Introduction
Named for Sir Charles Bell, the Scottish neurologist and anatomist who first described the condition, Bell palsy is the most common paralysis of the seventh cranial nerve, accounting for 38% to 83% of cases of facial weakness (see Image. Sir Charles Bell).[1][2][3][4] In 1821, Bell presented his paper titled On the Nerves: Giving an Account of Some Experiments on Their Structure and Functions, Which Lead to a New Arrangement of the System to the Royal Society, in which he described, among other things, the course and function of the facial nerves.[5] Prior to Bell's anatomical studies, it was not known that the seventh cranial nerve controlled the muscles of facial expression, and this nerve was occasionally sacrificed during treatments for facial pain, thereby resulting in hemifacial paralysis. Bell was a prolific researcher and artist, and his name remains associated with several anatomical structures and physical findings, including the Bell phenomenon, or palpebral oculogyric reflex, which elevates the globes in order to protect the cornea when the eyelids close. This reflex is particularly critical for patients with facial paralysis, whose corneas are at greater risk for exposure and ulceration in the absence of effective eye closure.
When Bell palsy occurs, patients typically present complaining of weakness and often "numbness" of one side of the face, usually preceded 1 to 2 days prior by a dull ache behind or within the ipsilateral ear. Patients may report a dry eye over the course of the day prior to presentation, noting drooling while brushing their teeth before going to bed, and potentially numbness of the tongue or a metallic taste in the mouth. Some patients will also describe ipsilateral ear pain with loud noises. Upon awakening, the patient or partner will frequently notice facial asymmetry and may be concerned about the possibility of a stroke, which often leads to an emergency department visit. Generally, however, distinguishing between a stroke and Bell palsy is very straightforward, and patients can be reassured and discharged with steroids, antivirals, and eye lubricant as long as there is no suspicion for Ramsay Hunt syndrome, Lyme disease, or any other conditions that may cause a facial nerve palsy.
Fortunately, over 80% of patients with Bell palsy will recover on their own, with 90% to 97% improving if provided with appropriate medical management in a timely fashion.[2][6][7] For this reason, the most important early intervention for most patients is corneal protection in the event that eye closure is impaired; this consists of artificial tears, eyelid taping, and potentially upper eyelid weight placement. For the patients unfortunate enough to recover incompletely, spastic and dyscoordinated facial contractions (synkinesis) will develop, for which a broad range of treatments is available, from physiotherapy to botulinum toxin injections and surgery.[8][9]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Historically, Bell palsy has been considered a diagnosis of exclusion, to be applied when no other causes of acute-onset facial paralysis are readily apparent. If a patient with hemifacial palsy were to present in the absence of an auriculofacial rash, history of a tick bite, other neurological deficits, and imaging or laboratory abnormalities, Bell palsy would be diagnosed. Ironically, however, the term "Bell palsy" is often misused in an overly nonspecific manner to refer to any hemifacial paralysis, regardless of etiology, for example, describing the manifestations of a coronavirus infection as "upper and lower respiratory symptoms with polyneuropathy and Bell palsy."[10]
While it remains difficult to pin down the exact cause of Bell palsy for any individual patient, there is evidence to suggest that Human herpesvirus 1 is responsible for most of the cases. This virus has been identified in the epineurial fluid of 79% of the patients with Bell palsy but in none of the patients with Ramsay Hunt or other facial palsy conditions in a 1996 study performed by Murakami et al.[11] Testing epineurial fluid for herpes simplex deoxyribonucleic acid by polymerase chain reaction is clinically impractical in most patients, but because of the ubiquity of this virus and its close relatives (other herpes simplex viruses, cytomegalovirus, Epstein-Barr virus), the diagnosis of Bell palsy is now typically made clinically if the history and physical examination are consistent.
Another unsolved mystery regarding Bell palsy is its association with coronavirus disease (COVID) 2019 and the immunizations to prevent this infection. Facial paralysis, as well as other cranial neuropathies, are known to accompany SARS-CoV-2 infection, particularly affecting the olfactory, oculomotor, and abducens nerves, but also the trigeminal, auditory, vagus, glossopharyngeal, and hypoglossal nerves.[12][13][14] More controversial, however, is the potential relationship between Bell palsy and the various available COVID vaccinations. A large metanalysis published by Rafati et al in 2023 provided results determining that the risk of developing Bell palsy after messenger ribonucleic acid (mRNA) vaccination was 3.6 times higher than in the unvaccinated population, but that there was no significant increase in the risk of Bell palsy with viral vector vaccines.[15] However, results from a 2021 study that compared the incidence of Bell palsy after immunization with the Pfizer/BioNTech mRNA vaccine to that after the CoronaVac/Sinovac inactivated virus vaccine found the incidence to be over twice as high with the CoronaVac/Sinovac injection.[16] Regardless, the chance of developing facial palsy is significantly higher (relative risk of 3.23) with COVID infection than it is after COVID vaccination.[15]
Epidemiology
The annual incidence of Bell palsy is 15 to 40 per 100,000 individuals, and the lifetime risk is 1 in 60, with a recurrence rate of 8% to 12%.[1][17] There is no sex, ethnic, or laterality predilection, and Bell palsy can occur at any age; there is a bimodal distribution with incidence peaks between 20 and 30 years and between 60 and 70.[2] There are multiple known risk factors for developing Bell palsy, including diabetes, pregnancy, preeclampsia, obesity, dental procedures, and, debatably, hypertension.[18][19][20] Pregnant patients and those with diabetes are specifically at higher risk for worse outcomes and are potentially more likely to present with worse paralysis than patients without diabetes and who aren't pregnant with Bell palsy.[3][21]
According to a 2022 paper published by Escalante et al, the most common severity of Bell palsy is House-Brackmann grade III (mild-moderate), accounting for 41.9% of patients. Grade VI palsy (total hemifacial paralysis) occurs in 20.1% of patients. House-Brackmann grades II (mild) and V (severe) each comprise 16.3% of patients, and grade IV accounts for only 5.4%. The same study also assessed the chance of recovery as a function of palsy severity and found that patients with House-Brackmann grade VI palsy had a 60% chance of recovery to grade I or II, and grade V patients had an 83% chance if provided steroids and antivirals. Patients with grade II to IV paralysis all recovered to grade I or II in this series.[2]
Pathophysiology
Bell palsy, which is a unilateral hemifacial palsy of rapid onset, results from inflammation of the facial nerve, typically originating in the labyrinthine segment (see Image. Bell Palsy). The labyrinthine segment is the second intratemporal segment of the facial nerve and the narrowest, with an average diameter of 0.7 mm and a length of 3 to 5 mm.[22] The narrowness of this portion of the facial nerve's canal through the temporal bone of the skull (the Fallopian canal) predisposes it to dysfunction, potentially due to edema and subsequent impairment of perfusion when inflammation occurs, with Bell palsy more likely to occur in patients who have narrow labyrinthine segments of the facial canal.[23]
Proximal to the labyrinthine segment is the canalicular segment, which runs in the internal auditory canal superior to the cochlear nerve and anterior to the superior vestibular nerve. The labyrinthine segment ends distally at the geniculate ganglion, where the greater superficial petrosal nerve branches off to supply parasympathetic innervation to the lacrimal gland and the mucous glands of the nasal cavity and palate. At the distal end of the geniculate ganglion, also known as the first genu of the facial nerve, the tympanic segment enters the middle ear and courses superior to the oval window before turning inferiorly at the second genu and entering the mastoid cavity. The mastoid segment courses downwards to the stylomastoid foramen, where the nerve exits the temporal bone on its path to the parotid gland, within which it divides into the five main branches that innervate the muscles of facial expression. These branches are the frontal, zygomatic, buccal, marginal mandibular, and cervical (see Image. The Facial Nerve).[24]
Bell palsy is thought to result from compression of the seventh cranial nerve within the labyrinthine segment of the Fallopian canal. Not only is this segment the narrowest along the nerve's course within the temporal bone, but it also appears to have the most tenuous blood supply, with very fine arterioles connecting the angiosomes supplied by the internal auditory branch of the anterior inferior cerebellar artery proximally and the petrosal branch of the middle meningeal artery distally.[25] The final segment of the intratemporal facial nerve is perfused by the stylomastoid artery, a branch of the posterior auricular artery. Further implicating the labyrinthine segment as the primary site of Bell palsy inflammation is the appearance of the nerve on magnetic resonance imaging (MRI): 59% of patients with acute Bell palsy have asymmetric enhancement of the labyrinthine segment, and a further 33% have enhancement of both the labyrinthine segment and the geniculate ganglion (see Image. Left-Sided Bell Palsy, Magnetic Resonance Image).[26]
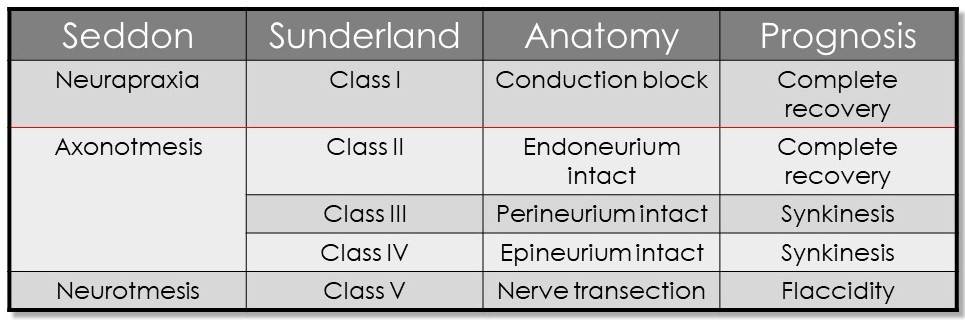
The severity of this inflammation may depend upon several factors, including the patient's immune status, blood glucose level, blood pressure, and age.[18][19] There is a hypothesis that edema of the nerve within the Fallopian canal compromises perfusion, leading to nerve dysfunction. The extent of the dysfunction and the prognosis for recovery depend on the degree of disruption of the internal architecture of the facial nerve, in which each axon has a myelin sheath and an endoneurial membrane surrounding it, with the axons grouped together into fascicles surrounded by perineurial membranes, and the fascicles together constituting the nerve itself, which is invested in epineurium (see Image. Neurology, Nerve Fascicle, Fasciculus, Epineurium).
A mild injury may only cause a temporary conduction block with or without focal demyelination, known as neuropraxia in the Seddon grading system, or a Sunderland class I injury. Actual axonal damage occurs in a Sunderland class II injury, but all of the internal architecture of the nerve is otherwise preserved, virtually ensuring that the axons regrow correctly along their original paths. In the case of a Sunderland class III injury, the axons undergo Wallerian degeneration as they do in a class II injury. However, the endoneurial membranes that surround the damaged axons are violated, which permits mild misdirection of the regenerating axons that may result in discoordinated movements and spasms (synkinesis). A Sunderland class IV injury adds perineurial damage to the class III injury, which results in disruption of the fascicular organization of the nerve and greater potential for synkinesis. Sunderland class II to IV injuries are collectively known as axonotmesis in the Seddon grading system, and a Sunderland class V injury is known as neuronotmesis.[27][28] Neuronotmesis is a complete transection of the nerve, including the epineurium, guaranteeing severe synkinesis even if the nerve is repaired microsurgically (see Image. Seddon and Sunderland Classifications of Nerve Injury).
History and Physical
Patients with Bell palsy present with progressive hemifacial paralysis that typically reaches a peak in severity within 24 to 72 hours of onset. Most patients will also report prodromal dull pain within or behind the ipsilateral ear. Other common associated symptoms include hyperacusis due to stapedius muscle weakness, a metallic taste on the ipsilateral side of the tongue or ipsilateral tongue numbness due to chorda tympani involvement, paradoxical eye dryness with tearing due to impairment of both eye closure and the lacrimal pump function of the orbicularis oculi muscle, ipsilateral nasal obstruction due to nasalis muscle weakness, oral incompetence and dysarthria due to orbicularis oris dysfunction, aesthetic self-consciousness because of facial asymmetry, and ipsilateral facial numbness. The latter may be a mischaracterization of facial weakness by some patients but may also represent a manifestation of the poorly understood connections between branches of the facial and trigeminal nerves that have been observed in laboratory studies.[29][30]
If the patient notes skin or mucosal rashes, an alternative diagnosis, such as Ramsay Hunt syndrome or Lyme disease, should be considered. Similarly, if the patient mentions significant, burning pain rather than a dull otalgia, a herpes zoster diagnosis may be appropriate even in the absence of a vesicular outbreak. Most importantly, there should not be an element of the history that implicates a specific etiology for facial paralysis, such as head trauma, otologic infection, or neoplasm.
Upon physical examination, all branches of the facial nerve are roughly equally impaired on the affected side of the face in patients with Bell palsy. In contrast, a cortical stroke will typically leave forehead movement intact on the affected side due to bilateral upper motor neuron contributions to the facial nucleus. A stroke will typically also present with other neurological signs or cranial neuropathies, while Bell palsy will not. Like Bell palsy though, a brainstem stroke will cause hemifacial weakness that does not preserve forehead movement; this too will most often present with other neurological symptoms as expected with a cortical stroke.
The unilaterality of Bell palsy is an important feature that helps to differentiate it from other facial paralyses, such as Lyme disease and Guillain-Barré syndrome, which often present with bilateral, albeit asymmetric, facial paralysis. Similarly, the presence of a cutaneous or mucosal rash of the head or face along with hemifacial palsy should shift focus to a diagnosis of Ramsay Hunt syndrome; a targetoid rash anywhere on the body may indicate Lyme disease. Patients presenting with chronic facial movement dysfunction from Bell palsy rather than acute, flaccid paralysis may report facial tension, fatigue, and pain instead of frank weakness, although they may mistake this synkinetic dyscoordination and asymmetry of facial expression for persistent paralysis. Another phenomenon that may accompany facial synkinesis is Bogorad syndrome, or "crocodile tears," in which aberrant reinnervation of the lacrimal gland leads to tearing that accompanies salivation.
An assessment of facial nerve function should be performed in a systematic fashion to ensure no findings are overlooked (see Video. Acute Bell Palsy with a Severe Presentation). Evaluating each extratemporal branch of the facial nerve individually is critical, both at rest and with movement. Resting forehead rhytid and brow symmetry, as well as symmetry with elevation, should be assessed; the examiner's thumb may be used to hold the glabella still to prevent transmission of movement from the normal side to the paralyzed brow if there is any doubt about the presence of movement on the affected side. The resting appearance of the palpebral fissures should be compared, and any increased opening on the affected side should be noted, as well as whether the increased opening is due to upper eyelid retraction or lower eyelid laxity. There may be asymmetry of the lateral periocular rhytids (crow's feet) as well, with the affected side having fewer or shallower wrinkles. Gentle eye closure (with a normal blink) and eye closure with full effort should be assessed, although it is not necessary to attempt to pry open the patient's eyes manually, as this maneuver has no prognostic or diagnostic value.
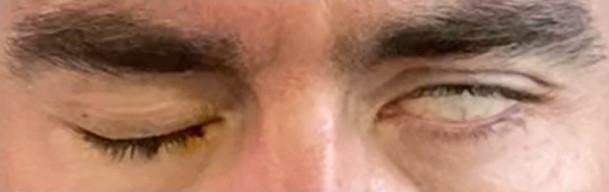
If eye closure is incomplete, the Bell phenomenon should be assessed to help determine the risk of corneal exposure. The Bell phenomenon is the reflex by which the globe rolls superolaterally to protect the cornea when the eye closes; this reflex may serve to protect the cornea even if eye closure is incomplete (see Image. Bell Phenomenon). The reflex is present in 70% to 80% of the population and tends to diminish with age.[31] If, on the other hand, eye closure is complete, the finding should be considered in the context of the rest of the history and physical examination. If several days have passed since the onset of paralysis and the rest of the hemiface also demonstrates some movement, the paralysis is likely only of mild to moderate severity. If there is slow but complete eye closure and no discernable movement in the rest of the hemiface with paralysis of recent onset, the eye closure is likely due to gravity rather than volitional movement and does not indicate any retained facial nerve function. Paralytic lagophthalmos in this situation will likely develop over the ensuing 24 to 48 hours.
Evaluation of midfacial motor function begins with a comparison of the nasolabial folds at rest. The affected side's fold may be shallower and/or more vertically oriented than the unaffected side. There may also be unilaterally diminished contraction of the nasalis muscle, although, like the eyebrows, this may be difficult to discern without placing a thumb in the center of the nose to prevent transmitted movement. A Cottle maneuver, in which the examiner's thumb is used to suspend the nasal base by retracting the cheek laterally, will identify any nasal valve collapse.
In the lower face, the oral commissure may be inferiorly malpositioned, and the philtrum may be pulled towards the unaffected side due to a lack of resting muscle tone on the affected side. The smile may be asymmetric, with decreased oral commissure excursion, upper lip elevation, and lower lip depression on the affected side. Lastly, there may be asymmetric platysmal contraction or even loss of resting platysmal banding on the affected side in an older patient.
The overall function can then be categorized using the House-Brackmann scale, which was published in 1985 as a means of describing acute otologic facial paralysis, although it also works well for documenting the severity of acute Bell palsy (see Table. House-Brackmann Facial Nerve Grading System). For patients with chronic facial movement disorders due to incompletely recovered Bell palsy, the use of a more detailed scale, such as the House-Brackmann 2.0, Sunnybrook, Yanagihara, or eFace, is appropriate and conveys additional useful information (see Staging section). These patients require a more detailed physical examination; after assessing resting symmetry and voluntary movement as described above, the location and degree of synkinesis should be evaluated. Doing so requires the same facial movements previously detailed, but the examiner directs attention to the regions of the face that are not requested to move. For example, with eye closure, the clinician will look for zygomaticus muscle and platysmal contraction, or with lip pucker, there may be involuntary winking (see Video. Post-Bell Palsy Synkinesis). Other common areas in which synkinesis is seen are the mentalis, depressor anguli oris, and frontalis muscles.
Table. House-Brackmann Facial Nerve Grading System
|
Grade I (Normal) |
Grade II (Mild dysfunction) |
Grade III (Mild-moderate dysfunction) |
Grade IV (Moderate dysfunction) |
Grade V (Severe dysfunction) |
Grade VI (Complete paralysis) |
|
| Resting Findings | Symmetric | Grossly symmetric | Grossly symmetric | Grossly symmetric |
Grossly asymmetric (ptosis of the brow, lower eyelid, oral commissure, effacement of nasolabial fold) |
Grossly asymmetric (often with a noticeable droop of the brow or oral commissure and effaced nasolabial fold, particularly in older patients) |
| Dynamic Findings | Symmetric |
Mild asymmetry Full eye closure with gentle effort |
Mild-moderate asymmetry Full eye closure only with full effort |
Moderate brow elevation and smile asymmetry Incomplete eye closure* |
Severe brow elevation and smile asymmetry Incomplete eye closure* |
No movement Incomplete eye closure*[32] |
*In the first 1 to 2 days after paralysis onset, eye closure on the affected side may remain complete due to gravity even if there is a severe facial nerve dysfunction. Lagophthalmos may present in a delayed fashion even if the rest of the face is paralyzed, and this preserved eye closure should not be considered in the overall House-Brackmann assessment.
Additional physical examination techniques may be employed to assess ocular health, such as slit-lamp evaluation with fluorescein to identify corneal defects and Schirmer testing to evaluate tear production. The Schirmer test involves placing a strip of filter paper in the fornix of the lower eyelid for 5 minutes and measuring how far the moisture has diffused at the end of that period; 10 mm or more is considered normal. Photographs, videos, and patient-reported quality-of-life instruments should also be completed in order to facilitate objective tracking of recovery.
The Sir Charles Bell Society of International Facial Paralysis Experts recommends obtaining the following photographs of every facial paralysis patient at every visit: in repose, elevation of eyebrows, gentle eye closure, tight eye closure, wrinkling the nose, small closed-mouth smile, large smile showing teeth, lip puckering, depressing the lower lip to show the bottom teeth, and a nasal base "worm's eye" view.[33] The same movements should be recorded on video as well. Lastly, an outcomes survey, such as the Facial Clinimetric Evaluation scale (FaCE), should be documented at each visit to track the effect of any interventions performed and monitor the patient's progress over time.[34]
Patients who present with incomplete paralysis less than 72 hours after palsy onset should have a follow-up visit within 1 week to determine whether the paralysis progresses to House-Brackmann grade VI and requires evaluation with electrodiagnostic testing (see Evaluation section). Patients should also be advised to follow up immediately if eye pain, photophobia, or a foreign body sensation develops, which can indicate a corneal abrasion and necessitate ophthalmological evaluation. Vesicles ipsilateral to the paralysis appearing on the ear, scalp, face, or in the mouth within a few days after initial presentation should trigger the diagnosis of Ramsay Hunt syndrome, which necessitates a higher dose of antivirals than Bell palsy and a prolonged steroid course.[35]
Evaluation
The history and physical examination determine the need for additional evaluation, which is not required in typical cases of Bell palsy. There is some evidence to suggest that a neutrophil-to-lymphocyte ratio higher than 3.53:1 is a reliable predictor of poor recovery, but this has not achieved widespread acceptance as a standard part of the evaluation of patients with Bell palsy, given the high spontaneous recovery rate and the cost of drawing blood.[36]
When a patient presents with House-Brackmann grade VI paralysis, some surgeons will offer facial nerve decompression under specific circumstances. While the outcomes of the operation are somewhat variable and the practice controversial, the most widely adopted candidacy criteria require electroneuronography (ENoG) and electromyography (EMG). ENoG is a form of evoked EMG in which a transcutaneous stimulator is placed over the stylomastoid foramen, and cutaneous electrodes on the face record the compound muscle action potentials that result from facial nerve stimulation. The muscles typically monitored are the orbicularis oculi and the zygomaticus major. The ENoG requires an unaffected side to act as a control for comparison with the affected side, and the test, therefore, works best when only 1 side of the face is weak, as is generally the case for Bell palsy. If the compound muscle action potential amplitude is reduced by 90% or more on the paralyzed side relative to the unaffected side, the ENoG is considered positive, and the EMG is then performed.[37]
The EMG following a positive ENoG is performed by placing percutaneous needle electrodes into the same muscles monitored with the ENoG and looking for motor units that contract when the patient attempts voluntary facial movement. If motor units are identified, the nerve injury is considered incomplete, but if no motor units are present, the patient meets the criteria for operative facial nerve decompression.[38] The procedure, depending on how it is performed, requires a craniotomy or, at the very least, a mastoidectomy, and many patients are either reluctant to proceed or are not good candidates due to medical comorbidities. For these patients, performing an ENoG can still be useful because of its prognostic value.[39] When treated medically, patients with House-Brackmann grade VI paralysis who meet decompression criteria are essentially guaranteed to develop synkinesis without surgery, whereas patients with House-Brackmann grade VI paralysis who do not meet decompression criteria have a roughly 60% chance of developing synkinesis.[2]
Laboratory investigations and imaging are more commonly performed in patients whose diagnosis of Bell palsy is questionable due to the presence of skin or mucosal lesions, other neurological symptoms, insidious paralysis onset, or repeated episodes of paralysis. Magnetic resonance imaging (MRI) can rule out space-occupying lesions, such as meningiomas or schwannomas, and contrast-enhanced computed tomography effectively assesses for geniculate ganglion vascular malformations, otologic pathology, and extratemporal masses.[40] Enhancement in the labyrinthine segment of the facial nerve, the cerebellopontine angle, the internal auditory canal, or the parotid gland is abnormal, while enhancement when limited to the geniculate ganglion and the tympanic and mastoid segments of the nerve is mostly likely normal "physiological" enhancement.[40][41] More recently, there has been increasing interest in using ultrasound to identify the location and severity of edema within the facial nerve, thereby providing prognostic information.[42] For patients with a history of travel to an endemic area, Lyme titers may be drawn, and numerous other conditions can cause facial paralysis as well, including human immunodeficiency virus infection, syphilis, West Nile virus, tuberculosis, leprosy, COVID, polio, and myriad autoimmune diseases.[1][43]
Historically, the maximal stimulation test (MST) and the nerve excitability test (NET) have been used to assess facial nerve function, but both have been largely supplanted by ENoG, which, despite the variability of its results, remains more objective and consistent than either the MST or NET. The NET involves stimulation of the face with an increasing electrical current and determining the difference between the lowest current amplitudes that produce visible contraction on each side. The MST is similar in that it requires a cooperative patient and the examiner's subjective evaluation of movement, but it involves stimulating the unaffected side with increasing electrical current until no additional contraction is achieved and then recording how much contraction is produced on the affected side with the same stimulation.[44] Various other studies may also be employed to evaluate the autonomic and sensory functions of the facial nerve, including saliva flow, taste testing, and stapedial reflex testing.
Treatment / Management
Acute Bell Palsy
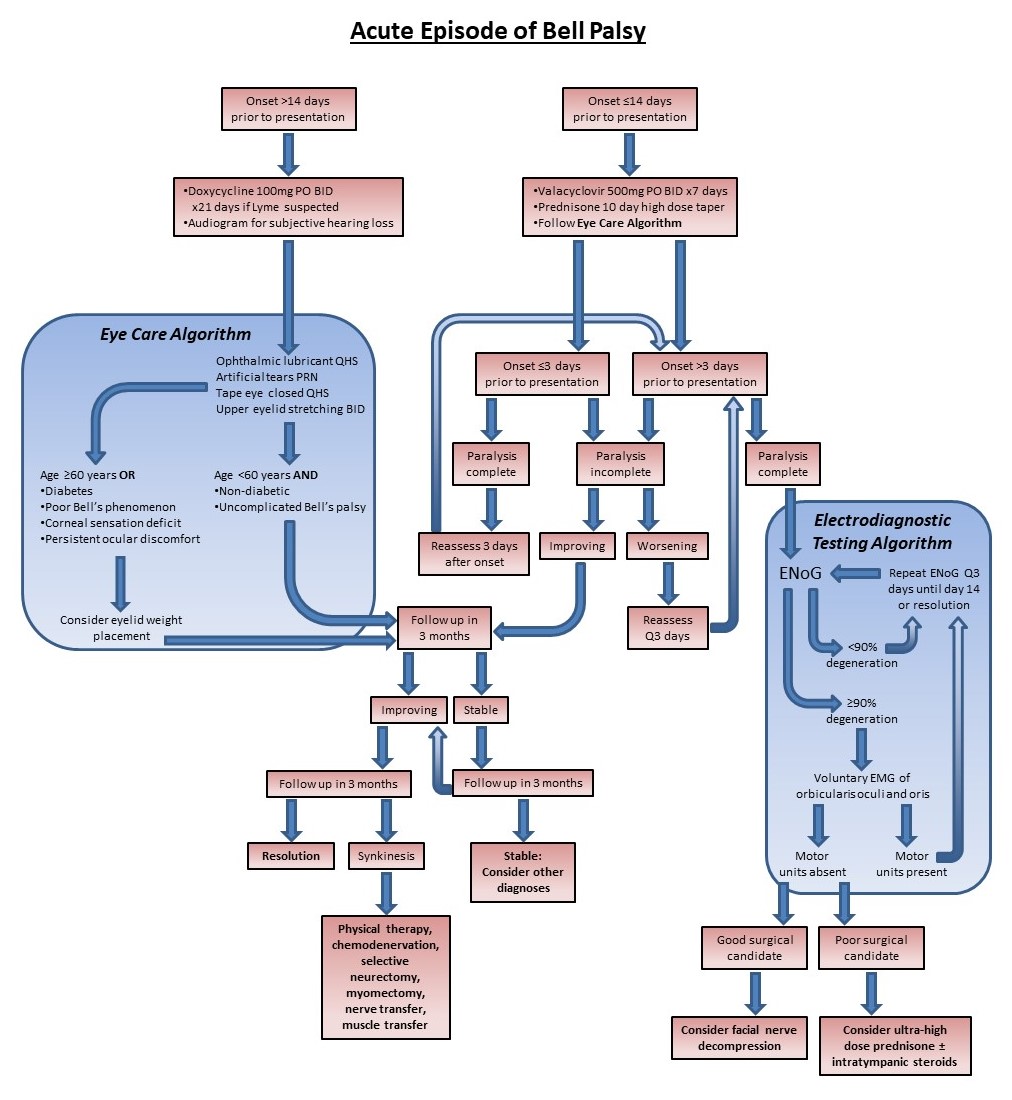
Once Bell palsy is diagnosed, management is very straightforward and follows a predictable algorithm (see Image. Management of Acute Bell Palsy). Steroids and antiviral medications are prescribed, with antihyperglycemic medications added or adjusted as necessary for patients with diabetes. Corneal protection is provided, and electrodiagnostic studies are performed if a House-Brackmann grade VI paralysis is present, with surgery offered if candidacy criteria are met. Patients at high risk for exposure keratopathy are given a scleral contact lens or undergo minor surgery, such as upper eyelid loading, tarsorrhaphy, or lateral tarsal strip canthopexy. Electrical stimulation has also been shown to improve outcomes when combined with medical management, expediting recovery and enhancing facial function.[45][46] Acupuncture, on the other hand, provides questionable benefits to patients with Bell palsy according to the poor quality studies conducted thus far; it does appear to be safe for those who wish to receive the treatment.[47][48](A1)
Medical management
Ideally, patients with acute Bell palsy should be seen as soon as possible and given corticosteroids within 72 hours of paralysis onset.[49] A common regimen is 1 mg/kg daily up to 60 mg of prednisone or an equivalent dose of prednisolone for 5 to 7 days with or without a taper. There is strong evidence that steroids alone improve Bell palsy outcomes, but there is some controversy regarding the addition of antivirals. A 2007 paper published by Sullivan et al reported that patients receiving prednisolone alone had a 94.4% chance of recovery to House-Brackmann grade I or II function by 9 months post-paralysis, but that the addition of acyclovir actually decreased the chance of a similar recovery to 92.7%. On the other hand, results from a study published that same year by Hato et al found that adding valacyclovir to a course of prednisolone improved the recovery rate to 96.5%.[50](A1)
In addition to steroids and antivirals, corneal protection during recovery is a critical intervention for patients without complete eye closure. Depending upon the severity, this may be an ocular lubricant at night only or periocular surgery. Most patients are well served with artificial tear drops to use throughout the day and lubricant ointment to use at night, with or without eyelid taping (see Video. Taping the Eyelid). Upper eyelid stretching 2 to 3 times per day is also very helpful for alleviating the unopposed contraction of the levator palpebrae superioris muscle that further hinders eye closure in the absence of a strong orbicularis oculi muscle (see Video. Stretching the Upper Eyelid). For patients who continue to have ocular discomfort despite these conservative measures or who are at high risk for exposure keratopathy (corneal anesthesia, absent Bell phenomenon, slow or prolonged recovery, only seeing eye), placement of a scleral contact lens or minor surgical procedures may be required to protect the cornea until a spontaneous eye blink is restored.
Periocular procedures
Due to the expense and time required to manufacture a custom scleral contact lens, periocular procedures are more often employed for patients with long-term flaccid facial paralysis due to nerve trauma or resection; scleral contact lenses can be very useful in cases of acute Bell palsy to prevent or treat exposure keratopathy.[51] Surgical options for improving eye closure include placement of a weight or a spring into the upper eyelid, tightening of the lower eyelid with medial or lateral canthopexy and tarsal strip, and tarsorrhaphy or tarsoconjunctival flap transfer (see Image. Interventions for Corneal Protection).(B3)
Of these options, the most common is upper eyelid loading, often performed with a gold or platinum weight. The weight can be placed onto the tarsal plate (pretarsal placement) via a supratarsal crease incision, as for blepharoplasty, or the weight can be located on the levator aponeurosis (postseptal placement), again via a supratarsal crease incision. The latter approach keeps the weight hidden behind the preaponeurotic fat so that its contour is not visible externally, but because the weight sits higher than the equator of the globe, it may paradoxically cause the eyelid to open when the patient is supine.[52] A pretarsal placement, approximately 2 mm superior to the lash line, provides a greater mechanical advantage and, therefore, employs a smaller weight, but the thin skin in this location usually renders the outline of the weight visible. In either case, the implant is typically centered over the medial limbus, which is the point of maximum lagophthalmos in most cases of facial paralysis (see Image. Pretarsal Upper Eyelid Weight Placement).[53]
The appropriate mass for the implant can be determined by trying a set of different adhesive weights on the patient's upper eyelid and identifying the one that provides the best eye closure while causing minimal blepharoptosis. For patients who are surgery-averse, the use of a skin color-matched adhesive weight is an alternative to surgical implantation, but the adhesive may be irritating to the thin skin of the upper eyelid, and weights often fall off and are lost due to their small size. For pretarsal placement, 1.2 g is a common size, while postseptal weights usually need to be slightly heavier. Heavier weights, particularly in a pretarsal location, can cause blepharoptosis even if complete eye closure is not achieved; patients should be informed of this lack of "Goldilocks" size preoperatively, ie, there is rarely an implant of just the right weight to produce complete eye closure without adverse effects.
Another common adverse event is astigmatism, which results from the mass pressing downward on the globe and distorting it. Fortunately, most astigmatism tends to correct itself spontaneously once the weight is removed. One way to minimize the astigmatism is to place an articulated chain rather than a solid weight, although doing so increases the risk of implant extrusion over time, and the thickness of a chain relative to that of a solid weight makes its contour more visible.[54] Material is also an important consideration, as, despite its inert characteristics, gold can still cause tissue reactions in 3.2% to 9.5% of the population; platinum is less reactive (<1%) and slightly denser, therefore possessing a thinner profile and potentially being less apparent under the skin.[53][55][56][57]
When lower eyelid laxity produces paralytic ectropion, loading the upper eyelid will not be sufficient to provide complete eye closure, and repositioning the lower eyelid will be required. This can be accomplished by tightening the eyelid at the medial canthus, the lateral canthus, or both, with the lateral tarsal strip procedure most commonly performed (see Image. Tarsal Strip-Lateral Canthopexy). Some degree of overcorrection is required with this technique, as the lower eyelid begins to loosen fairly quickly, and the overcorrected appearance will not last long. As a caveat, if the cornea protrudes substantially beyond the infraorbital rim, tightening the lower lid may cause a paradoxical retraction; therefore, this "negative vector" configuration must be identified preoperatively. When addressing paralytic lagophthalmos, some surgeons prefer to address the upper and lower eyelids simultaneously, and this can be accomplished by means of a tarsorrhaphy, either with a temporary suture or with a reversible surgical procedure that adheres the upper and lower eyelids together along the grey line (see Image. Interventions for Corneal Protection). In either case, the aesthetic implications of a tarsorrhaphy are significant, and many patients decline this option for that reason. A more cosmetically acceptable means of linking the upper and lower eyelids together, thereby decreasing the height of the palpebral fissure, is the tarsoconjunctival flap, which preserves the separation of the upper and lower lash lines and does not obstruct vision (see Image. Interventions for Corneal Protection).
Facial nerve decompression
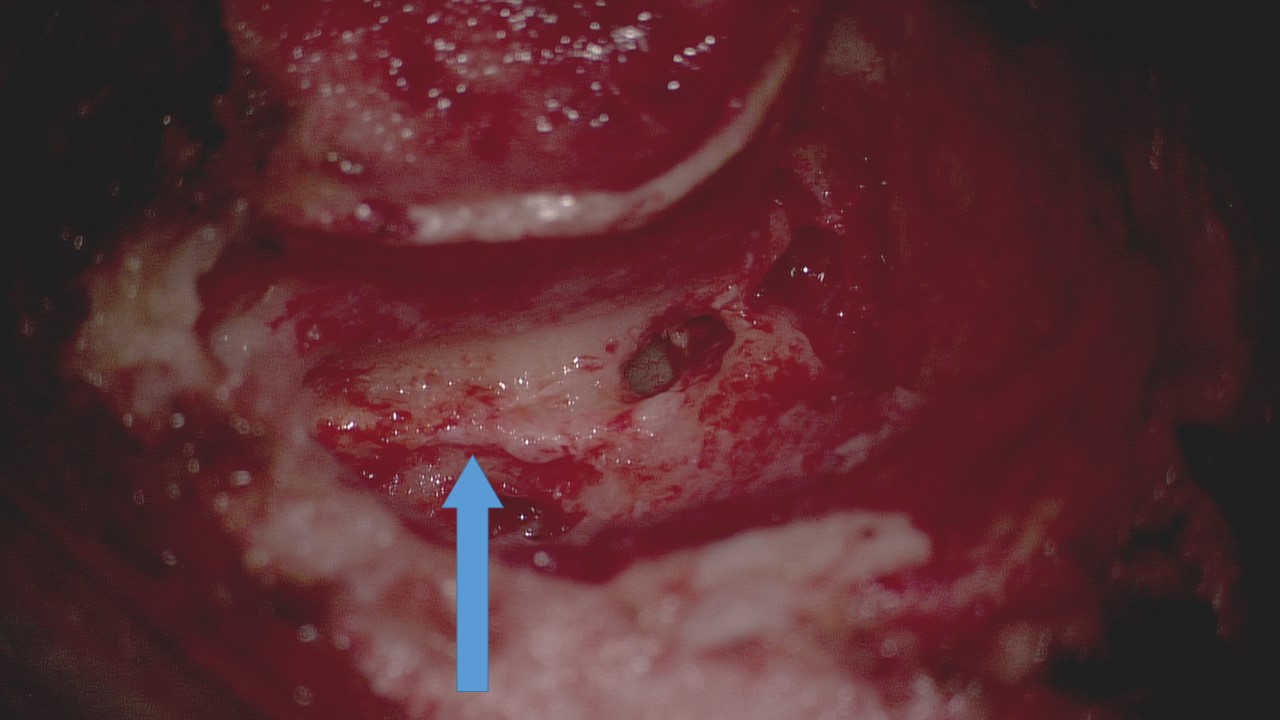
Surgery on the facial nerve itself, such as grafting or transfer, is not commonly performed in cases of acute Bell palsy; decompression of the facial nerve may be offered to patients who meet the electrodiagnostic criteria enumerated above and who are both willing and able to undergo the operation.[49] Decompression of the tympanic through the mastoid segments of the facial nerve may be accomplished via a postauricular mastoidectomy approach (see Image. Transmastoid Facial Nerve Decompression), although some surgeons do not consider this to be a thorough decompression because the neuritis in Bell palsy usually involves the labyrinthine segment, which may be best accessed via a middle fossa craniotomy. Accessing the labyrinthine segment of the facial nerve via a transmastoid approach may require removal of the incus and subsequent ossicular chain reconstruction in some cases, which can adversely impact postoperative hearing. Nevertheless, some authors do stand by the transmastoid technique because it spares the patient a craniotomy and intensive care unit admission; outcomes of this procedure are not consistently better than those after non-operative management, however.[58][59][60] (A1)
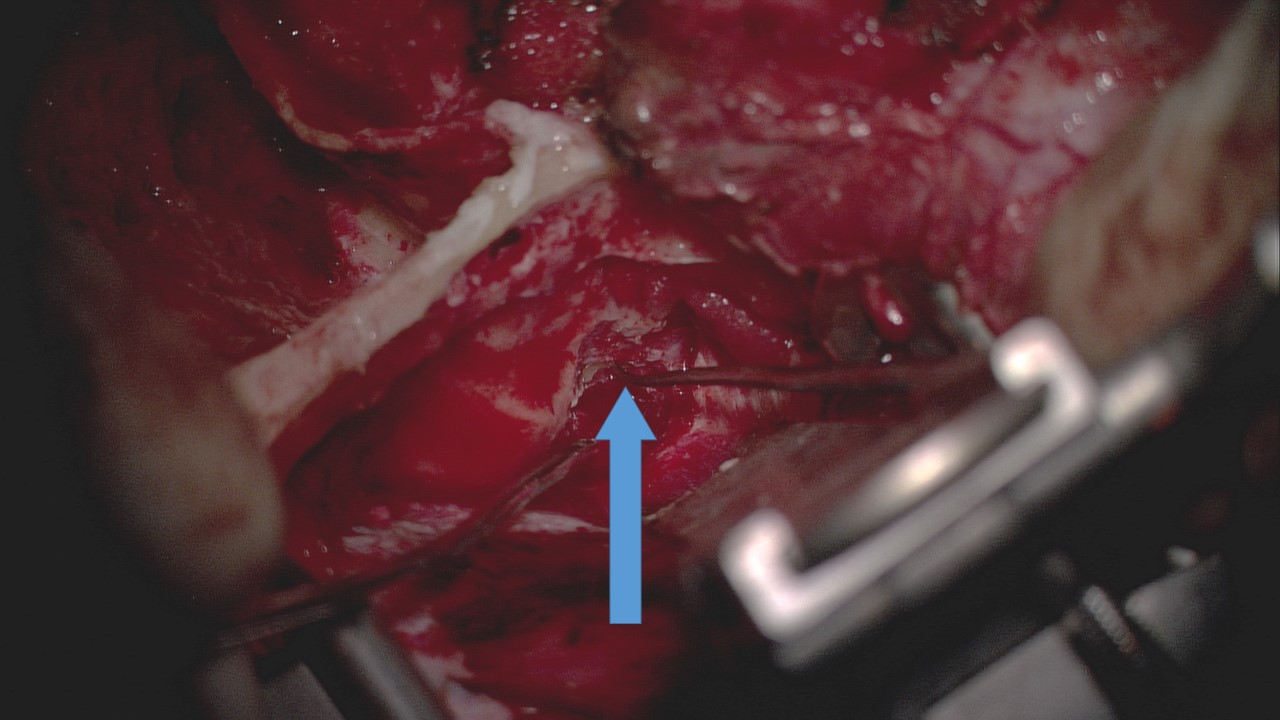
More recently, some surgeons have begun to employ a transcanal endoscopic approach to facial nerve decompression; this minimally invasive procedure provides somewhat limited access to the facial nerve, although the geniculate ganglion can be exposed effectively. Large studies with consistent resulting data are lacking with this technique, however.[61] To access the more proximal facial nerve, ie, the meatal foramen and canalicular portions, a middle fossa decompression approach is often employed or added to the transmastoid operation (see Image. Middle Fossa Craniotomy Approach to Facial Nerve Decompression).[62][63] Regardless of the approach selected, the earlier intervention appears to result in better outcomes.[38][59][60] Patients who meet criteria for facial nerve decompression but are not willing or able to undergo surgery may be offered alternative medical management, including ultra-high-dose oral corticosteroids (up to 200 mg of prednisone per day), intratympanic dexamethasone injections, and operative electrical stimulation to improve chances of satisfactory recovery.[64][65][66](A1)
Chronic facial nerve dysfunction
While every patient with true Bell palsy recovers facial motor function to some extent, many will develop synkinesis, particularly if presenting with severe or total hemifacial paralysis. The clinician is responsible for identifying these aberrant facial movements, as patients may not recognize the difference between the initial flaccid weakness and the hypertonicity that follows it, characterizing the chronic movement disorder as a continuation of the acute palsy rather than as a different, but related, clinical entity. Nevertheless, persistent facial asymmetry and mimetic dysfunction can have major adverse impacts on quality of life, causing patients to seek care. Physiotherapy and chemodenervation are sufficient for most individuals, although operative options do exist for patients who are dissatisfied with the results of conservative measures or who would prefer to avoid a prolonged course of regular botulinum toxin injections.
Physiotherapy
Facial rehabilitation therapy comprises a wide range of techniques, including neuromuscular reeducation, surface EMG biofeedback, mime therapy, video self-modeling, massage, stretching, and relaxation.[67][68][69] Neuromuscular reeducation (NMR) consists of exercises to produce small but controlled movements on the affected side of the face while concentrating on symmetry and avoiding synkinetic muscle contraction.[70] This can be performed with a mirror or EMG biofeedback that provides an audible tone when contraction is detected in the desired muscle, such as the zygomaticus major. Patients with more severe synkinesis may need to begin the course of therapy with relaxation, stretching, massage, and meditation before progressing to a point at which beginning NMR is even feasible. Exercises, such as NMR, require dozens of repetitions a few times a day to help build the neural pathways that will improve mimetic muscle coordination and symmetry of movement. These same techniques are also applied to patients who undergo nerve or muscle transfer for facial reanimation, as they need to learn to use the transferred nerve or muscle to produce the desired movement, typically biting down to smile.[71](B2)
Chemodenervation
The use of botulinum toxin injection to alleviate spasms and dyscoordinated or asymmetric movement due to facial palsy has been well-documented and found to be effective in both pediatric and adult populations.[72][73][74] There are currently several commercially available botulinum toxin preparations, but the most commonly used one is onabotulinumtoxinA, which is also the best studied, although any can be used effectively.[75] Several muscles typically become problematic with the development of synkinesis, and these are, therefore, most often the primary targets for chemodenervation. The orbicularis oculi is often injected to alleviate involuntary eye closure, particularly with mouth movement. Injections into this muscle are usually performed laterally, just deep to the skin, close to—but not medial to—the lateral orbital rim to prevent inadvertent weakening of the lateral rectus muscle and ensuing diplopia. A good starting dose is 2 aliquots of 5 units of onabotulinumtoxinA, or its equivalent in a different botulinum toxin preparation. When lower eyelid spasms persist despite lateral orbicularis oculi injections, small aliquots of 1 to 2 units may also be placed in the pretarsal portion of the inferior orbicularis oculi muscle (see Image. Injection of Botulinum Toxin for Facial Synkinesis). Care should be taken to avoid causing lower lid laxity that may result in lagophthalmos or blepharoptosis from medial injection of the upper eyelid that weakens the levator palpebrae superioris muscle. When injecting the orbicularis oculi muscle in addition to other facial muscles, it may be helpful to inject the orbicularis oculi first, while the needle is sharpest, because the thin skin in this region of the face is sensitive and prone to bruising. A fine, short needle on a small syringe is best for ensuring precise doses of toxin are administered to the desired targets; a 30- or 33-gauge needle is recommended on a 1 cc syringe. For onabotulinumtoxinA, diluting a 100-unit vial with 2 ccs of sterile, normal saline with preservatives provides 50 units in each 1 cc syringe, or 5 units per 0.1 cc, which makes keeping track of doses during injections comparatively straightforward. The preservatives in the saline improve comfort during injection, as they have a mild local anesthetic effect.(A1)
Other targets frequently injected include the platysma muscle, which, along with the depressor anguli oris muscle, can pull the oral commissure downwards when a patient is trying to smile, thus turning the expression into an unpleasant sneer. Twenty units, 4 injections of 5 units each, make a good starting point for treating the platysma. The mentalis, treated with 5 units, is also a common injection site because it tends to wrinkle with eye closure or brow elevation in patients with synkinesis (see Image. Botulinum Toxin Injection for Facial Synkinesis). Injecting the midface can be challenging, with some clinicians treating the zygomaticus major or upper lip elevators if they contract involuntarily or are hyperactive at rest and others electing to forego these injections because of the risk of causing an upper lip or oral commissure droop. Some clinicians prefer instead to treat the buccinator muscle to alleviate some midfacial tension as well as accidental cheek biting during mastication; this injection is typically performed via an intraoral approach.[76][77] While not strictly for synkinesis, injection of the depressor labii inferioris muscle on the unaffected side, also with 5 units, may improve smiling symmetry by correcting the crooked appearance of the lower lip caused by unilateral depression. Other less common targets include the posterior belly of the digastric muscle, which can cause pain when hypertonic, and the lacrimal gland, which helps to alleviate Bogorad syndrome.[78][79][80][81](B3)
Before injection of onabotulinumtoxinA, patients should be counseled that effects will not be appreciable immediately as it may take 3 to 5 days for any improvement to occur and 1 to 2 weeks to reach maximum effect. The duration of the improvement is 3 to 4 months, and patients, therefore, typically schedule injection appointments at that interval to avoid significant recurrence of synkinesis between treatments. These intervals are, however, somewhat variable among individual patients and may be shorter or longer if a different botulinum toxin preparation is used. Over time, typically several years, repeat injections of large doses of botulinum toxin may lead to neutralizing antibody development that generally manifests as a shorter duration of effect after an injection.[82] This can be addressed by increasing the doses up to a point or by switching to a different preparation of the toxin. Many patients are satisfied with the symptom improvement they achieve with chemodenervation with or without physiotherapy and do not proceed to surgical intervention. To maximize the effect of these nonsurgical interventions, however, it is imperative to start physiotherapy prior to botulinum toxin treatments so that patients have the opportunity to practice their facial exercises before the injections additionally impair muscle movement.
Myomectomy
Patients who have undergone botulinum toxin injections with good results but who desire a longer-term solution or who are losing the effectiveness of the injections due to antibody development may be candidates for myomectomy procedures, in which a segment of muscle is removed to reduce synkinetic contraction. Common targets include the platysma and the depressor anguli oris, in which diminished function will improve smile excursion, and the orbicularis oculi, whose myomectomy will reduce involuntary eye closure and squinting. A full-thickness horizontal strip of platysma muscle may be excised under local anesthesia through a 2 cm lateral transverse cervical incision, interrupting visible platysmal bands and the abnormal contraction that pulls down on the corner of the mouth.[83] The platysma is a large muscle, however, and over the course of several months, it will often restore its continuity, leading to the return of some of its synkinetic activity. Resection of a portion of the depressor anguli oris muscle, also to permit greater movement of the oral commissure during smiling, is performed via an intraoral approach. This procedure involves a 3 cm vertical incision made along the lateral margin of the orbicularis oris muscle, with dissection proceeding through the buccinator, a portion of which is also excised, before reaching the depressor anguli oris, the majority of which is then resected as a strip perpendicular to the vector of contraction.[84] Less commonly performed is resection of a strip of orbicularis oculi muscle from the lower eyelid, with or without its innervating branches of the facial nerve, via a skin-muscle flap lower blepharoplasty approach.[85]
Selective neurectomy
Like removing portions of abnormally-contracting muscles, resection of segments of nerve branches that innervate these muscles can supplement or replace long-term botulinum toxin injections, or be performed as an adjunct to myomectomy. As with myomectomy, selective neurectomy is often performed to limit involuntary eye closure or improve the symmetry of the smile, but unlike myomectomy, an extraparotid facial nerve dissection is required, and thus general anesthesia is necessary (see Image. Selective Neurectomy for Facial Synkinesis). A 2-step highly selective neurectomy is a technique that identifies zygomatic branches of the facial nerve that innervate the orbicularis oculi muscle, employing a facial nerve exploration under general anesthesia. Loops of the identified branches are then externalized via percutaneous stab incisions for resection after the patient has emerged from anesthesia, thus permitting precise titration of the number of neurectomies required to balance reduction of involuntary eyelid contraction against creating lagophthalmos.[86] (B2)
Patients report early symptomatic improvement with this technique, but nerve branches to the orbicularis oculi muscle tend to regenerate after a few months, thereby negating the effects of the surgery.[87] In the lower face, however, longer-term results appear more achievable with modified selective neurectomy of the branches that control the lower facial depressor muscles, as described by Azzizadeh et al.[88] When performing selective neurectomy, it is important to maintain excellent communication between the surgeon and the anesthesia provider, as identification of nerve branches requires not only that the patient remain still, but also that the patient remain still in the absence of paralytic drugs, the use of which would prevent electronic nerve stimulators from facilitating nerve branch identification via muscle contraction. Additionally, it may be prudent to inject the surgical field preoperatively with epinephrine only, rather than lidocaine and epinephrine, as local anesthetic medications occasionally diffuse deeply enough to block action potential conduction of the motor nerves in question and, therefore, prevent their identification with an electronic stimulator. Selective neurectomy may be combined with myomectomy to maximize relief of synkinesis and may also be combined with nerve or muscle transfer.[89]
Nerve transfer
While historically employed for restoring function in cases of flaccid facial paralysis, typically either traumatic or iatrogenic, nerve transfer may also be used to improve coordination of a particular muscle or muscle group by "disconnecting" it from the synkinetic control of the facial nerve and reinnervating it with a different motor nerve, such as the hypoglossal or masseteric nerve (the latter being a branch of the mandibular division of the trigeminal nerve). For reanimation of the flaccid face, nerve transfer is ideally performed within the first 6 to 12 months after the onset of paralysis to ensure that viable muscle remains to reinnervate, as irreversible muscle atrophy and fibrosis occur with prolonged denervation. In the case of synkinesis, however, the muscles are not denervated but rather aberrantly reinnervated, and therefore, there is no time constraint for surgical intervention.
Nerve transfer for smile rehabilitation most often involves rerouting the nerve to the masseter into the facial nerve's buccal branch that controls the zygomaticus major muscle.[90] This procedure may be performed independently or in combination with selective neurectomy or myomectomy.[89] The procedure is performed via a modified Blair incision, as one might use for a parotidectomy or facelift, and as with selective neurectomy, long-acting paralytics and preoperative injection of lidocaine should be avoided (see Image. Masseteric Nerve Transfer). The masseteric nerve is reliably located at a point 3 cm anterior to the tragus, 1 cm inferior to the zygomatic arch, and 1.5 cm deep to the parotidomasseteric fascia.[91][92] Once exposed, it is transected as distally (inferiorly) as possible, just proximal to its branch point, to provide as much length as possible to reflect the cut end of the proximal segment out of the masseter muscle to facilitate the neurorrhaphy; proximal circumferential dissection of the nerve up to the zygomatic arch will further improve its mobility.(B2)
The buccal branch that innervates the zygomaticus major muscle is consistently found at Zuker point, located halfway along a line between the root of the helix and the oral commissure.[93] This buccal branch courses deep to the parotid fascia but is superficial and just superior to the masseteric nerve, in the same plane as the parotid duct and the transverse facial vessels, which are located inferior to it. The buccal branch is transected as proximally as possible without including branches to other muscles. The use of a nerve stimulator will verify the correct receiving buccal branch for the nerve transfer. Provided the masseteric nerve is mobilized adequately, it should reach the distal segment of the buccal branch without tension. Both nerves are roughly 2 mm in diameter. The neurorrhaphy is generally performed under an operating microscope, using 9-0 or 10-0 interrupted nylon sutures on a cutting or spatula-tip needle. Several weeks to months may pass before seeing any improvement in the patient's smile, and the patient may not notice anything until the surgeon points out the movement. Initially, contraction of the zygomaticus major muscle will require intentional biting to trigger an action potential in the masseteric nerve, but over time, most patients can smile without the need to clench the jaw if they comply with the postoperative physiotherapy regimen.[94] Significant improvements in smile symmetry and oral commissure excursion (approximately 5 mm) are expected with a successful nerve transfer.[89][90]
Muscle transfer
For patients in whom nerve transfer has failed or who desire greater smile excursion than is anticipated with nerve transfer, muscle transfer techniques, such as those used for smile reanimation in a flaccidly paralyzed face, may be employed.[95] Of these, the best studied is the gracilis free muscle transfer, which takes a portion of the gracilis muscle from the medial thigh and transfers it into the face between the modiolus of the oral commissure and the temporalis fascia. This location and vector allow the gracilis to assume the function of the zygomaticus major muscle, thus permitting the patient to produce a close-lipped smile (see Image. Gracilis Free Flap for Facial Reanimation).
The adductor artery and vena comitans in the gracilis' vascular pedicle are typically anastomosed to the facial artery and vein, while the obturator nerve to the gracilis is usually coapted to the masseteric nerve, although a cross-face nerve graft may be used instead of the masseteric nerve or added via an end-to-side neurorrhaphy.[96] The monitoring of flap perfusion may be achieved with an implantable Doppler sensor, duplex ultrasound the day after surgery, or a handheld Doppler probe.[97] As with a masseteric nerve transfer, postoperative physiotherapy is critical, and the patient will initially have to bite down to smile, although this movement may not be required in the long term.(B2)
More recently, the use of dual-vector cervical strap muscle-free flaps has been described for smile restoration and, like the gracilis, has been applied to the reanimation of the flaccid face and smile rehabilitation in the context of synkinesis.[98] The advantage of the sterno-omohyoid flap over the gracilis is that it introduces less bulk into the face, thereby minimizing postoperative volume asymmetry, and it also provides 2 contractile muscle bellies that can be used to produce a smile with the dental show, replacing both the zygomaticus major and the levator labii superioris muscles' functions (see Image. Sterno-Omohyoid Flap for Facial Reanimation). This flap also relies upon the facial vessels for blood supply, although the flap's vessels, the superior thyroid artery and vein, are less reliable than the gracilis' adductor vessels, making the strap muscle flap potentially more challenging. The ansa cervicalis provides the innervation for the strap flap, and because there are 2 ends to this loop of nerve, both the masseteric nerve and a cross-face nerve graft may be coapted simultaneously in an end-to-end fashion.[99] As with nerve transfers, muscle transfer may be combined with myomectomy and selective neurectomy.(B2)
Differential Diagnosis
The differential diagnosis for acute hemifacial palsy is broad and includes infectious, neoplastic, vascular, traumatic, toxic, and metabolic etiologies.[1] In most cases, however, a patient presenting with rapid-onset, hemifacial weakness without other neurological or dermatological signs or symptoms and without a history of trauma, chemical exposure, or severe metabolic derangement will be experiencing Bell palsy.
The most common condition that may be mistaken for Bell palsy is Ramsay Hunt syndrome, particularly when it presents as zoster sine herpete, ie, hemifacial palsy without a rash but still with significant pain.[100] Lyme disease is another fairly common cause of acute facial paralysis, although it can usually be identified by the pathognomonic targetoid rash, a history of tick bite or travel to an endemic area, and potentially bilateral facial weakness. Other viral infections, such as Epstein-Barr virus, cytomegalovirus, and human immunodeficiency virus, may also present with unilateral facial paralysis but often also cause vague systemic symptoms, such as fevers, fatigue, and malaise. Tuberculosis and COVID may also cause facial paralysis, but typically with accompanying respiratory symptoms. Neoplasms, such as facial nerve schwannoma and parotid gland mucoepidermoid carcinoma, as well as autoimmune disorders, such as Sjögren syndrome, are more likely to cause an insidious onset of facial paralysis but may nevertheless present acutely.[101][102]
Cerebrovascular accident is often considered high on the differential diagnosis when a patient presents with Bell palsy, leading to unnecessary imaging. A patient with acute onset hemifacial weakness in the absence of other neurological symptoms or vital sign instability is unlikely to be experiencing cerebral ischemia, particularly if the forehead is paralyzed. A brainstem stroke, unlike a cortical stroke, will cause hemifacial weakness that includes the forehead but will typically also cause other cranial neuropathies as well as vertigo, nausea, and vomiting. Recurrent episodes of hemifacial paralysis may represent Bell palsy, which recurs in 8% to 12% of affected patients. Still, patients with more than 2 episodes without other risk factors should be evaluated further with imaging and laboratory studies, as facial nerve schwannomas and autoimmune diseases, such as Melkersson-Rosenthal syndrome, may present this way.[1]
Sometimes, risk factors may not be apparent, even with a detailed history and physical examination. For example, a patient may deny a history of head trauma but fail to mention self-contained underwater breathing apparatus (scuba) diving or air travel, either of which can result in otic barotrauma and subsequent facial paralysis.[103][104] Similarly, a patient with a distant cancer may suffer facial paralysis as a result of brain metastasis or chemotherapy. Misadventures with botulinum toxin injections can cause facial paralysis as well, which some patients may be reluctant to disclose. Last, otologic disease should not be overlooked, whether in the context of acute otitis media or a chronic cholesteatoma.
Staging
The original House-Brackmann scale is simple and easy to use for surgeons, emergency medicine providers, and primary care clinicians, but it provides very little detail, particularly concerning the function of individual zones of the face or synkinetic movement. While it is a sound staging system for acute Bell palsy, there are a number of more detailed classification systems that are better suited to describing the chronic facial movement dysfunction that may follow Bell palsy or other forms of facial paralysis that may affect different zones of the face differently, such as facial nerve trauma, iatrogenic injury, congenital anomalies, poliomyelitis, and multiple sclerosis.
The first facial paralysis grading scheme published was that of Naoaki Yanagihara in 1976, requiring patients to perform 9 facial movements and a resting evaluation. The strength of each is graded as 4 points (normal or nearly normal), 2 points (weak), or 0 points (no movement).[105] Thus, a score of 40 is normal, and a score of 0 corresponds to a House-Brackmann grade of VI. The 9 movements are eyebrow elevation, gentle eye closure, forceful eye closure, closure of the affected eye only, wrinkling the nose, puffing out the cheek, whistling, smiling, and depressing the lower lip. Scoring Bell palsy with this system requires a thorough facial nerve examination. This scale is still commonly used in Asia.
In 1996, Ross and her colleagues published the Sunnybrook Facial Grading System, named for the Sunnybrook Health Science Center in Toronto, Canada.[106] This scale goes 1 step beyond that of Yanagihara, evaluating resting asymmetry, voluntary movement, and synkinetic movement separately for each facial zone, thus providing a very detailed assessment of a patient's facial function, but at the expense of the rapidity and convenience of the House-Brackmann scale. For resting asymmetry, the Sunnybrook system individually scores the palpebral fissure height, nasolabial fold depth, and oral commissure position, with a maximum of 15 points for asymmetry across all 3 zones. For voluntary movement, eyebrow elevation, gentle eye closure, open mouth smile, snarl, and lip pucker are evaluated, with 100 points indicating normal function. Synkinetic movement is evaluated with the same facial expressions, with 15 points for severe synkinesis in all zones. The resting asymmetry and synkinesis scores are subtracted from the voluntary movement score to determine the composite Sunnybrook score.
An update to the House-Brackmann Facial Nerve Grading System with the "Facial Nerve Grading System 2.0" was devised by Vrabec et al in 2009.[107] This scale, like the Sunnybrook system, assesses the eyebrow, the eye, the nasolabial fold, and the oral commissure separately, assigning a score between 1 (normal symmetry) and 6 (no movement) for each region's function that takes into account both movement and resting symmetry. Up to 3 more points can be added to the total for disfiguring synkinesis, with 2 points indicating obvious synkinesis and 1 point signifying slight synkinesis. Because no synkinesis points can be scored when there is no movement, the maximum score is 24 points, which equates to a House-Brackmann grade VI on the original scale.
More recently, technology has been leveraged to improve the granularity and the convenience of grading facial functions. Massachusetts Eye & Ear Infirmary's eFACE application was released in 2015 and validated by a group of international facial nerve experts in 2017.[108][109] This app-based approach to evaluating facial paralysis uses multiple Likert scales to assess resting symmetry, voluntary movement, and synkinetic movement. Then, it provides a set of scores that reproducibly quantify function in terms of static symmetry, dynamic symmetry, and synkinesis, as well as regional function for the periocular area, lower face and neck, midface and smile, and smile alone (see Image. The eFACE Software Application). Further development of the software grading concept resulted in the auto-eFACE, which analyzes a set of standardized photographs to evaluate the severity of facial dysfunction using the eFACE scale.[110]
Numerous other facial nerve grading scales, such as the FAME, MoReSS, Sydney, and Toronto systems, have been developed and compared with the original House-Brackmann Facial Nerve Grading System, with many of them providing good interrater reliability and correlation with the House-Brackmann scale.[111][112][113][114][115] However, the Sir Charles Bell Society recommends using the Sunnybrook scale because of its high reliability and ability to evaluate resting asymmetry, flaccid palsy, and synkinesis.[116]
Prognosis
Bell palsy resolves completely without treatment in approximately 80% of cases, with the remaining patients developing synkinesis to some degree.[6] Oral corticosteroids with or without antivirals increase the chance of recovery from 90% to 97%.[2][7] Recurrence does occur in 8% to 12% of affected individuals, with a mean latency of 10 years between episodes.[1][17][117] Cases of multiple recurrent Bell palsy may be due to narrow Fallopian canals that exacerbate vascular insufficiency caused by nerve inflammation and edema within the enclosed space of the canal.
Risk factors associated with the development of synkinesis include complete paralysis, age 60 years or older, and decreased salivation or taste on the ipsilateral side. While some patients are typically young and healthy with incomplete palsy and may exhibit complete recovery in as few as 2 weeks, most patients will take several months to a year to recover. The longer it takes for clinical recovery to begin and the slower the recovery once it does, the more likely synkinesis will occur. Similarly, patients who present with more severe paralysis are more likely to have worse outcomes. Escalante et al reported that House-Brackmann grade V paralysis evolves into synkinesis in 17% of patients and House-Brackmann grade VI paralysis does so in 40% of patients; conversely, no patients with a severity of House-Brackmann grade II developed synkinesis in their series.[2] Other factors that adversely affect outcomes include diabetes, pregnancy, history of a recent dental procedure, progression to complete paralysis within 24 hours of onset, and loss of more than 90% of the compound muscle action potential amplitude on electroneuronography, ENoG.[3][20][21][38][39] Debate remains regarding the impact of hypertension on recovery from Bell palsy, with some studies reporting worse outcomes and others no difference between hypertensive and non-hypertensive patients.[19][118][119] Regardless of risk factors, the mean time to development of synkinesis is roughly 5 months for patients who fail to recover completely.[2]
Complications
Despite its high spontaneous recovery rate, Bell palsy may cause synkinesis in approximately 15% of patients and corneal exposure keratopathy in about 40% of patients.[2][120] Beyond physical sequelae, psychological distress and quality-of-life decrements are known to occur with facial paralysis, particularly for women, older patients, and in cases of long-standing paralysis.[121] Fortunately, keratopathy and decreased quality of life tend to resolve as facial function recovers.
Postoperative and Rehabilitation Care
Physiotherapy remains a critical element of the treatment of severe Bell palsy, whether as an aspect of nonoperative management in conjunction with botulinum toxin injection or as part of the postoperative recovery regimen. Facial rehabilitation therapy, provided by physical therapists, occupational therapists, or speech-language pathologists, may involve stretching, massages, and exercises performed in the mirror to improve facial muscle coordination. In some cases, transcutaneous electrical stimulation is also employed. Postoperatively, therapy most often centers on helping the patient use a transferred nerve, with or without a transferred muscle, to produce a smile on the paralyzed side and to integrate it with the smile on the unaffected side to create a symmetric expression.
Consultations
Most cases of acute Bell palsy are managed successfully by emergency medicine or primary care professionals, who can prescribe the appropriate medications and educate patients regarding corneal protection. Patients who have House-Brackmann grade VI paralysis should be seen as soon as possible by a neurologist for electrodiagnostic testing and then referred to an otologist for consideration for facial nerve decompression, if appropriate. If an intratympanic steroid injection is planned, an otologist or otolaryngologist will be required. If conservative corneal protection interventions are inadequate, an ophthalmologist, plastic surgeon, or facial plastic surgeon may be needed to place an eyelid weight or perform another periocular procedure. In some cases, an adjustment disorder may develop, particularly in young women or patients with a history of depression; mental health evaluation may be appropriate for these patients.
For patients with chronic facial dysfunction after Bell palsy, facial rehabilitation can be performed by a physical therapist, occupational therapist, or speech-language pathologist. A plastic surgeon, neurologist, or facial plastic surgeon may offer botulinum toxin injections for synkinesis, or the injections may be performed by a trained injection nurse, nurse practitioner, or physician assistant in 1 of those specialties. Plastic or facial plastic surgeons typically perform surgical interventions, including myomectomy, neurectomy, nerve transfer, and muscle transfer.
Deterrence and Patient Education
The most critical intervention for optimizing facial functional outcomes in Bell palsy is the prescription of oral corticosteroids, although patients should be strongly encouraged to take antiviral medications as well. That said, most patients will recover completely or nearly completely from Bell palsy with or without medical management, so the argument can be made that the most important intervention in cases of acute Bell palsy is the protection of the cornea with eyedrops, ointment, taping, and eyelid stretching exercises. Patients should be counseled strongly that inadequate eye care can result in long-term ocular sequelae despite the temporary nature of the Bell palsy itself. Beyond that, while there are no known effective strategies for preventing the occurrence or recurrence of Bell palsy, maintaining tight glucose control for diabetic patients may improve outcomes or even decrease the risk of developing the condition.
Enhancing Healthcare Team Outcomes
Due to its effects on emotional expression, oral competence, articulation, ocular health, and quality of life, Bell palsy can have myriad consequences for severely affected patients. Clinicians must be skilled in accurately diagnosing and managing Bell palsy, including recognizing early signs, applying corticosteroid and antiviral treatments, and managing chronic complications like synkinesis. Physicians, advanced practitioners, and nurses should be adept at monitoring patient progress and coordinating care with other specialties. Pharmacists play a crucial role in ensuring safe medication management and patient education. While an otolaryngologist or neurologist is typically tasked with managing the patient overall if the paralysis is severe or complicated, an interprofessional team of healthcare providers should be engaged to optimize outcomes for patients with Bell palsy.[122][123]
Patients with House-Brackmann grade VI paralysis have the greatest need for an effective interprofessional team because they require prompt referral for electrodiagnostic testing and may require major surgery shortly after diagnosis. These patients are also at greatest risk for developing synkinesis and are, therefore, most likely to require facial rehabilitation therapy. Likewise, patients with House-Brackmann grade VI paralysis typically take the longest to recover and are most likely to require intervention to prevent corneal injury. Having a team assembled whose members effectively communicate and collaborate will expedite and streamline care for patients with Bell palsy, ensuring timely and appropriate intervention while minimizing complications.[124][125]
Media
(Click Image to Enlarge)
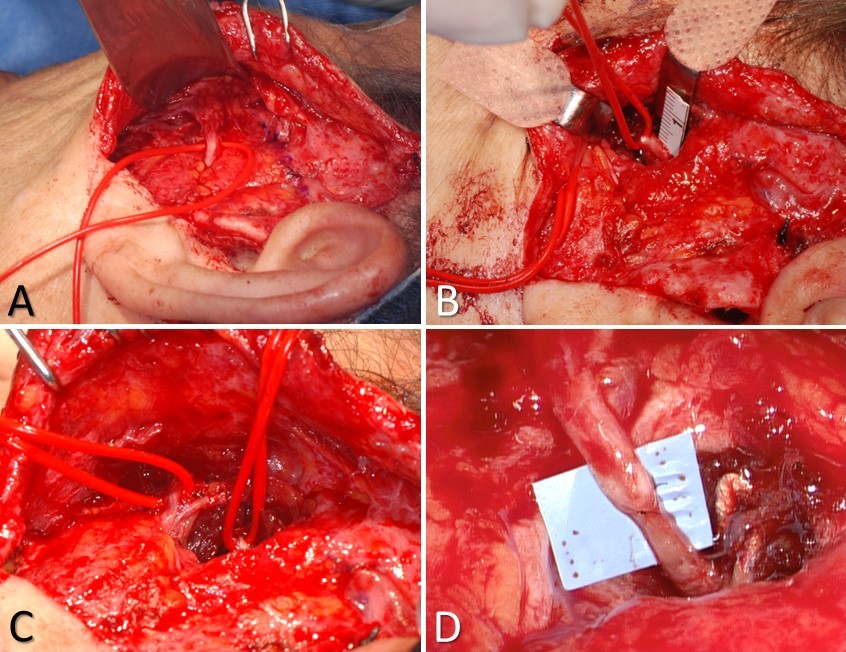
Masseteric Nerve Transfer. A) the buccal branch controlling the zygomaticus major muscle is identified at Zuker's point (halfway between the helical root and the oral commissure), B) the masseteric nerve is identified 3 cm anterior to the tragus, 1 cm inferior to the zygomatic arch, and 1.5 cm deep to the masseteric fascia; C) the 2 nerves are typically located within ~1.5 cm of each other, which obviates the need for an interposition graft; D) the completed nerve transfer, using 10-0 nylon epineurial sutures to coapt the proximal stump of the masseteric nerve to the distal stump of the buccal branch.
Contributed by MH Hohman, MD, FACS
(Click Image to Enlarge)
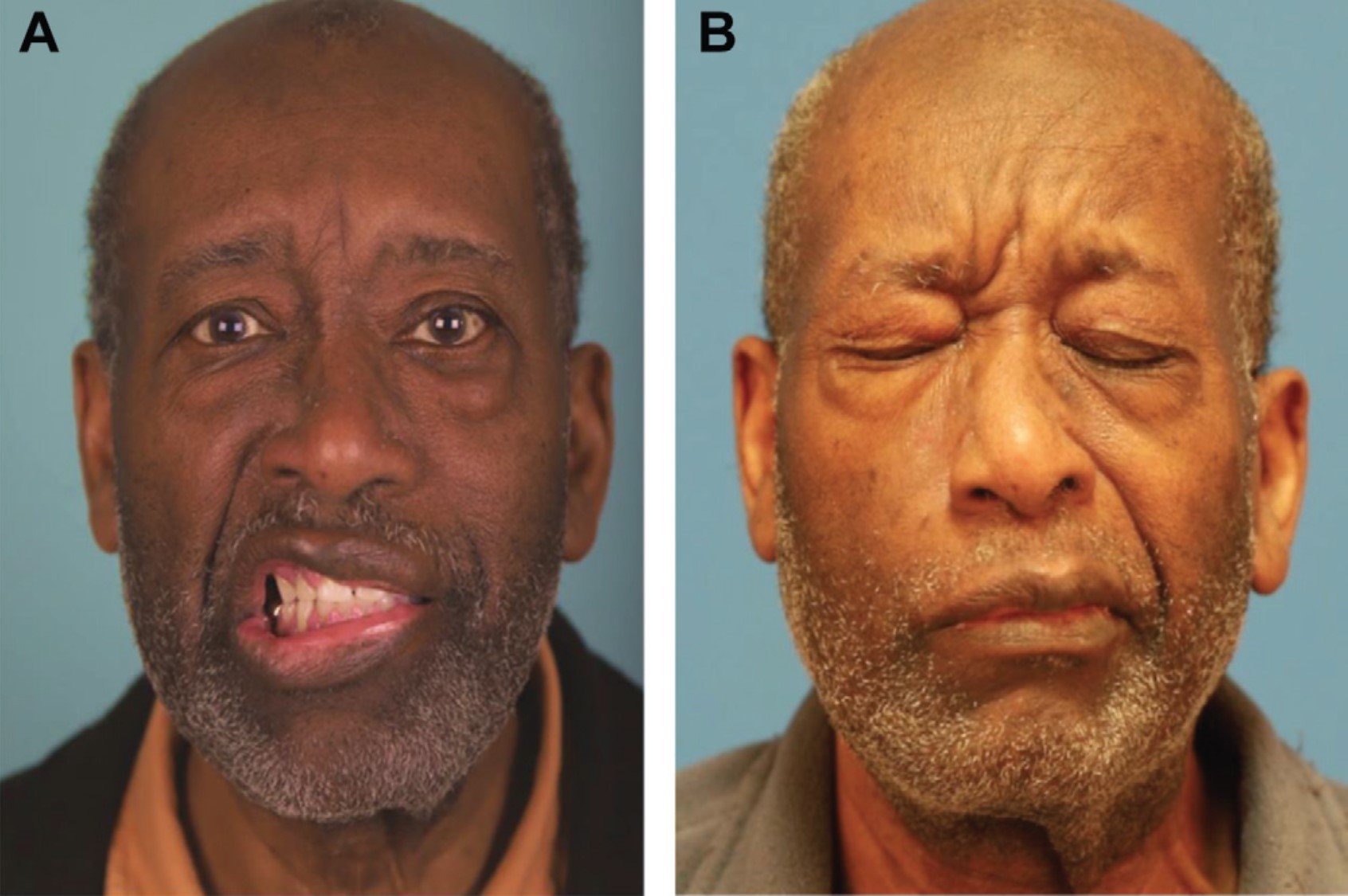
Bell Palsy. A) This patient presented with House-Brackmann grade VI left-sided facial paralysis that met the criteria for facial nerve decompression. B) A 3-year follow-up photograph demonstrating midfacial and platysmal synkinesis with eye closure on the left side, despite facial nerve decompression.
Contributed by MH Hohman, MD, FACS
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
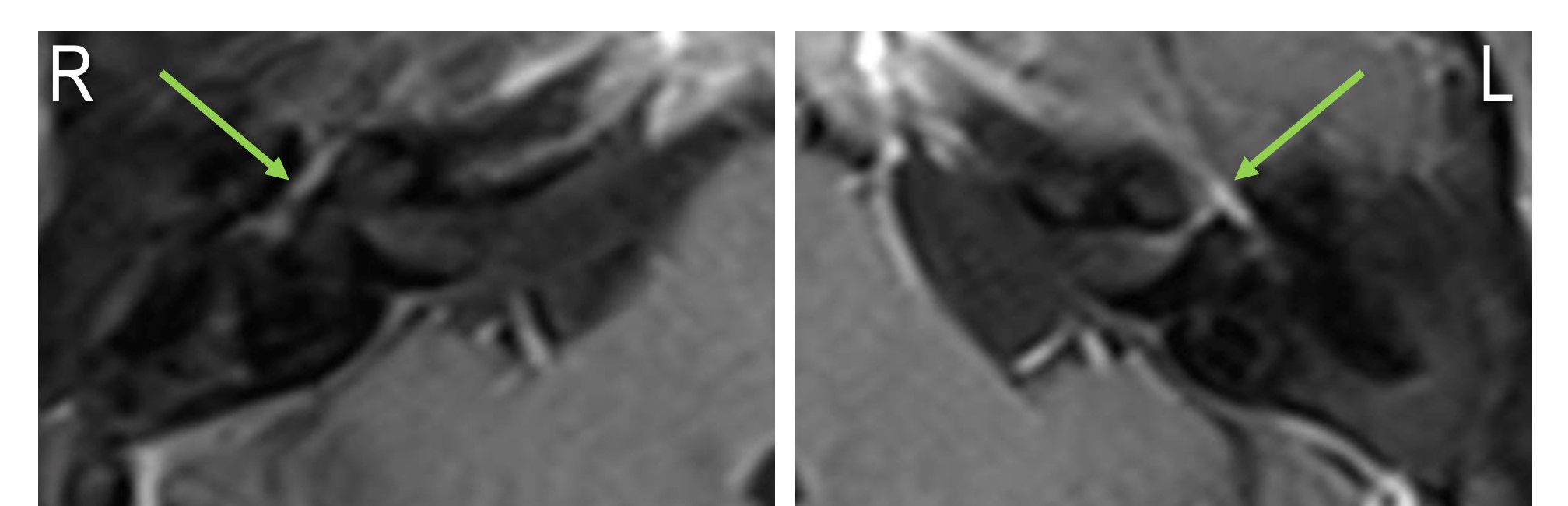
Left-Sided Bell Palsy, Magnetic Resonance Image. On the patient's right side, there is normal physiological enhancement of the facial nerve due to its epineurial and perineurial blood vessels. Normal enhancement may be seen in the geniculate ganglion, tympanic segment, and mastoid segment of the facial nerve. On the patient's left side, the enhancement is stronger than on the right and extends into the labyrinthine segment and the fundus of the internal auditory canal. Enhancement of the facial nerve in the labyrinthine segment, cerebellopontine angle, internal auditory canal, or parotid gland is abnormal.
Contributed by J Costello, DO
(Click Image to Enlarge)
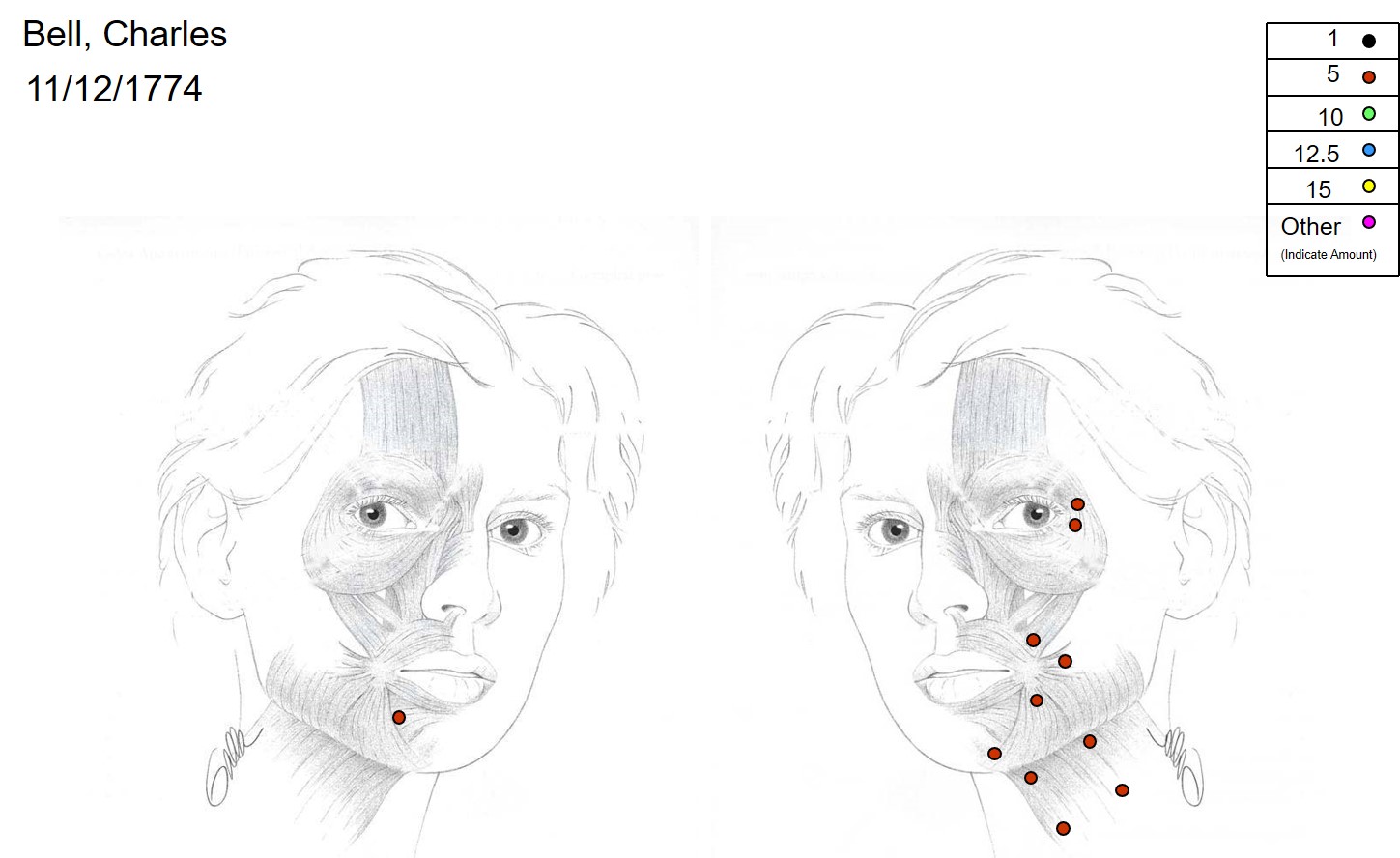
Botulinum Toxin injection for Facial Synkinesis. Common targets include the orbicularis oculi, zygomaticus major, buccinator, depressor anguli oris, mentalis, platysma, and the contralateral depressor labii inferioris, which helps reduce lower lip crookedness during smiling.
Contributed by TA Hadlock, MD; the Sir Charles Bell Society
(Click Image to Enlarge)
Selective Neurectomy for Facial Synkinesis. The procedure is performed through either a parotidectomy or a facelift incision. The individual facial nerve branches are dissected, and their functions are determined with a nerve stimulator. One- to 2-cm segments are cut out of the ones that innervate the hypertonic muscles, and the cut ends are clipped or sutured into collagen nerve caps to help prevent regrowth. Selective neurectomy can be combined with myomectomies and nerve transfer to optimize outcomes.
Contributed by MH Hohman, MD, FACS
(Click Video to Play)
Taping the Eyelid. Taping the eyelid closed at night helps prevent exposure keratopathy and corneal abrasion from paralytic lagophthalmos. In some cases, taping during the day may also be beneficial.
Contributed by MH Hohman, MD, FACS
(Click Video to Play)
Stretching the Upper Eyelid. Stretching the upper eyelid for 60 seconds 2-3 times daily can enhance gravity-assisted eye closure and help prevent exposure keratopathy in patients with facial paralysis.
Contributed by MH Hohman, MD FACS
(Click Image to Enlarge)
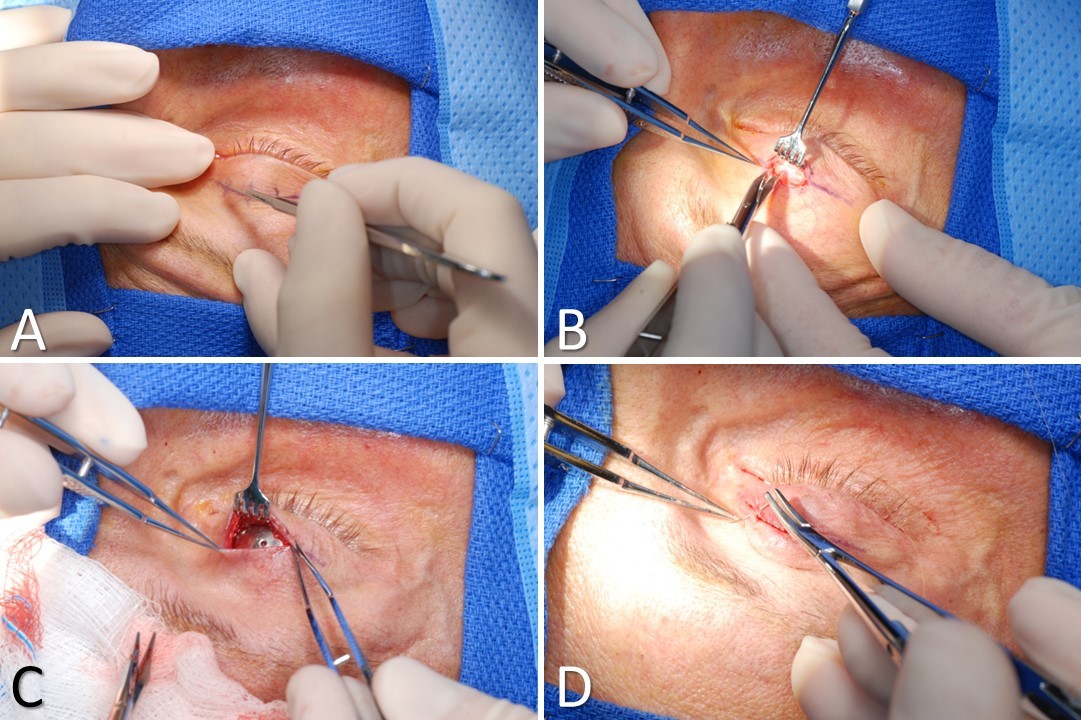
Pretarsal Upper Eyelid Weight Placement. A) The incision is made in the supratarsal crease. B) Dissection is performed through the orbicularis oculi muscle until the tarsal plate is identified. C) The weight is placed into a tight pocket parallel to and 2 mm superior to the inferior edge of the tarsal plate. Hemostasis is achieved, and the weight is sutured in place with 6-0 polypropylene. D) The wound is closed in layers, approximating the muscle and then the skin.
Contributed by MH Hohman, MD, FACS
(Click Image to Enlarge)
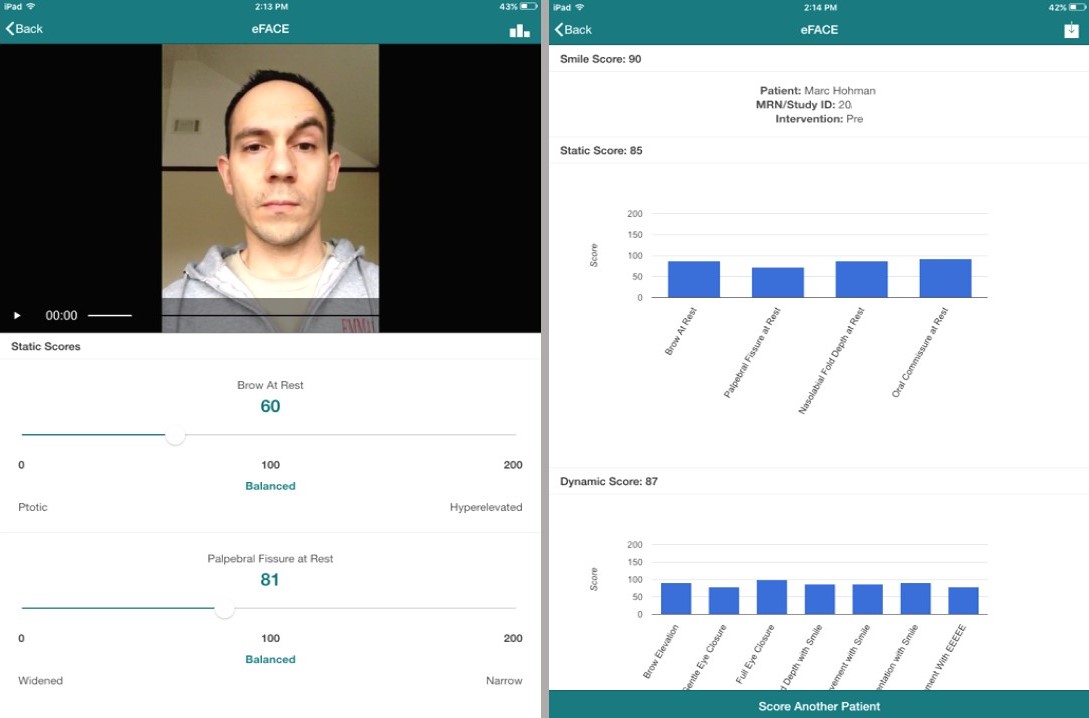
The eFACE Software Application. Screenshots of the Massachusetts Eye & Ear Infirmary eFACE software used on a tablet computer. The app is a validated instrument that permits quantification of function and symmetry of individual facial features at rest, with voluntary movement, and with involuntary movement.
Contributed by MH Hohman, MD, FACS
(Click Image to Enlarge)
(Click Image to Enlarge)
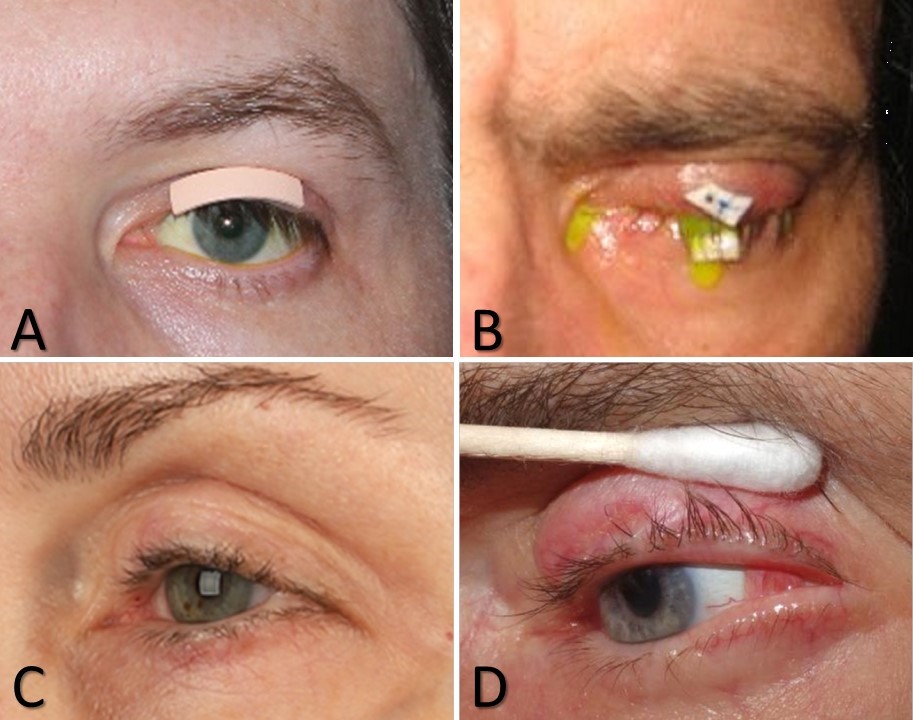
Interventions for Corneal Protection. A) Adhesive weight that may be used for sizing an implantable weight or as an external weight itself. B) Temporary tarsorrhaphy suture placed over a foam bolster. This patient recently underwent a slit-lamp examination with fluorescein. C) Lateral tarsorrhaphy. D) Tarsoconjunctival flap.
Contributed by V Yakopson, MD, FACS
(Click Image to Enlarge)
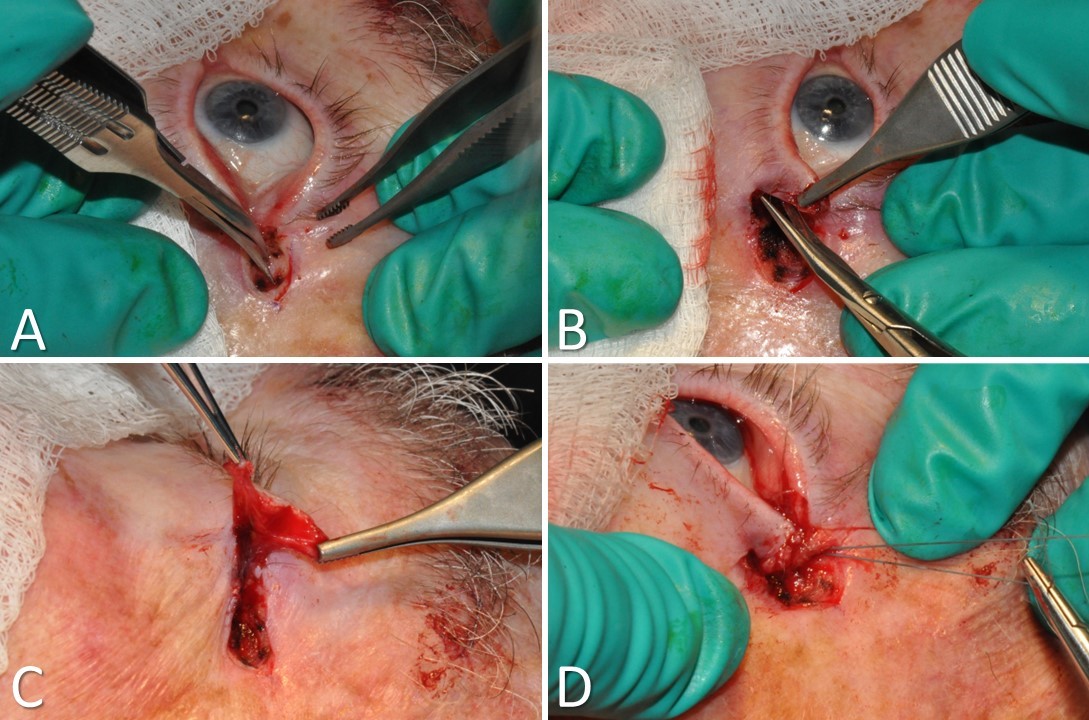
Tarsal Strip-Lateral Canthopexy. A) A lateral canthotomy and inferior cantholysis are performed. B) A full-thickness incision is made just inferior to the inferior tarsal plate. C) The skin and muscle are separated from the underlying tarsal plate and removed. The conjunctiva is scraped off the tarsal strip as well. D) The lateral aspect of the denuded tarsal strip is suspended superoposteriorly to the periorbita above Whitnall’s tubercle. A sharp lateral canthal angle is then restored with a suture, and the wound is closed.
Contributed by TA Hadlock, MD, and MH Hohman, MD, FACS
(Click Image to Enlarge)
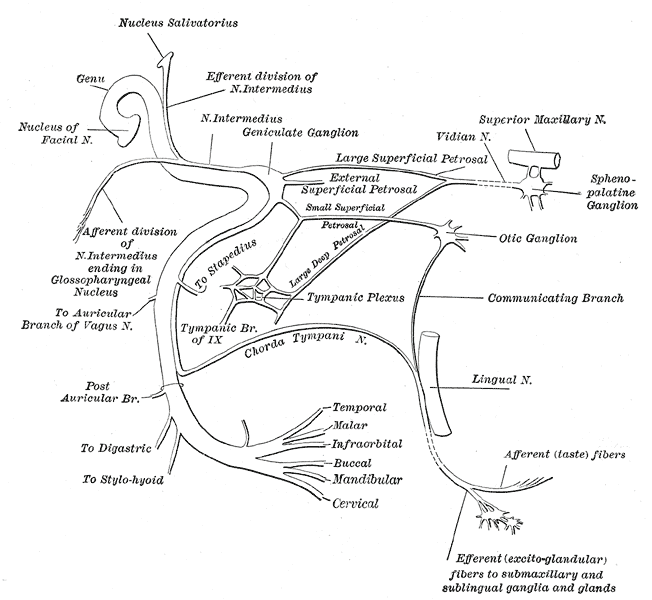
The Facial Nerve. This figure depicts the plan of the facial and intermediate nerves and their communication with other nerves.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
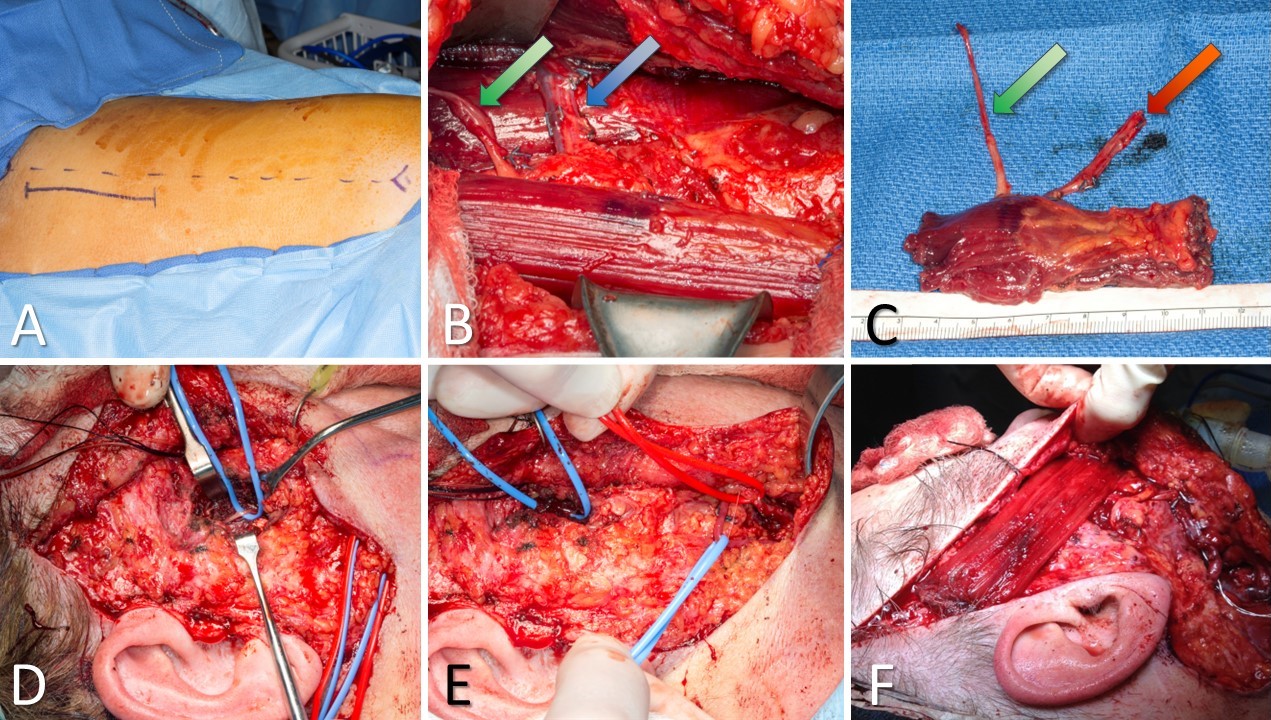
Gracilis Free Flap for Facial Reanimation. A) The incision is marked 2 cm posterior to a line drawn between the medial tibial epicondyle and the insertion of the adductor tendons on the pubis. B) The gracilis muscle is exposed, with the obturator nerve (green arrow) and the adductor artery with its 2 venae comitantes (blue arrow). C) The gracilis free flap harvested and thinned, with the obturator nerve (green arrow) and the adductor artery with its 2 venae comitantes (red arrow). D) Masseteric nerve isolated for coaptation to the obturator nerve on the gracilis flap. E) Facial artery (red loop) and facial vein (blue loop) isolated for anastomosis to the adductor artery and a vena comitans on the gracilis flap. F) Gracilis flap inset between the modiolus of the oral commissure and the temporalis fascia. An implantable Doppler probe has been placed to permit monitoring of perfusion to this buried flap.
Contributed by MH Hohman, MD; SE Bevans, MD; JM Robitschek, MD; and WJ Harsha, MD
(Click Image to Enlarge)
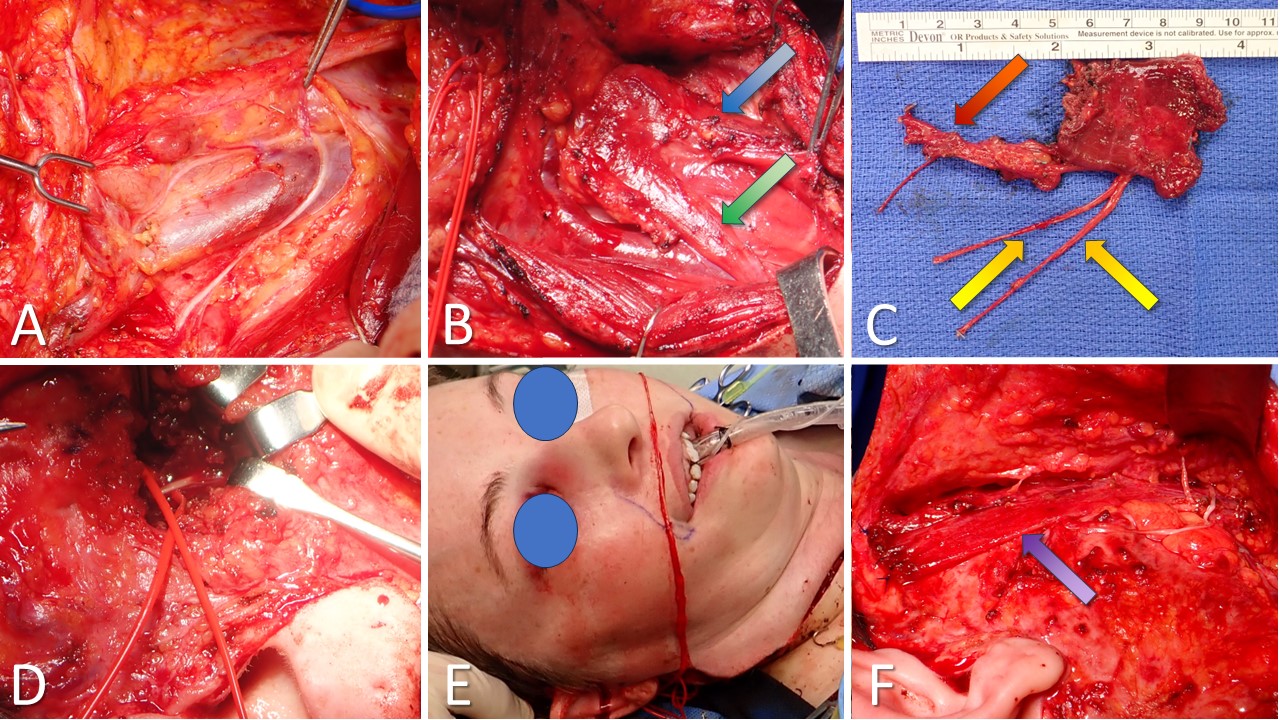
Sterno-Omohyoid Flap for Facial Reanimation. A) The ansa cervicalis, which innervates the sternohyoid and omohyoid muscles, runs along the internal jugular vein. B) Omohyoid (green arrow) and sternohyoid (blue arrow), with the facial artery and vein isolated with vessel loops. C) Flap harvested with both ends of the ansa (yellow arrows), superior thyroid artery (red arrow), and vein for anastomosis to the facial vessels. D) Masseteric nerve isolated for coaptation to 1 end of the ansa. E) A cross-face sural nerve graft is passed from a buccal branch on the nonparalyzed side for coaptation into the other end of the ansa. F) Flap inset with sternohyoid (purple arrow) between the modiolus of the oral commissure and temporalis fascia (replacing zygomaticus major) and omohyoid (too medial to be seen in this photo) between the upper lip and inferior orbital rim (replacing levator labii superioris). An implantable Doppler probe is placed to monitor perfusion to this buried flap.
Contributed by MH Hohman, MD, FACS; AG Vincent, MD, FACS; KG Anderson, MD; MW Herr, MD
(Click Image to Enlarge)

Injection of Botulinum Toxin for Facial Synkinesis. This patient is receiving an injection in the pretarsal portion of the lower eyelid orbicularis oculi muscle for recurrent spasms resulting from a facial nerve tumor. Tension is applied to the facial skin using a 4x4-inch gauze pad, which facilitates needle entry and improves patient comfort. The syringe has a 1 cc capacity and a 33 gauge needle. The botulinum toxin has been diluted to a concentration of 5 units/0.1 cc or 50 units/syringe.
Contributed by MH Hohman, MD, FACS
(Click Video to Play)
Acute Bell Palsy with a Severe Presentation. Patient with House-Brackmann grade VI. Note the resting effacement of the left nasolabial fold, laxity of the left lower eyelid at rest, and the Bell phenomenon in the left eye. There is no movement of the left hemiface except for transmitted traction from right-sided movements.
Contributed by MH Hohman, MD, FACS
(Click Video to Play)
Post-Bell Palsy Synkinesis. Patient after House-Brackmann grade VI paralysis. Note the essentially symmetric resting appearance (with slight deepening of the left nasolabial fold). There is involuntary movement of the midface and platysma with forceful eye closure and involuntary eye closure with lip puckering.
Contributed by MH Hohman, MD, FACS
(Click Image to Enlarge)
(Click Image to Enlarge)
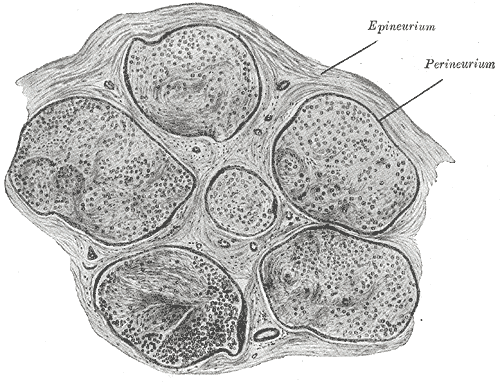
Neurology, Nerve Fascicle, Fasciculus, Epineurium. In the peripheral nervous system, nerve fascicles, epineurium, perineurium, and endoneurium are all layers of connective tissue that surround and support nerve fibers.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)

Sir Charles Bell. Scottish surgeon and anatomist.
Contributed by Granger, Public Domain, via Wikimedia Commons
References
Hohman MH, Hadlock TA. Etiology, diagnosis, and management of facial palsy: 2000 patients at a facial nerve center. The Laryngoscope. 2014 Jul:124(7):E283-93. doi: 10.1002/lary.24542. Epub 2014 Jan 15 [PubMed PMID: 24431233]
Level 2 (mid-level) evidenceEscalante DA, Malka RE, Wilson AG, Nygren ZS, Radcliffe KA, Ruhl DS, Vincent AG, Hohman MH. Determining the Prognosis of Bell's Palsy Based on Severity at Presentation and Electroneuronography. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2022 Jan:166(1):151-157. doi: 10.1177/01945998211004169. Epub 2021 Mar 30 [PubMed PMID: 33784203]
Peitersen E. Bell's palsy: the spontaneous course of 2,500 peripheral facial nerve palsies of different etiologies. Acta oto-laryngologica. Supplementum. 2002:(549):4-30 [PubMed PMID: 12482166]
Adour KK, Byl FM, Hilsinger RL Jr, Kahn ZM, Sheldon MI. The true nature of Bell's palsy: analysis of 1,000 consecutive patients. The Laryngoscope. 1978 May:88(5):787-801 [PubMed PMID: 642672]
Berkowitz C. DEFINING A DISCOVERY: PRIORITY AND METHODOLOGICAL CONTROVERSY IN EARLY NINETEENTH-CENTURY ANATOMY. Notes and records of the Royal Society of London. 2014 Dec 20:68(4):357-72 [PubMed PMID: 27494015]
Level 3 (low-level) evidenceSullivan FM, Swan IR, Donnan PT, Morrison JM, Smith BH, McKinstry B, Davenport RJ, Vale LD, Clarkson JE, Hammersley V, Hayavi S, McAteer A, Stewart K, Daly F. Early treatment with prednisolone or acyclovir in Bell's palsy. The New England journal of medicine. 2007 Oct 18:357(16):1598-607 [PubMed PMID: 17942873]
Level 1 (high-level) evidenceHato N, Yamada H, Kohno H, Matsumoto S, Honda N, Gyo K, Fukuda S, Furuta Y, Ohtani F, Aizawa H, Aoyagi M, Inamura H, Nakashima T, Nakata S, Murakami S, Kiguchi J, Yamano K, Takeda T, Hamada M, Yamakawa K. Valacyclovir and prednisolone treatment for Bell's palsy: a multicenter, randomized, placebo-controlled study. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2007 Apr:28(3):408-13 [PubMed PMID: 17414047]
Level 1 (high-level) evidenceJowett N, Hadlock TA. Contemporary management of Bell palsy. Facial plastic surgery : FPS. 2015 Apr:31(2):93-102. doi: 10.1055/s-0035-1549040. Epub 2015 May 8 [PubMed PMID: 25958893]
Miller MQ, Hadlock TA. Beyond Botox: Contemporary Management of Nonflaccid Facial Palsy. Facial plastic surgery & aesthetic medicine. 2020 Mar/Apr:22(2):65-70. doi: 10.1089/fpsam.2020.0009. Epub [PubMed PMID: 32130060]
Spencer CR, Irving RM. Causes and management of facial nerve palsy. British journal of hospital medicine (London, England : 2005). 2016 Dec 2:77(12):686-691 [PubMed PMID: 27937022]
Murakami S, Mizobuchi M, Nakashiro Y, Doi T, Hato N, Yanagihara N. Bell palsy and herpes simplex virus: identification of viral DNA in endoneurial fluid and muscle. Annals of internal medicine. 1996 Jan 1:124(1 Pt 1):27-30 [PubMed PMID: 7503474]
Finsterer J, Scorza FA, Scorza C, Fiorini A. COVID-19 associated cranial nerve neuropathy: A systematic review. Bosnian journal of basic medical sciences. 2022 Feb 1:22(1):39-45. doi: 10.17305/bjbms.2021.6341. Epub 2022 Feb 1 [PubMed PMID: 34392827]
Level 1 (high-level) evidenceTonkal A, Alamri AA, AlMaghrabi SJ, Mozahim NF, Mozahim SF, Alsubaie SA, Alsehly AA, Alshuaibi RO, Alotaibi LA, Qashgari FS. Cranial Nerve Impairment Associated With COVID-19 Infections: A Systematic Review. Cureus. 2022 Nov:14(11):e31997. doi: 10.7759/cureus.31997. Epub 2022 Nov 28 [PubMed PMID: 36589199]
Level 1 (high-level) evidenceMolaverdi G, Kamal Z, Safavi M, Shafiee A, Mozhgani SH, Ghobadi MZ, Goudarzvand M. Neurological complications after COVID-19: A narrative review. eNeurologicalSci. 2023 Dec:33():100485. doi: 10.1016/j.ensci.2023.100485. Epub 2023 Nov 15 [PubMed PMID: 38077923]
Level 3 (low-level) evidenceRafati A, Pasebani Y, Jameie M, Yang Y, Jameie M, Ilkhani S, Amanollahi M, Sakhaei D, Rahimlou M, Kheradmand A. Association of SARS-CoV-2 Vaccination or Infection With Bell Palsy: A Systematic Review and Meta-analysis. JAMA otolaryngology-- head & neck surgery. 2023 Jun 1:149(6):493-504. doi: 10.1001/jamaoto.2023.0160. Epub [PubMed PMID: 37103913]
Level 1 (high-level) evidenceWan EYF, Chui CSL, Lai FTT, Chan EWY, Li X, Yan VKC, Gao L, Yu Q, Lam ICH, Chun RKC, Cowling BJ, Fong WC, Lau AYL, Mok VCT, Chan FLF, Lee CK, Chan LST, Lo D, Lau KK, Hung IFN, Leung GM, Wong ICK. Bell's palsy following vaccination with mRNA (BNT162b2) and inactivated (CoronaVac) SARS-CoV-2 vaccines: a case series and nested case-control study. The Lancet. Infectious diseases. 2022 Jan:22(1):64-72. doi: 10.1016/S1473-3099(21)00451-5. Epub 2021 Aug 16 [PubMed PMID: 34411532]
Level 2 (mid-level) evidenceDe Diego-Sastre JI, Prim-Espada MP, Fernández-García F. [The epidemiology of Bell's palsy]. Revista de neurologia. 2005 Sep 1-15:41(5):287-90 [PubMed PMID: 16138286]
Zhao H, Zhang X, Tang YD, Zhu J, Wang XH, Li ST. Bell's Palsy: Clinical Analysis of 372 Cases and Review of Related Literature. European neurology. 2017:77(3-4):168-172. doi: 10.1159/000455073. Epub 2017 Jan 25 [PubMed PMID: 28118632]
Level 3 (low-level) evidenceSavadi-Oskouei D, Abedi A, Sadeghi-Bazargani H. Independent role of hypertension in Bell's palsy: a case-control study. European neurology. 2008:60(5):253-7. doi: 10.1159/000151701. Epub 2008 Aug 29 [PubMed PMID: 18756090]
Level 2 (mid-level) evidenceGaudin RA, Remenschneider AK, Phillips K, Knipfer C, Smeets R, Heiland M, Hadlock TA. Facial palsy after dental procedures - Is viral reactivation responsible? Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2017 Jan:45(1):71-75. doi: 10.1016/j.jcms.2016.11.002. Epub 2016 Nov 17 [PubMed PMID: 27939042]
Phillips KM, Heiser A, Gaudin R, Hadlock TA, Jowett N. Onset of bell's palsy in late pregnancy and early puerperium is associated with worse long-term outcomes. The Laryngoscope. 2017 Dec:127(12):2854-2859. doi: 10.1002/lary.26569. Epub 2017 Mar 27 [PubMed PMID: 28349542]
Gupta S, Mends F, Hagiwara M, Fatterpekar G, Roehm PC. Imaging the facial nerve: a contemporary review. Radiology research and practice. 2013:2013():248039. doi: 10.1155/2013/248039. Epub 2013 May 23 [PubMed PMID: 23766904]
Celik O, Eskiizmir G, Pabuscu Y, Ulkumen B, Toker GT. The role of facial canal diameter in the pathogenesis and grade of Bell's palsy: a study by high resolution computed tomography. Brazilian journal of otorhinolaryngology. 2017 May-Jun:83(3):261-268. doi: 10.1016/j.bjorl.2016.03.016. Epub 2016 Apr 29 [PubMed PMID: 27217008]
Seneviratne SO, Patel BC. Facial Nerve Anatomy and Clinical Applications. StatPearls. 2024 Jan:(): [PubMed PMID: 32119456]
Balkany T, Fradis M, Jafek BW, Rucker NC. Intrinsic vasculature of the labyrinthine segment of the facial nerve--implications for site of lesion in Bell's palsy. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1991 Jan:104(1):20-3 [PubMed PMID: 1900625]
Seok JI, Park JH, Park JA, Do Y. Contrast-enhanced MRI findings of patients with acute Bell palsy within 7 days of symptom onset: A retrospective study. Medicine. 2023 Dec 1:102(48):e36337. doi: 10.1097/MD.0000000000036337. Epub [PubMed PMID: 38050278]
Level 2 (mid-level) evidenceSeddon HJ. A Classification of Nerve Injuries. British medical journal. 1942 Aug 29:2(4260):237-9 [PubMed PMID: 20784403]
Sunderland S. The anatomy and physiology of nerve injury. Muscle & nerve. 1990 Sep:13(9):771-84 [PubMed PMID: 2233864]
Li C, Jiang XZ, Zhao YF. [Connection of trigeminal nerve and facial nerve branches and its clinical significance]. Shanghai kou qiang yi xue = Shanghai journal of stomatology. 2009 Oct:18(5):545-50 [PubMed PMID: 19907866]
Level 2 (mid-level) evidenceHeaton JT, Sheu SH, Hohman MH, Knox CJ, Weinberg JS, Kleiss IJ, Hadlock TA. Rat whisker movement after facial nerve lesion: evidence for autonomic contraction of skeletal muscle. Neuroscience. 2014 Apr 18:265():9-20. doi: 10.1016/j.neuroscience.2014.01.038. Epub 2014 Jan 28 [PubMed PMID: 24480367]
Yilmaz OF, Oguz H. Ocular surface lesions in clinical grades of Bell's phenomenon. Medical hypothesis, discovery & innovation ophthalmology journal. 2023 Winter:12(4):177-186. doi: 10.51329/mehdiophthal1484. Epub 2024 Jan 31 [PubMed PMID: 38601052]
House JW, Brackmann DE. Facial nerve grading system. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1985 Apr:93(2):146-7 [PubMed PMID: 3921901]
Santosa KB, Fattah A, Gavilán J, Hadlock TA, Snyder-Warwick AK. Photographic Standards for Patients With Facial Palsy and Recommendations by Members of the Sir Charles Bell Society. JAMA facial plastic surgery. 2017 Jul 1:19(4):275-281. doi: 10.1001/jamafacial.2016.1883. Epub [PubMed PMID: 28125753]
Kahn JB, Gliklich RE, Boyev KP, Stewart MG, Metson RB, McKenna MJ. Validation of a patient-graded instrument for facial nerve paralysis: the FaCE scale. The Laryngoscope. 2001 Mar:111(3):387-98 [PubMed PMID: 11224766]
Level 1 (high-level) evidenceCoulson S, Croxson GR, Adams R, Oey V. Prognostic factors in herpes zoster oticus (ramsay hunt syndrome). Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2011 Aug:32(6):1025-30. doi: 10.1097/MAO.0b013e3182255727. Epub [PubMed PMID: 21725270]
Level 2 (mid-level) evidenceKim HS, Jung J, Dong SH, Kim SH, Jung SY, Yeo SG. Association Between High Neutrophil to Lymphocyte Ratio and Delayed Recovery From Bell's Palsy. Clinical and experimental otorhinolaryngology. 2019 Aug:12(3):261-266. doi: 10.21053/ceo.2018.01018. Epub 2018 Dec 15 [PubMed PMID: 30545211]
Fisch U. Surgery for Bell's palsy. Archives of otolaryngology (Chicago, Ill. : 1960). 1981 Jan:107(1):1-11 [PubMed PMID: 7469872]
Gantz BJ, Rubinstein JT, Gidley P, Woodworth GG. Surgical management of Bell's palsy. The Laryngoscope. 1999 Aug:109(8):1177-88 [PubMed PMID: 10443817]
Fisch U. Prognostic value of electrical tests in acute facial paralysis. The American journal of otology. 1984 Oct:5(6):494-8 [PubMed PMID: 6393772]
Mower S. Bell's palsy: excluding serious illness in urgent and emergency care settings. Emergency nurse : the journal of the RCN Accident and Emergency Nursing Association. 2017 Apr 13:25(1):32-39. doi: 10.7748/en.2017.e1628. Epub [PubMed PMID: 28403702]
Al-Noury K, Lotfy A. Normal and pathological findings for the facial nerve on magnetic resonance imaging. Clinical radiology. 2011 Aug:66(8):701-7. doi: 10.1016/j.crad.2011.02.012. Epub 2011 Apr 22 [PubMed PMID: 21514926]
Lo YL, Fook-Chong S, Leoh TH, Dan YF, Lee MP, Gan HY, Chan LL. High-resolution ultrasound in the evaluation and prognosis of Bell's palsy. European journal of neurology. 2010 Jun 1:17(6):885-9. doi: 10.1111/j.1468-1331.2010.02950.x. Epub 2010 Feb 11 [PubMed PMID: 20158516]
Al-Hashimi I, Okon E. West Nile Virus: A Neglected Cause of Bell's Palsy? Cureus. 2024 Jun:16(6):e62486. doi: 10.7759/cureus.62486. Epub 2024 Jun 16 [PubMed PMID: 39015861]
Guntinas-Lichius O, Volk GF, Olsen KD, Mäkitie AA, Silver CE, Zafereo ME, Rinaldo A, Randolph GW, Simo R, Shaha AR, Vander Poorten V, Ferlito A. Facial nerve electrodiagnostics for patients with facial palsy: a clinical practice guideline. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2020 Jul:277(7):1855-1874. doi: 10.1007/s00405-020-05949-1. Epub 2020 Apr 8 [PubMed PMID: 32270328]
Level 1 (high-level) evidenceTuncay F, Borman P, Taşer B, Ünlü İ, Samim E. Role of electrical stimulation added to conventional therapy in patients with idiopathic facial (Bell) palsy. American journal of physical medicine & rehabilitation. 2015 Mar:94(3):222-8. doi: 10.1097/PHM.0000000000000171. Epub [PubMed PMID: 25171666]
Di Pietro A, Cameron M, Campana V, Leyes L, Zalazar Cinat JAI, Lochala C, Johnson CZ, Hilldebrand A, Loyo M. Efficacy of adding selective electrical muscle stimulation to usual physical therapy for Bell's palsy: immediate and six-month outcomes. European journal of translational myology. 2023 Oct 24:33(4):. doi: 10.4081/ejtm.2023.11630. Epub 2023 Oct 24 [PubMed PMID: 37877154]
Li P, Qiu T, Qin C. Efficacy of Acupuncture for Bell's Palsy: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PloS one. 2015:10(5):e0121880. doi: 10.1371/journal.pone.0121880. Epub 2015 May 14 [PubMed PMID: 25974022]
Level 1 (high-level) evidenceChen N, Zhou M, He L, Zhou D, Li N. Acupuncture for Bell's palsy. The Cochrane database of systematic reviews. 2010 Aug 4:2010(8):CD002914. doi: 10.1002/14651858.CD002914.pub5. Epub 2010 Aug 4 [PubMed PMID: 20687071]
Level 1 (high-level) evidenceBaugh RF, Basura GJ, Ishii LE, Schwartz SR, Drumheller CM, Burkholder R, Deckard NA, Dawson C, Driscoll C, Gillespie MB, Gurgel RK, Halperin J, Khalid AN, Kumar KA, Micco A, Munsell D, Rosenbaum S, Vaughan W. Clinical practice guideline: Bell's palsy. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2013 Nov:149(3 Suppl):S1-27. doi: 10.1177/0194599813505967. Epub [PubMed PMID: 24189771]
Level 1 (high-level) evidenceHato N, Sawai N, Teraoka M, Wakisaka H, Takahashi H, Hinohira Y, Gyo K. Valacyclovir for the treatment of Bell's palsy. Expert opinion on pharmacotherapy. 2008 Oct:9(14):2531-6. doi: 10.1517/14656566.9.14.2531. Epub [PubMed PMID: 18778190]
Level 3 (low-level) evidenceGire A, Kwok A, Marx DP. PROSE treatment for lagophthalmos and exposure keratopathy. Ophthalmic plastic and reconstructive surgery. 2013 Mar-Apr:29(2):e38-40. doi: 10.1097/IOP.0b013e3182674069. Epub [PubMed PMID: 23034688]
Level 3 (low-level) evidenceRozen S, Lehrman C. Upper eyelid postseptal weight placement for treatment of paralytic lagophthalmos. Plastic and reconstructive surgery. 2013 Jun:131(6):1253-1265. doi: 10.1097/PRS.0b013e31828be961. Epub [PubMed PMID: 23714789]
Silver AL, Lindsay RW, Cheney ML, Hadlock TA. Thin-profile platinum eyelid weighting: a superior option in the paralyzed eye. Plastic and reconstructive surgery. 2009 Jun:123(6):1697-1703. doi: 10.1097/PRS.0b013e3181a65a56. Epub [PubMed PMID: 19483568]
Bergeron CM, Moe KS. The evaluation and treatment of upper eyelid paralysis. Facial plastic surgery : FPS. 2008 May:24(2):220-30. doi: 10.1055/s-2008-1075838. Epub [PubMed PMID: 18470834]
Fowler J Jr, Taylor J, Storrs F, Sherertz E, Rietschel R, Pratt M, Mathias CG, Marks J, Maibach H, Fransway A, DeLeo V, Belsito D. Gold allergy in North America. American journal of contact dermatitis : official journal of the American Contact Dermatitis Society. 2001 Mar:12(1):3-5 [PubMed PMID: 11244133]
Fleming C, Lucke T, Forsyth A, Rees S, Lever R, Wray D, Aldridge R, MacKie R. A controlled study of gold contact hypersensitivity. Contact dermatitis. 1998 Mar:38(3):137-9 [PubMed PMID: 9536404]
Fowler JF Jr, Perryman JH, Quinlan B. Positive patch-test reactions to platinum are rare. Dermatitis : contact, atopic, occupational, drug. 2008 May-Jun:19(3):146-7 [PubMed PMID: 18627687]
Inagaki A, Takahashi M, Murakami S. Facial and hearing outcomes in transmastoid nerve decompression for Bell's palsy, with preservation of the ossicular chain. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2021 Mar:46(2):325-331. doi: 10.1111/coa.13671. Epub 2020 Dec 8 [PubMed PMID: 33236466]
Yanagihara N, Hato N, Murakami S, Honda N. Transmastoid decompression as a treatment of Bell palsy. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2001 Mar:124(3):282-6 [PubMed PMID: 11240992]
Casazza GC, Schwartz SR, Gurgel RK. Systematic Review of Facial Nerve Outcomes After Middle Fossa Decompression and Transmastoid Decompression for Bell's Palsy With Complete Facial Paralysis. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2018 Dec:39(10):1311-1318. doi: 10.1097/MAO.0000000000001979. Epub [PubMed PMID: 30239428]
Level 1 (high-level) evidenceWang Z, Chai Y, Chen Z, Wu H, Wang Z. Endoscopic transcanal facial nerve decompression in Bell's palsy: A pilot study. American journal of otolaryngology. 2022 Jan-Feb:43(1):103167. doi: 10.1016/j.amjoto.2021.103167. Epub 2021 Jul 23 [PubMed PMID: 34371460]
Level 3 (low-level) evidenceCannon RB, Gurgel RK, Warren FM, Shelton C. Facial nerve outcomes after middle fossa decompression for Bell's palsy. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2015 Mar:36(3):513-8. doi: 10.1097/MAO.0000000000000513. Epub [PubMed PMID: 25058839]
Andresen NS, Sun DQ, Hansen MR. Facial nerve decompression. Current opinion in otolaryngology & head and neck surgery. 2018 Oct:26(5):280-285. doi: 10.1097/MOO.0000000000000478. Epub [PubMed PMID: 30138146]
Level 3 (low-level) evidenceFujiwara T, Namekawa M, Kuriyama A, Tamaki H. High-dose Corticosteroids for Adult Bell's Palsy: Systematic Review and Meta-analysis. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2019 Sep:40(8):1101-1108. doi: 10.1097/MAO.0000000000002317. Epub [PubMed PMID: 31290805]
Level 1 (high-level) evidenceInagaki A, Katsumi S, Sekiya S, Murakami S. Intratympanic steroid therapy for Bell's palsy with poor prognostic results. Scientific reports. 2021 Apr 13:11(1):8058. doi: 10.1038/s41598-021-87551-x. Epub 2021 Apr 13 [PubMed PMID: 33850231]
North M, Weishaar J, Leonetti JP. Intraoperative electrical stimulation for persistent, post-traumatic facial paralysis. Ear, nose, & throat journal. 2023 Apr 24:():1455613221115145. doi: 10.1177/01455613221115145. Epub 2023 Apr 24 [PubMed PMID: 37092954]
Cronin GW, Steenerson RL. The effectiveness of neuromuscular facial retraining combined with electromyography in facial paralysis rehabilitation. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2003 Apr:128(4):534-8 [PubMed PMID: 12707657]
Nakamura K, Toda N, Sakamaki K, Kashima K, Takeda N. Biofeedback rehabilitation for prevention of synkinesis after facial palsy. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2003 Apr:128(4):539-43 [PubMed PMID: 12707658]
Martineau S, Rahal A, Piette E, Moubayed S, Marcotte K. The "Mirror Effect Plus Protocol" for acute Bell's palsy: A randomized controlled trial with 1-year follow-up. Clinical rehabilitation. 2022 Oct:36(10):1292-1304. doi: 10.1177/02692155221107090. Epub 2022 Jun 19 [PubMed PMID: 35722671]
Lindsay RW, Robinson M, Hadlock TA. Comprehensive facial rehabilitation improves function in people with facial paralysis: a 5-year experience at the Massachusetts Eye and Ear Infirmary. Physical therapy. 2010 Mar:90(3):391-7. doi: 10.2522/ptj.20090176. Epub 2010 Jan 21 [PubMed PMID: 20093325]
Level 2 (mid-level) evidencevan Veen MM, Dusseldorp JR, Quatela O, Baiungo J, Robinson M, Jowett N, Hadlock TA. Patient experience in nerve-to-masseter-driven smile reanimation. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2019 Aug:72(8):1265-1271. doi: 10.1016/j.bjps.2019.03.037. Epub 2019 Apr 11 [PubMed PMID: 31060989]
de Sanctis Pecora C, Shitara D. Botulinum Toxin Type A to Improve Facial Symmetry in Facial Palsy: A Practical Guideline and Clinical Experience. Toxins. 2021 Feb 18:13(2):. doi: 10.3390/toxins13020159. Epub 2021 Feb 18 [PubMed PMID: 33670477]
Haykal S, Arad E, Bagher S, Lai C, Hohman M, Hadlock T, Zuker RM, Borschel GH. The role of botulinum toxin a in the establishment of symmetry in pediatric paralysis of the lower lip. JAMA facial plastic surgery. 2015 May-Jun:17(3):174-8. doi: 10.1001/jamafacial.2015.10. Epub [PubMed PMID: 25742503]
Mehta RP, Hadlock TA. Botulinum toxin and quality of life in patients with facial paralysis. Archives of facial plastic surgery. 2008 Mar-Apr:10(2):84-7. doi: 10.1001/archfaci.10.2.84. Epub [PubMed PMID: 18347234]
Level 2 (mid-level) evidenceThomas AJ, Larson MO, Braden S, Cannon RB, Ward PD. Effect of 3 Commercially Available Botulinum Toxin Neuromodulators on Facial Synkinesis: A Randomized Clinical Trial. JAMA facial plastic surgery. 2018 Mar 1:20(2):141-147. doi: 10.1001/jamafacial.2017.1393. Epub [PubMed PMID: 28973094]
Level 1 (high-level) evidenceKanerva M. Buccinator synkinesis treated by botulinum toxin in facial palsy and hemifacial spasms. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2021 Jul:74(7):1464-1469. doi: 10.1016/j.bjps.2020.12.002. Epub 2020 Dec 5 [PubMed PMID: 33358465]
Patel PN, Owen SR, Norton CP, Emerson BT, Bronaugh AB, Ries WR, Stephan SJ. Outcomes of Buccinator Treatment With Botulinum Toxin in Facial Synkinesis. JAMA facial plastic surgery. 2018 May 1:20(3):196-201. doi: 10.1001/jamafacial.2017.1385. Epub [PubMed PMID: 28973100]
Pescarini E, Butler DP, Perusseau-Lambert A, Nduka C, Kannan RY. Targeted chemodenervation of the posterior belly of the digastric muscle for the management of jaw discomfort in facial synkinesis. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2021 Dec:74(12):3437-3442. doi: 10.1016/j.bjps.2021.05.045. Epub 2021 Jun 9 [PubMed PMID: 34233854]
Longino ES, Davis SJ, Landeen KC, Kimura KS, Sharma RK, Ortiz AS, Yang SF, Patel PN, Stephan SJ. Chemodenervation of the Posterior Belly of the Digastric Muscle in Facial Synkinesis. Facial plastic surgery & aesthetic medicine. 2023 Sep-Oct:25(5):378-383. doi: 10.1089/fpsam.2022.0207. Epub 2022 Sep 6 [PubMed PMID: 36067327]
Krivda K, Clabeaux C, Yakopson V. Crocodile tear syndrome treated with lacrimal gland incobotulinum toxin A injection: a report of two cases. Digital journal of ophthalmology : DJO. 2023:29(4):97-1000. doi: 10.5693/djo.02.2023.09.001. Epub 2023 Dec 28 [PubMed PMID: 38344060]
Level 3 (low-level) evidencePattanayak S, Sharma PK, Samikhya S, Khuntia I, Patra K. Transconjunctival botulinum toxin injection into the lacrimal gland in crocodile tears syndrome. Indian journal of ophthalmology. 2022 Apr:70(4):1339-1342. doi: 10.4103/ijo.IJO_2909_21. Epub [PubMed PMID: 35326051]
. High Prevalence of Neutralizing Antibodies After Long-term Botulinum Neurotoxin Therapy. Neurology. 2022 Feb 22:98(8):341. doi: 10.1212/WNL.0000000000013258. Epub 2021 Dec 22 [PubMed PMID: 34937776]
Henstrom DK, Malo JS, Cheney ML, Hadlock TA. Platysmectomy: an effective intervention for facial synkinesis and hypertonicity. Archives of facial plastic surgery. 2011 Jul-Aug:13(4):239-43. doi: 10.1001/archfacial.2011.43. Epub [PubMed PMID: 21768558]
Derakhshan A, Miller MQ, Malka R, Gadkaree SK, Hadlock TA. Releasing the Smile: Depressor Anguli Oris Excision in the Context of Managing Nonflaccid Facial Palsy. Plastic and reconstructive surgery. 2022 Feb 1:149(2):261e-269e. doi: 10.1097/PRS.0000000000008807. Epub [PubMed PMID: 35077425]
Yoshioka N. Selective orbicularis neuromyectomy for postparetic periocular synkinesis. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2015 Nov:68(11):1510-5. doi: 10.1016/j.bjps.2015.06.015. Epub 2015 Jun 27 [PubMed PMID: 26187811]
Hohman MH, Lee LN, Hadlock TA. Two-step highly selective neurectomy for refractory periocular synkinesis. The Laryngoscope. 2013 Jun:123(6):1385-8. doi: 10.1002/lary.23873. Epub 2013 Jan 11 [PubMed PMID: 23315713]
Level 2 (mid-level) evidencevan Veen MM, Dusseldorp JR, Hadlock TA. Long-term outcome of selective neurectomy for refractory periocular synkinesis. The Laryngoscope. 2018 Oct:128(10):2291-2295. doi: 10.1002/lary.27225. Epub 2018 Apr 18 [PubMed PMID: 29668050]
Azizzadeh B, Irvine LE, Diels J, Slattery WH, Massry GG, Larian B, Riedler KL, Peng GL. Modified Selective Neurectomy for the Treatment of Post-Facial Paralysis Synkinesis. Plastic and reconstructive surgery. 2019 May:143(5):1483-1496. doi: 10.1097/PRS.0000000000005590. Epub [PubMed PMID: 30807497]
Vincent AG, Bevans SE, Robitschek JM, Wind GG, Hohman MH. Masseteric-to-Facial Nerve Transfer and Selective Neurectomy for Rehabilitation of the Synkinetic Smile. JAMA facial plastic surgery. 2019 Dec 1:21(6):504-510. doi: 10.1001/jamafacial.2019.0689. Epub [PubMed PMID: 31465094]
Gray ML, Hu S, Gorbea E, Mashkevich G. Masseteric-zygomatic nerve transfer for the management of eye closure-smile excursion synkinesis. American journal of otolaryngology. 2020 Jul-Aug:41(4):102479. doi: 10.1016/j.amjoto.2020.102479. Epub 2020 Apr 4 [PubMed PMID: 32359868]
Klebuc MJA. Facial reanimation using the masseter-to-facial nerve transfer. Plastic and reconstructive surgery. 2011 May:127(5):1909-1915. doi: 10.1097/PRS.0b013e31820e9138. Epub [PubMed PMID: 21532419]
Level 2 (mid-level) evidenceBorschel GH, Kawamura DH, Kasukurthi R, Hunter DA, Zuker RM, Woo AS. The motor nerve to the masseter muscle: an anatomic and histomorphometric study to facilitate its use in facial reanimation. Journal of plastic, reconstructive & aesthetic surgery : JPRAS. 2012 Mar:65(3):363-6. doi: 10.1016/j.bjps.2011.09.026. Epub 2011 Oct 10 [PubMed PMID: 21992936]
Dorafshar AH, Borsuk DE, Bojovic B, Brown EN, Manktelow RT, Zuker RM, Rodriguez ED, Redett RJ. Surface anatomy of the middle division of the facial nerve: Zuker's point. Plastic and reconstructive surgery. 2013 Feb:131(2):253-257. doi: 10.1097/PRS.0b013e3182778753. Epub [PubMed PMID: 23357986]
Hontanilla B, Cabello A. Spontaneity of smile after facial paralysis rehabilitation when using a non-facial donor nerve. Journal of cranio-maxillo-facial surgery : official publication of the European Association for Cranio-Maxillo-Facial Surgery. 2016 Sep:44(9):1305-9. doi: 10.1016/j.jcms.2016.06.031. Epub 2016 Jul 9 [PubMed PMID: 27460946]
Lindsay RW, Bhama P, Weinberg J, Hadlock TA. The success of free gracilis muscle transfer to restore smile in patients with nonflaccid facial paralysis. Annals of plastic surgery. 2014 Aug:73(2):177-82. doi: 10.1097/SAP.0b013e3182a0df04. Epub [PubMed PMID: 24051452]
Bhama PK, Weinberg JS, Lindsay RW, Hohman MH, Cheney ML, Hadlock TA. Objective outcomes analysis following microvascular gracilis transfer for facial reanimation: a review of 10 years' experience. JAMA facial plastic surgery. 2014 Mar-Apr:16(2):85-92. doi: 10.1001/jamafacial.2013.2463. Epub [PubMed PMID: 24481538]
Level 2 (mid-level) evidenceVakharia KT, Henstrom D, Lindsay R, Cunnane MB, Cheney M, Hadlock T. Color Doppler ultrasound: effective monitoring of the buried free flap in facial reanimation. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2012 Mar:146(3):372-6. doi: 10.1177/0194599811427377. Epub 2012 Jan 18 [PubMed PMID: 22261491]
Hohman MH, Krivda JS, Herr MW, Anderson KG, Bevans SE, Montgomery EA, Robitschek JM, Vincent AG. Composite Sterno-Omohyoid Functional Muscle Transfer for Dual-Vector Smile Reanimation: A Case Series. Facial plastic surgery & aesthetic medicine. 2024 Jul-Aug:26(4):418-423. doi: 10.1089/fpsam.2023.0067. Epub 2023 Nov 10 [PubMed PMID: 37948552]
Level 2 (mid-level) evidenceVincent AG, Bevans SE, Robitschek JM, Groom KL, Herr MW, Hohman MH. Sterno-omohyoid Free Flap for Dual-Vector Dynamic Facial Reanimation. The Annals of otology, rhinology, and laryngology. 2020 Feb:129(2):195-200. doi: 10.1177/0003489419875473. Epub 2019 Oct 3 [PubMed PMID: 31578078]
Crouch AE, Hohman MH, Moody MP, Andaloro C. Ramsay Hunt Syndrome. StatPearls. 2024 Jan:(): [PubMed PMID: 32491341]
Quesnel AM, Lindsay RW, Hadlock TA. When the bell tolls on Bell's palsy: finding occult malignancy in acute-onset facial paralysis. American journal of otolaryngology. 2010 Sep-Oct:31(5):339-42. doi: 10.1016/j.amjoto.2009.04.003. Epub 2009 Jun 24 [PubMed PMID: 20015776]
Level 3 (low-level) evidenceMontgomery EA, Patel JA, Boone RE, Teixeira JC, Vincent AG, Hohman MH. Rare Manifestation of Sjögren's Syndrome as Unilateral Facial Paralysis: A Case Report and Literature Review. Military medicine. 2023 Jul 22:188(7-8):e2805-e2808. doi: 10.1093/milmed/usac272. Epub [PubMed PMID: 36106512]
Level 3 (low-level) evidenceHyams AF, Toynton SC, Jaramillo M, Stone LR, Bryson PJ. Facial baroparesis secondary to middle-ear over-pressure: a rare complication of scuba diving. The Journal of laryngology and otology. 2004 Sep:118(9):721-3 [PubMed PMID: 15509373]
Level 3 (low-level) evidenceGrossman A, Ulanovski D, Barenboim E, Azaria B, Goldstein L. Facial nerve palsy aboard a commercial aircraft. Aviation, space, and environmental medicine. 2004 Dec:75(12):1075-6 [PubMed PMID: 15619863]
Level 3 (low-level) evidenceYanagihara N. [On standardised documentation of facial palsy (author's transl)]. Nihon Jibiinkoka Gakkai kaiho. 1977 Aug 20:80(8):799-805 [PubMed PMID: 915593]
Ross BG, Fradet G, Nedzelski JM. Development of a sensitive clinical facial grading system. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1996 Mar:114(3):380-6 [PubMed PMID: 8649870]
Vrabec JT, Backous DD, Djalilian HR, Gidley PW, Leonetti JP, Marzo SJ, Morrison D, Ng M, Ramsey MJ, Schaitkin BM, Smouha E, Toh EH, Wax MK, Williamson RA, Smith EO, Facial Nerve Disorders Committee. Facial Nerve Grading System 2.0. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2009 Apr:140(4):445-50. doi: 10.1016/j.otohns.2008.12.031. Epub [PubMed PMID: 19328328]
Level 1 (high-level) evidenceBanks CA, Bhama PK, Park J, Hadlock CR, Hadlock TA. Clinician-Graded Electronic Facial Paralysis Assessment: The eFACE. Plastic and reconstructive surgery. 2015 Aug:136(2):223e-230e. doi: 10.1097/PRS.0000000000001447. Epub [PubMed PMID: 26218397]
Banks CA, Jowett N, Azizzadeh B, Beurskens C, Bhama P, Borschel G, Coombs C, Coulson S, Croxon G, Diels J, Fattah A, Frey M, Gavilan J, Henstrom D, Hohman M, Kim J, Marres H, Redett R, Snyder-Warwick A, Hadlock T. Worldwide Testing of the eFACE Facial Nerve Clinician-Graded Scale. Plastic and reconstructive surgery. 2017 Feb:139(2):491e-498e. doi: 10.1097/PRS.0000000000002954. Epub [PubMed PMID: 28121888]
Miller MQ, Hadlock TA, Fortier E, Guarin DL. The Auto-eFACE: Machine Learning-Enhanced Program Yields Automated Facial Palsy Assessment Tool. Plastic and reconstructive surgery. 2021 Feb 1:147(2):467-474. doi: 10.1097/PRS.0000000000007572. Epub [PubMed PMID: 33235050]
Ojha PT, Nagendra S, Ansari A, Kadam ND, Mathur A, Gopinathan N, Ojha N, Patel H, Bansode A, Kalel O, Morwani KP, Jagtap D, Kumar S, Vij V. Validation of a New Graphic Facial Nerve Grading System: FAME Scale. Annals of Indian Academy of Neurology. 2022 May-Jun:25(3):464-472. doi: 10.4103/aian.aian_662_21. Epub 2022 Apr 6 [PubMed PMID: 35936632]
Level 1 (high-level) evidencede Ru JA, Braunius WW, van Benthem PP, Busschers WB, Hordijk GJ. Grading facial nerve function: why a new grading system, the MoReSS, should be proposed. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2006 Oct:27(7):1030-6 [PubMed PMID: 17006355]
Lee LN, Susarla SM, H Hohman M, Henstrom DK, Cheney ML, Hadlock TA. A comparison of facial nerve grading systems. Annals of plastic surgery. 2013 Mar:70(3):313-6. doi: 10.1097/SAP.0b013e31826acb2c. Epub [PubMed PMID: 23241802]
Coulson SE, Croxson GR, Adams RD, O'Dwyer NJ. Reliability of the "Sydney," "Sunnybrook," and "House Brackmann" facial grading systems to assess voluntary movement and synkinesis after facial nerve paralysis. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2005 Apr:132(4):543-9 [PubMed PMID: 15806042]
Kayhan FT, Zurakowski D, Rauch SD. Toronto Facial Grading System: interobserver reliability. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2000 Feb:122(2):212-5 [PubMed PMID: 10652392]
Fattah AY, Gurusinghe ADR, Gavilan J, Hadlock TA, Marcus JR, Marres H, Nduka CC, Slattery WH, Snyder-Warwick AK, Sir Charles Bell Society. Facial nerve grading instruments: systematic review of the literature and suggestion for uniformity. Plastic and reconstructive surgery. 2015 Feb:135(2):569-579. doi: 10.1097/PRS.0000000000000905. Epub [PubMed PMID: 25357164]
Level 1 (high-level) evidenceCai Z, Li H, Wang X, Niu X, Ni P, Zhang W, Shao B. Prognostic factors of Bell's palsy and Ramsay Hunt syndrome. Medicine. 2017 Jan:96(2):e5898. doi: 10.1097/MD.0000000000005898. Epub [PubMed PMID: 28079835]
Jörg R, Milani GP, Simonetti GD, Bianchetti MG, Simonetti BG. Peripheral facial nerve palsy in severe systemic hypertension: a systematic review. American journal of hypertension. 2013 Mar:26(3):351-6. doi: 10.1093/ajh/hps045. Epub 2013 Jan 7 [PubMed PMID: 23382485]
Level 1 (high-level) evidenceJeong J, Yoon SR, Lim H, Oh J, Choi HS. Risk factors for Bell's palsy based on the Korean National Health Insurance Service National Sample Cohort data. Scientific reports. 2021 Dec 3:11(1):23387. doi: 10.1038/s41598-021-02816-9. Epub 2021 Dec 3 [PubMed PMID: 34862431]
Bhate M, Das AV, Singh S. Characteristics of facial nerve palsy in 112 children and risk factors for ocular complications. Journal of AAPOS : the official publication of the American Association for Pediatric Ophthalmology and Strabismus. 2023 Jun:27(3):141.e1-141.e5. doi: 10.1016/j.jaapos.2023.03.003. Epub 2023 May 6 [PubMed PMID: 37156335]
Kleiss IJ, Hohman MH, Susarla SM, Marres HA, Hadlock TA. Health-related quality of life in 794 patients with a peripheral facial palsy using the FaCE Scale: a retrospective cohort study. Clinical otolaryngology : official journal of ENT-UK ; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2015 Dec:40(6):651-6. doi: 10.1111/coa.12434. Epub [PubMed PMID: 25858429]
Level 2 (mid-level) evidenceEviston TJ, Croxson GR, Kennedy PG, Hadlock T, Krishnan AV. Bell's palsy: aetiology, clinical features and multidisciplinary care. Journal of neurology, neurosurgery, and psychiatry. 2015 Dec:86(12):1356-61. doi: 10.1136/jnnp-2014-309563. Epub 2015 Apr 9 [PubMed PMID: 25857657]
Butler DP, Grobbelaar AO. Facial palsy: what can the multidisciplinary team do? Journal of multidisciplinary healthcare. 2017:10():377-381. doi: 10.2147/JMDH.S125574. Epub 2017 Sep 25 [PubMed PMID: 29026314]
Hadlock TA, Greenfield LJ, Wernick-Robinson M, Cheney ML. Multimodality approach to management of the paralyzed face. The Laryngoscope. 2006 Aug:116(8):1385-9 [PubMed PMID: 16885741]
Level 2 (mid-level) evidenceGlass GE, Tzafetta K. Bell's palsy: a summary of current evidence and referral algorithm. Family practice. 2014 Dec:31(6):631-42. doi: 10.1093/fampra/cmu058. Epub 2014 Sep 10 [PubMed PMID: 25208543]