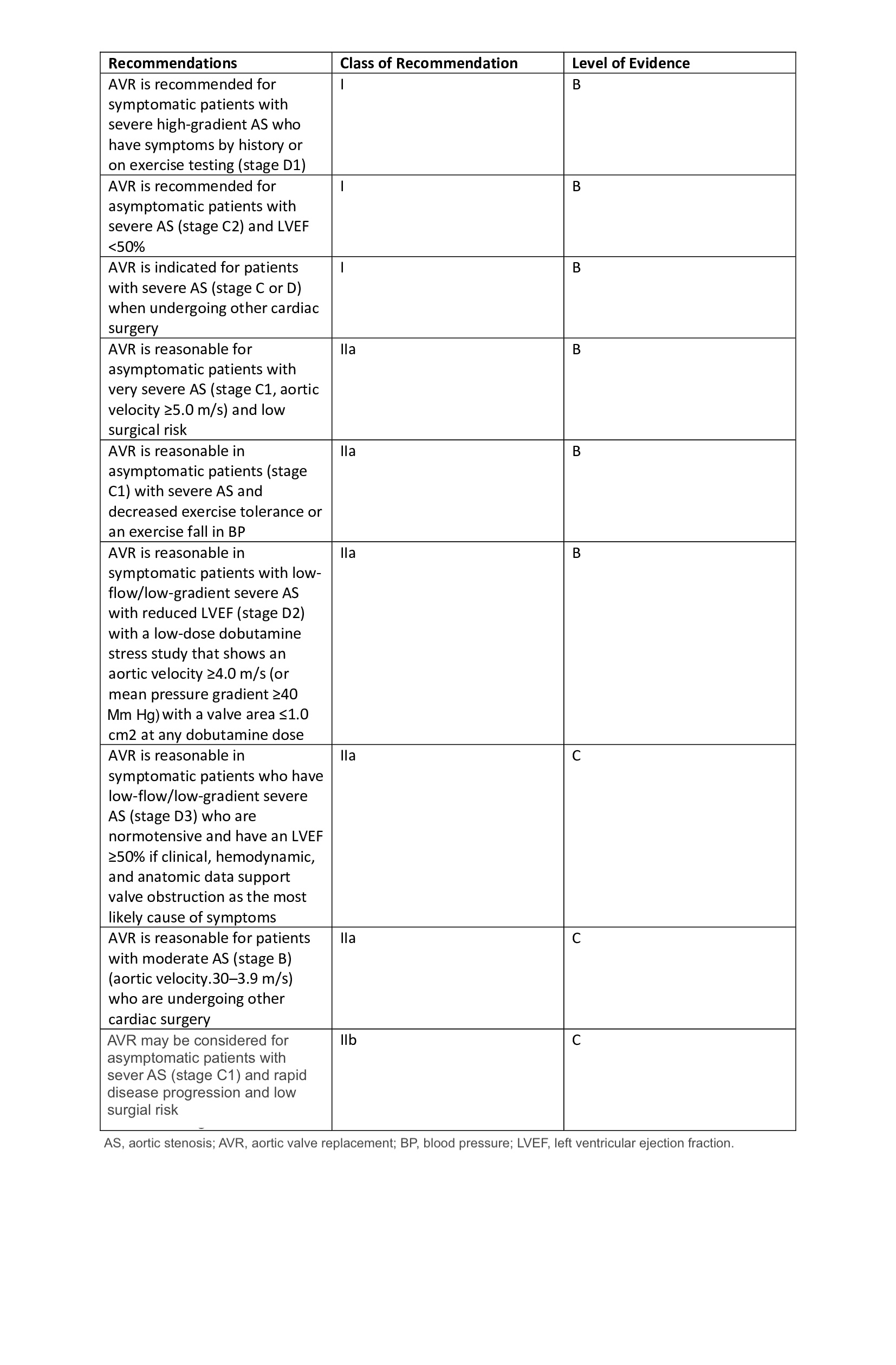
- Intermediate to prohibitive surgical risk patients with severe AS (class I recommendation level B evidence)
- A valve-in-valve procedure for a failed prior bioprosthetic valve (class I recommendation, level B evidence)
In mid-2019 the US FDA approved TAVR for low surgical risk patients. While society guidelines have not yet included this patient population in TAVR indications and practice guidelines (as of the time of this writing), we expect indications to expand to include these patients. Currently, guidelines recommend SAVR for these patients if the patient is a surgical candidate. TAVR is not approved for use in aortic valve insufficiency (AI), although there have been reports of off-label use of TAVR in these patients.[3] However, several studies are now being conducted to challenge this recommendation.[4]
Balloon Aortic Valvuloplasty Indications:
This procedure was first introduced in 1986 as a non-invasive option for the treatment of aortic stenosis. Unfortunately due to the high failure rate with early restenosis and poor long term survival rates the procedure is rarely performed. Typically, this procedure is offered as a palliative option for patients with contraindications to TAVR or SAVR. Occasionally, it is offered to hemodynamically unstable patients as a bridge to SAVR or TAVR intervention. BAV is often performed at the time of TAVR to aid with valve implantation.
Contraindications
TAVR Contraindications:
There are several contraindications to the use of TAVR. It is not recommended for any patient with a life expectancy under 12 months. Other contraindications include the absence of a Heart Team on-site, lack of cardiac surgical backup on-site, and other major co-morbidities that would limit post-operative quality of life such as profound dementia. Other factors for consideration would include a myocardial infarction less than one month before, a severe pulmonary disease, such as pulmonary hypertension with right ventricular dysfunction, severely depressed left ventricular function less than 20% ejection fraction, abnormal size of native aortic annulus less than 18 or larger than 25 mm. Also, the presence of an intracardiac mass, thrombus or vegetation, intracardiac anatomy preventing successful catheterization, or severe mitral regurgitation or insufficiency should be considered. In addition, patients unable to tolerate anticoagulation, stroke/TIA within six months of procedure, patients requiring emergent surgical intervention, patients on dialysis, those with elevated serum creatinine higher than 3 mg/dL, congenital bicuspid, unicuspid, or noncalcified aortic valve, hypertrophic cardiomyopathy (HOCM), significant aortic disease, or the presence of mixed aortic valve disease. There is debate on the feasilibity of TAVR in patients with mixed stenosis/insufficiency.[5]-[6]
BAV Contraindications:
Contraindications for percutaneous aortic valve balloon valvuloplasty include moderate to severe aortic insufficiency, an intracardiac mass, active endocarditis, and the presence of a contraindication to anti-thrombolytic therapy in the perioperative setting.[7]
Equipment
TAVR can be performed in either a hybrid operating room or catheterization laboratory. Fluoroscopy, transesophageal echocardiography (TEE), perfusion standby, and appropriate procedure-specific instrumentation (wires, catheters, introducers, surgical instruments) as per institution protocol are required. BAV is generally performed in the catheterization laboratory with fluoroscopy and/or TEE.
Personnel
TAVR Personnel
Current personnel requirements in the US for TAVR include a cardiac surgeon and interventional cardiologist performing the procedure jointly. Other intraoperative personnel necessary include anesthesia (preferably cardiac anesthesia) with TEE capability, perfusionist standby, and operating room/catheterization laboratory support staff including scrub technicians/nurses.
BAV Personnel
BAV personnel required for completion of this procedure include an interventional cardiologist trained in BAV and appropriate catheterization laboratory staff. Cardiac anesthesia with TEE capability is preferable.
Preparation
TAVR Preparation
Heart Team evaluation of any proposed TAVR patient is required. This consists of an interprofessional group including interventional cardiology and cardiac surgery. There must be Heart Team consensus for recommending TAVR over SAVR. The Heart Team must also review all relevant procedural planning aspects including valve sizing and planned vascular access. Preoperative diagnostic studies include echocardiogram (TTE or TEE), coronary artery evaluation with angiography, and TAVR-protocol computed tomography (CT) scanning. These studies help to evaluate the severity of aortic valve disease, anatomy of the aortic valve and root apparatus, size of the annulus and aortic root sinuses, degree of valve calcification, vascular access options including the lower and upper extremities, and functional status of other cardiac structures including ventricular function. [8] Valve sizing can be accomplished with the use of dedicated software programs such as 3Mensio to aid with precise measurement. Laboratory workup should include an assessment of the baseline kidney function, liver function, hemoglobin, glucose, and type and cross-match. A pulmonary function test and arterial blood gas analysis may be performed in patients with known or suspected pulmonary disease. A detailed airway evaluation is crucial to assess if the patient will tolerate general anesthesia and to avoid preventable complications. A thorough review of the patient's preoperative medications should also be done.
The patient is instructed to continue medications as they would for any other surgical procedure. Antiplatelet agents such as aspirin and clopidogrel may be already part of a patient’s list of drugs, or they may be newly administered before the procedure. Aspirin in a dose of 75 to 300 mg should be delivered before the procedure if there are no contraindications to the use.[9] Currently, there are no standardized postoperative antiplatelet use protocols, although many centers will start dual antiplatelet therapy for a limited duration postoperatively. On the day of the operation, approximately one hour before the procedure, pretreatment with intravenous antibiotics should be administered. There are no guidelines for post-operative antibiotic therapy, but some reports in the literature report prophylactic antibiotics for three to seven days following the procedure.
BAV Preparation
Prior to BAV a TTE is performed to assess the anatomy and severity of the AV disease. The aortic valve area and the mean transaortic valve gradient are noted. A coronary angioplasty is also performed prior to BAV and in the evaluate for and treat for coronary artery disease.
Technique or Treatment
TAVR Technique
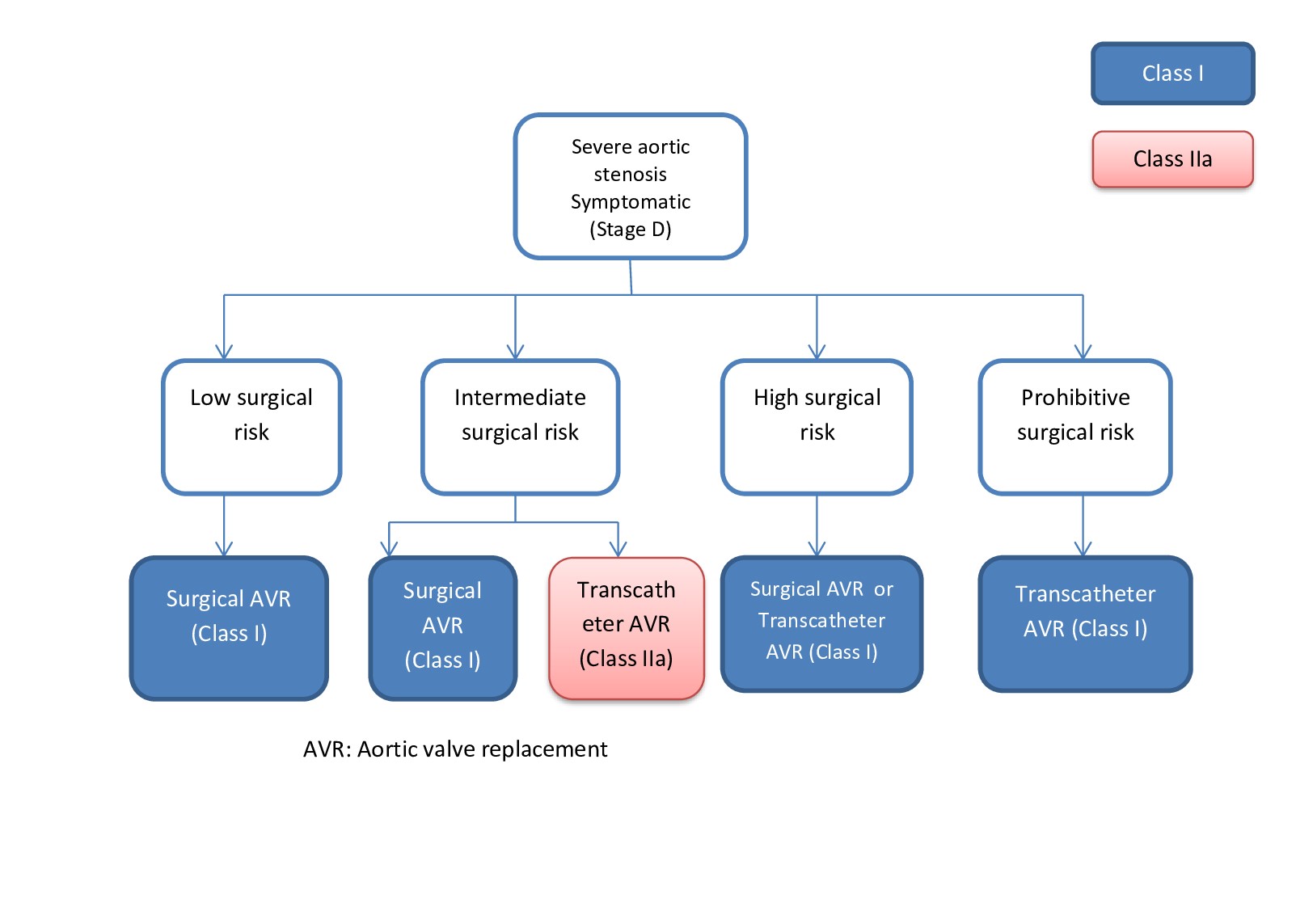
The most important aspects to decide prior to the day of surgery is the valve size and vascular access. Various approaches include transfemoral, transaxillary, transapical, transaortic, and transcarotid.[10] [11] Percutaneous transfemoral approach is the most common and least invasive, while other approaches must be considered if the lower extremity or iliac vessels are of inadequate size, excessively tortuous, or heavily calcified. Of the alternative approaches, the transaxillary is the next most common.
For the transfemoral approach, the vessels can be accessed either percutaneously or via open surgical cutdown. For the percutaneous route, the contralateral common femoral artery and vein are accessed and small caliber sheaths (such as 5 Fr and 6 Fr) are placed. A temporary pacing wire is inserted through this venous access and positioned into the right heart for pacing during valve deployment. A pigtail catheter is placed via this arterial access and positioned in one of the aortic sinus cusps. The operative side common femoral artery is then accessed using fluoroscopic guidance and a micropuncture needle. Two pre-deployed percutaneous vessel closure suture devices are placed for future use. Exchange length guidewires and catheters are then placed per institutional routine. Heparin is administered. The aortic valve is crossed with a guidewire and then exchanged to a soft wire over an exchange catheter. The delivery sheath (if the selected TAVR device uses a sheath) is inserted. The new device is loaded onto the delivery device and appropriate orientation is confirmed. BAV can be performed now if desired. The TAVR valve and delivery device are then inserted and positioned appropriately across the aortic annulus under fluoroscopy. Depending on the specific valve type, rapid ventricular pacing may be necessary. Self-expanding valve prostheses may not required rapid pacing. The valve is then deployed under fluoroscopic guidance. Once deployed, TEE is performed to evaluate positioning, valve function, and assess for paravalvular leak. If TEE shows acceptable findings, the delivery device is then removed. All other catheters are removed sequentially. The temporary pacing wire can be removed or left in place if there is any concern for heart block or conduction abnormalities. The delivery sheath is removed and the pre-deployed suture closure devices are secured.
For the transaxillary approach, an infraclavicular incision is made and soft tissue dissected to expose the axillary artery. The brachial plexus structures are often overlying the artery in this location and must be clearly identified and gently retracted to avoid injury. Furthermore, the use of electrocautery in this area should be avoided again to minimize the risk of injury to the nerve structures. Once the artery is exposed, proximal and distal control are obtained. Heparin is given. Pursestring sutures are placed at the site of planned access. Once the artery is accessed and wire placed via Seldinger technique, the remainder of the procedure can proceed as listed above for Transfemoral approach.
For the transpaical approach, an echocardiogram probe is placed on the left inframammary region to ascertain the exact location of the LV apex. An inframammary skin incision is made and soft tissue dissected to expose the intercostal space. A finder needle can be used to confirm location. The intercostal space and pericardium are opened to expose the LV apex. A pursestring suture is placed and LV cavity accessed using Seldinger technique. One note of caution for this approach: the valve must be loaded in the appropriate orientation. The orientation for the transapical approach is opposite that of all other approaches since the access direction is antegrade.
The transaortic approach requires an upper hemi-sternotomy to expose adequate ascending aorta. A pursestring is placed and aorta accessed again using Seldinger technique. The remainder of the procedure proceeds as described above.
BAV Technique
Access is obtained similar to the TAVR procedure described above. After obtaining access, a balloon catheter is introduced and positioned across the stenotic aortic valve. Aortic valvuloplasty is then performed with balloon inflation with the aim to increase aortic valve area (AVA) and reduce transaortic pressure gradient. To obtain the mean transaortic pressure gradient, a pigtail catheter is used to measure this gradient before and after valvuloplasty. The goal of this procedure is to reduce the pressure gradient by at least a half. If the initial attempt fails to achieve the gradient change then balloon inflation can be repeated. [12]
Complications
TAVR Complications
Studies have demonstrated vascular complications, arrhythmias/conduction abnormalities, and stroke were the most common major complications following TAVR.[13] Death, coronary obstruction, aortic root rupture, and major hemorrhage were also reported, although less frequently than the former.[14] According to a study done by Ribeiro and colleagues, the risk of coronary artery obstruction was four times higher in patients following valve-in-valve TAVR.[15] Other documented complications include worsening aortic stenosis or regurgitation, mispositioning of the valve, device embolus, kidney disease or myocardial infarction. PARTNER 2 Trial results showed a significantly higher rate of kidney disease with the use of TAVR versus SAVR.[16]
BAV Complications
BAV does not improve long term survival. Therefore, it is normally only recommended as a palliative measure or a bridge to TAVR or SAVR. Other common complications following BAV including prolonged hospitalizations, the requirement for blood transfusions, acute kidney injury and/or failure, serious vascular complications (most common), profound hypotension, cardiac tamponade, permanent pacemaker, moderate to severe aortic regurgitation, coronary occlusion or dissection, stroke, or intraprocedural death. [17]
Clinical Significance
TAVR
The advent of the transcatheter approach in managing aortic valve disease was a monumental turning point in the field of cardiovascular disease. TAVR has provided a treatment option for those deemed prohibitive risk for traditional SAVR, and offers a much less invasive and morbid option for those at lower surgical risk.
BAV
The BAV was the first transcatheter intervention introduced for the treatment of severe aortic valve disease. Although it is no longer recommended as monotherapy, except as a palliative measure, it opened the door for more advanced therapies to be developed such as the formerly discussed TAVR. BAV is still performed today on patients as a bridge therapy for TAVR or SAVR and for palliative care as no long term survival benefit post-procedure has been established.
Enhancing Healthcare Team Outcomes
Percutaneous transcatheter management of aortic valve disease requires a skilled interprofessional team of healthcare professionals that includes a cardiac nurse, laboratory technologists, a pharmacist and some physicians in different specialties. Without proper pre-operative, intra-operative, and post-operative management life-threatening complications can and will occur. Therefore, it is imperative that the interprofessional team works as a unit with reliable communication to optimize patient-centered care. From the moment an aortic valve defect is identified, the discovering clinician has a responsibility to coordinate care including the following:
- Obtain an echocardiogram, electrocardiography, chest radiograph, and detailed physical exam including general labs
- Monitor the patient for signs and symptoms of respiratory distress, cardiac arrhythmias, and cardiovascular collapse
- Consult with the interventional cardiologist and cardiothoracic surgeon to assess the need for catheter versus surgical intervention [level 1]
- Consult with the structuralist about imaging tests to ensure that they meet the requirements for surgical or catheter management
- Consult with the intensivist about ICU care and monitoring while in the hospital with the assistance of cardiac specialty nurses trained nurses assisting with coordination of care, family education, and reporting untoward changes in vital signs and arrhythmias.
- The cardiac pharmacist specialty should assist with checking for drug-drug interactions, appropriate dosing, and assisting the team with monitoring therapeutic effect.
The management of aortic valve disease is a dynamic, ever-changing field. New technology comes up every day. It is crucial for the team to be up-to-date with the new upcoming technology and guidelines.
Media
(Click Image to Enlarge)
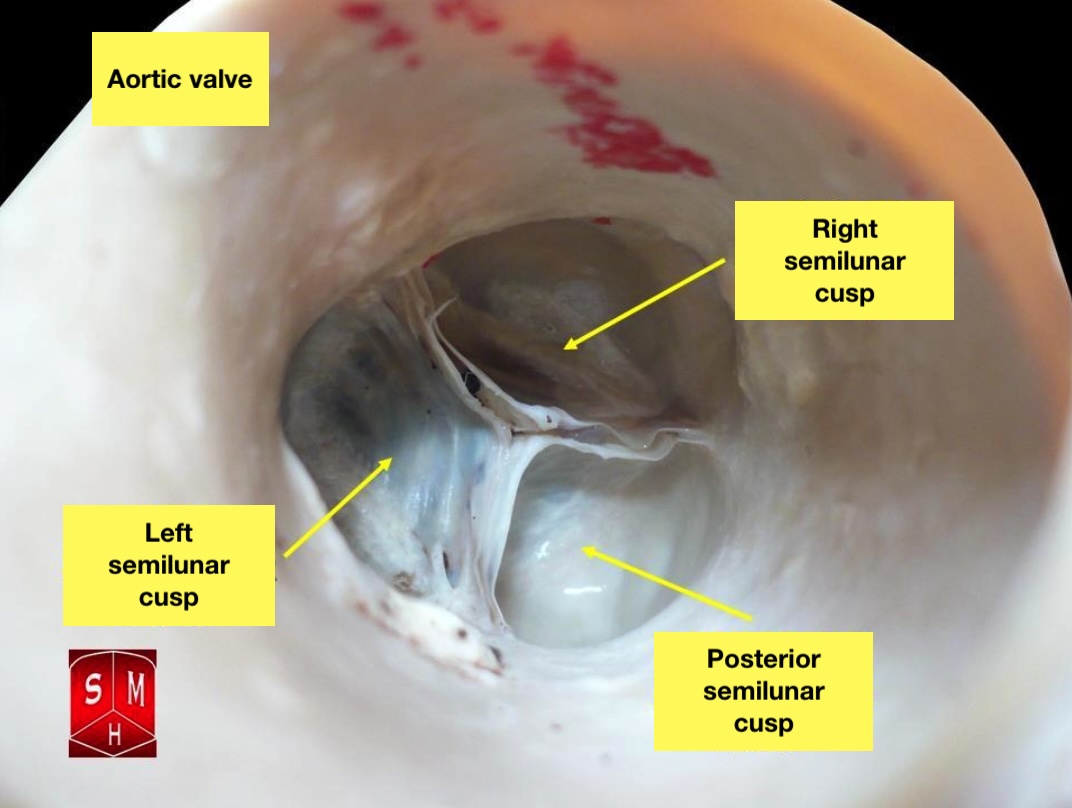
The Aortic Valve. The aortic valve resides centrally between the ascending aorta and the left ventricular outflow tract.
Anatomist90, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, Derumeaux G, Anselme F, Laborde F, Leon MB. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002 Dec 10:106(24):3006-8 [PubMed PMID: 12473543]
Level 3 (low-level) evidenceSakata Y, Syed Z, Salinger MH, Feldman T. Percutaneous balloon aortic valvuloplasty: antegrade transseptal vs. conventional retrograde transarterial approach. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2005 Mar:64(3):314-21 [PubMed PMID: 15736255]
Level 2 (mid-level) evidenceAbdelghani M, Cavalcante R, Miyazaki Y, de Winter RJ, Tijssen JG, Sarmento-Leite R, Mangione JA, Abizaid A, Lemos PA, Serruys PW, de Brito FS Jr. Transcatheter aortic valve implantation for mixed versus pure stenotic aortic valve disease. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2017 Nov 20:13(10):1157-1165. doi: 10.4244/EIJ-D-17-00328. Epub [PubMed PMID: 28691910]
Barker CM,Reardon MJ, Should TAVR Replace Surgery for Aortic Stenosis in Low- to Intermediate-Risk Patients? The Canadian journal of cardiology. 2017 Sep; [PubMed PMID: 28843323]
Arai T, Lefèvre T. Who is the right patient for TAVI? Journal of cardiology. 2014 Mar:63(3):178-81. doi: 10.1016/j.jjcc.2013.11.005. Epub 2013 Dec 12 [PubMed PMID: 24332753]
Rezq A, Godino C, Montorfano M, Covello D, Colombo A. Comprehensive multidisciplinary patient assessment and selection before TAVI procedure. Minerva cardioangiologica. 2014 Apr:62(2):177-91 [PubMed PMID: 24686996]
Olasińska-Wiśniewska A, Trojnarska O, Grygier M, Lesiak M, Grajek S. Percutaneous balloon aortic valvuloplasty in different age groups. Postepy w kardiologii interwencyjnej = Advances in interventional cardiology. 2013:9(1):61-7. doi: 10.5114/pwki.2013.34029. Epub 2013 Mar 21 [PubMed PMID: 24570692]
Level 3 (low-level) evidencePatel PA, Fassl J, Thompson A, Augoustides JG. Transcatheter aortic valve replacement--part 3: the central role of perioperative transesophageal echocardiography. Journal of cardiothoracic and vascular anesthesia. 2012 Aug:26(4):698-710. doi: 10.1053/j.jvca.2012.03.017. Epub 2012 May 11 [PubMed PMID: 22578977]
Grube E, Schuler G, Buellesfeld L, Gerckens U, Linke A, Wenaweser P, Sauren B, Mohr FW, Walther T, Zickmann B, Iversen S, Felderhoff T, Cartier R, Bonan R. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. Journal of the American College of Cardiology. 2007 Jul 3:50(1):69-76 [PubMed PMID: 17601548]
Level 2 (mid-level) evidenceDapunt OE, Luha O, Ebner A, Sonecki P, Spadaccio C, Sutherland FW. New Less Invasive Approach for Direct Aortic Transcatheter Aortic Valve Replacement Using Novel CoreVista Transcervical Access System. JACC. Cardiovascular interventions. 2016 Apr 11:9(7):750-3. doi: 10.1016/j.jcin.2016.01.035. Epub [PubMed PMID: 27056316]
Walther T, Dewey T, Borger MA, Kempfert J, Linke A, Becht R, Falk V, Schuler G, Mohr FW, Mack M. Transapical aortic valve implantation: step by step. The Annals of thoracic surgery. 2009 Jan:87(1):276-83. doi: 10.1016/j.athoracsur.2008.08.017. Epub [PubMed PMID: 19101311]
Kogoj P, Devjak R, Bunc M. Balloon aortic valvuloplasty (BAV) as a bridge to aortic valve replacement in cancer patients who require urgent non-cardiac surgery. Radiology and oncology. 2014 Mar:48(1):62-6. doi: 10.2478/raon-2013-0078. Epub 2014 Jan 22 [PubMed PMID: 24587781]
Rodés-Cabau J, Sacco RL. Neurological Complications Following Aortic Valve Replacement: TAVR Better Than SAVR, But Room for Improvement. Journal of the American College of Cardiology. 2018 Oct 30:72(18):2120-2122. doi: 10.1016/j.jacc.2018.06.080. Epub [PubMed PMID: 30360821]
Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ, PARTNER Trial Investigators. Transcatheter versus surgical aortic-valve replacement in high-risk patients. The New England journal of medicine. 2011 Jun 9:364(23):2187-98. doi: 10.1056/NEJMoa1103510. Epub 2011 Jun 5 [PubMed PMID: 21639811]
Level 1 (high-level) evidenceRibeiro HB, Webb JG, Makkar RR, Cohen MG, Kapadia SR, Kodali S, Tamburino C, Barbanti M, Chakravarty T, Jilaihawi H, Paradis JM, de Brito FS Jr, Cánovas SJ, Cheema AN, de Jaegere PP, del Valle R, Chiam PT, Moreno R, Pradas G, Ruel M, Salgado-Fernández J, Sarmento-Leite R, Toeg HD, Velianou JL, Zajarias A, Babaliaros V, Cura F, Dager AE, Manoharan G, Lerakis S, Pichard AD, Radhakrishnan S, Perin MA, Dumont E, Larose E, Pasian SG, Nombela-Franco L, Urena M, Tuzcu EM, Leon MB, Amat-Santos IJ, Leipsic J, Rodés-Cabau J. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. Journal of the American College of Cardiology. 2013 Oct 22:62(17):1552-62. doi: 10.1016/j.jacc.2013.07.040. Epub 2013 Aug 14 [PubMed PMID: 23954337]
Level 3 (low-level) evidenceBeohar N, Kirtane AJ, Blackstone E, Waksman R, Holmes D Jr, Minha S, Alli O, Suri RM, Svensson LG, Leon M, Kodali S. Trends in Complications and Outcomes of Patients Undergoing Transfemoral Transcatheter Aortic Valve Replacement: Experience From the PARTNER Continued Access Registry. JACC. Cardiovascular interventions. 2016 Feb 22:9(4):355-363. doi: 10.1016/j.jcin.2015.10.050. Epub 2016 Jan 20 [PubMed PMID: 26803420]
Ben-Dor I, Pichard AD, Satler LF, Goldstein SA, Syed AI, Gaglia MA Jr, Weissman G, Maluenda G, Gonzalez MA, Wakabayashi K, Collins SD, Torguson R, Okubagzi P, Xue Z, Kent KM, Lindsay J, Waksman R. Complications and outcome of balloon aortic valvuloplasty in high-risk or inoperable patients. JACC. Cardiovascular interventions. 2010 Nov:3(11):1150-6. doi: 10.1016/j.jcin.2010.08.014. Epub [PubMed PMID: 21087751]
Level 2 (mid-level) evidence