Introduction
Bilirubin is an important metabolite of heme (ferroprotoporphyrin IX), a coordination complex coordinating iron in various proteins. It is a potentially toxic substance. However, the body has developed mechanisms for its safe detoxification and disposition. Bilirubin and its metabolites also provide a distinctive yellow color to bile and stool and, to a lesser degree, urine. This topic summarizes the mechanism of heme metabolism and bilirubin synthesis.[1][2][3]
Formation of Bilirubin
Bilirubin is derived from 2 main sources. Roughly 80% of bilirubin is made from the breakdown of hemoglobin in senescent red blood cells and prematurely destroyed erythroid cells in the bone marrow. The remainder originates from the turnover of various heme-containing proteins found in other tissues, primarily the liver and muscles. These proteins include myoglobin, cytochromes, catalase, peroxidase, and tryptophan pyrrolase.[4][5] Approximately 4 mg/kg body weight of bilirubin is produced daily.
Cellular Level
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Cellular Level
Cellular Heme Metabolism
Heme is a ring of 4 pyrroles joined by carbon bridges and a central iron atom. Bilirubin is generated by a 2-stage sequential catalytic degradation reaction that primarily occurs in the reticuloendothelial system's cells, notably the spleen. Other cells include phagocytes and the Kupffer cells of the liver. Heme is taken up into These cells take up the heme, and enzyme heme oxygenase acts on them. The enzyme liberates the chelated iron by catalyzing the oxidation of the alpha-carbon bridge. This reaction produces an equimolar amount of carbon monoxide, which is excreted by the lungs and leads to the formation of the green pigment biliverdin. This green pigment is acted upon further by the nicotinamide adenine dinucleotide phosphate (NADPH) dependent enzyme, biliverdin reductase. This process releases an orange-yellow pigment known as bilirubin. Heme oxygenase, as mentioned above, is present in high concentrations in the liver's Kupffer cells and the reticuloendothelial system's cells. Heme oxygenase is the rate-limiting factor in bilirubin production.
The final structure is highly compacted by hydrogen bonding rendering the molecule essentially insoluble in aqueous solutions at neutral pH. The fully bonded structure of bilirubin is designated as bilirubin IX-alpha-ZZ. Bilirubin, insoluble in an aqueous solution, is carried in circulation bound to albumin, a reversible and covalent type of bonding.
Development
Metabolism of Bilirubin
- Albumin binding: Once bilirubin is released into the plasma, it is taken up by albumin, a transporter throughout the body. The binding affinity for albumin to bilirubin is extremely high, and under ideal conditions, no free (non-albumin bound) unconjugated bilirubin is seen in the plasma. To a lesser degree, especially in states of hypoalbuminemia, binding also occurs with high-density lipoprotein. The binding of albumin limits the escape of bilirubin from the vascular space, minimizes glomerular filtration, and prevents its precipitation and deposition in tissues. When the albumin-bilirubin complex reaches the liver, the highly permeable hepatic circulation allows the complex to reach the sinusoidal surface of the hepatocyte. This allows the pigment to disassociate from the albumin and enter the liver. This process is relatively inefficient, with the first pass clearance of bilirubin being approximately 20%. This inefficient process allows for always having the ability to measure a concentration of unconjugated bilirubin bound to albumin in the venous circulation. The binding of albumin to bilirubin is reversible.
- Hepatic transport mechanisms: Bilirubin is taken up into the hepatocytes from the liver sinusoids by 2 different mechanisms: passive diffusion and receptor-mediated endocytosis. The passive diffusion process is not energy-consuming and, as a result, follows a concentration gradient, making the flow bi-directional. Active transporter uptake of unconjugated bilirubin from the hepatic sinusoids is mediated by carrier proteins that are not well understood. Most of the unconjugated bilirubin entering the hepatocytes is extracted in the periportal region. A fraction of conjugated and unconjugated bilirubin within the hepatocyte is transported back into the sinusoidal space, and this fraction is once again taken up downstream to the sinusoidal flow. The 1A mediates the uptake, and 1B members of the organic anion transporting polypeptide family (OATP). These polypeptides are encoded by the genes: SLCO1B1 and SLCO1B3. Conjugated bilirubin that escapes reuptake into the hepatocyte is excreted in the urine. Bilirubin binding to glutathione S-transferases increases net uptake and minimizes the efflux of internalized bilirubin.
Hepatocyte Conjugation
Conjugation is mandatory to render bilirubin aqueous soluble and facilitate its secretion across the canalicular membrane and excretion into bile. Bilirubin is conjugated within the hepatocyte to glucuronic acid by a family of enzymes termed uridine-diphosphoglucuronic glucuronosyltransferase (UDPGT). The process of glucuronidation is 1 of the many crucial detoxification mechanisms of the human body. Many different isoforms of UDPGT exist, but the physiologically important isoform in bilirubin glucuronidation is UDPGT1A1. The enzyme esterifies 2 glucuronide moieties to bilirubin's propionic acid side chains. Under normal conditions, bilirubin diglucuronide is the predominant molecule synthesized. However, if the conjugation system is overwhelmed under conditions of excessive bilirubin synthesis, most bilirubin may be conjugated as bilirubin monoglucuronide. The ratio of mono-conjugated to the dis-conjugated pigment in bile is 1:4. Conjugation of bilirubin to the water-soluble form involves the disruption of the hydrogen bonds, an essential process for its elimination by the liver and kidney. This is achieved by glucuronic acid conjugating bilirubin's propionic acid side chains (See Image. Metabolic Pathway for Bilirubin in the Hepatocyte).
- Excretion of conjugated bile: Conjugated bilirubin and other substances destined to be excreted in bile are actively transported across the bile canalicular membrane of the hepatocyte. The concentration gradient is very high and can reach 1:1000. At least 4 known canalicular transporters participate in the excretion of conjugated bilirubin. However, the multidrug resistance-associated protein 2 (MRP2) appears to play the dominant role in the canalicular secretion of conjugated bilirubin. A portion of conjugated bilirubin is transported into the sinusoids and portal circulation by MRP3, which can undergo hepatocyte reuptake via the sinusoidal proteins, organic anion transport protein 1B1 and 1B3 (OATP1B1 and OATP1B3). Thus, some conjugated and unconjugated bilirubin may escape the hepatocyte cytosol into the plasma, which binds to albumin and gets transported around the body. However, only conjugated bilirubin can enter the bile. The conjugated bilirubin is then secreted into canalicular bile and drains into the small intestine. The rate-limiting step in bilirubin throughput is the hepatic excretory capacity of conjugated bilirubin. Part of the conjugated bilirubin may accumulate in serum when the hepatic excretion of the conjugated bilirubin is impaired, as in prolonged biliary obstruction or intrahepatic cholestasis. This conjugated bilirubin fraction gets covalently bound to albumin and is called delta bilirubin, delta fraction, or biliprotein. As the delta bilirubin is bound to albumin, its clearance from serum takes about 12-14 days (equivalent to the half-life of albumin) in contrast to the usual 2 to 4 hours (half-life of bilirubin).
The conjugation process alters the physicochemical properties of bilirubin, giving it many special properties. Most importantly, it makes the molecule water-soluble, allowing it to be transported in bile without a protein carrier. Conjugation also increases the size of the molecule. Conjugation prevents bilirubin from passively being reabsorbed by the intestinal mucosa due to its hydrophilicity and large molecular size. Thus, conjugation works to promote the elimination of potentially toxic metabolic waste products. Furthermore, conjugation modestly decreases the affinity of bilirubin for albumin.
- Degradation in the digestive tract: Conjugated bilirubin is not reabsorbed from the proximal intestine as mentioned above; in comparison, unconjugated bilirubin is partially reabsorbed across the lipid membrane of the small intestinal epithelium and undergoes enterohepatic circulation. Within the proximal small intestine, there is no additional bilirubin metabolism, and very little deconjugation occurs. In stark contrast, when the conjugated bilirubin reaches the distal ileum and colon, it is rapidly reduced and deconjugated by colonic flora to a series of molecules termed urobilinogen. The major urobilinoids seen in stool are known as urobilinogen and stercobilinogen, the nature and relative proportion of which depends on the presence and composition of the gut bacterial flora. These substances are colorless but turn orange-yellow after oxidation to urobilin, giving stool its distinctive color.
Related Testing
Measurement of Serum Bilirubin
Serum bilirubin is measured spectrophotometrically when the molecule reacts with diazo reagents, causing the breakdown of the tetrapyrrole into 2 azodipyrroles. This reaction is termed as the “Van den Bergh.” Unconjugated bilirubin reacts slowly with the diazo reagent as the central carbon bridge of bilirubin is buried within the hydrogen bonds. In contrast, conjugated bilirubin lacks these hydrogen bonds, and the reaction occurs rapidly even without accelerators. Adding accelerators such as caffeine or methanol disrupts the hydrogen bonds, and the reaction is quickly completed yielding the total bilirubin value. Unconjugated bilirubin is measured by subtracting the direct-reacting fraction from total bilirubin. Potential sources of error include plasma lipids, drugs such as propranolol, and several other endogenous substances. These interfere with the diazo assay and can potentially produce an unreliable result.
Clinical Significance
As unconjugated bilirubin is always bound to albumin in serum, it cannot be filtered by the glomeruli (in the absence of glomerular disease). Thus, unconjugated bilirubin is never found in urine, even when an elevated level of unconjugated bilirubin is in circulation. Jaundice that occurs with unconjugated hyperbilirubinemia is termed acholuric because the urine is not darkened. Dark urine, however, occurs when there is an excretion of an excess of water-soluble, conjugated bilirubin. This is seen in conjugated hyperbilirubinemia and signifies the presence of either liver or biliary disease. Thus the presence of bilirubin in urine helps identify subtle hepatobiliary dysfunction leading to conjugated hyperbilirubinemia, even when the measured concentration of conjugated bilirubin in serum is only slightly elevated. An exception to this rule is when bilirubinuria is not detected in a patient with prolonged cholestasis and marked jaundice. This is due to the formation of delta bilirubin or conjugated bilirubin tightly bound to serum albumin. The absence of bilirubinuria in such patients should not cause any difficulty in diagnosing conjugated hyperbilirubinemia, as the patient is jaundiced and serum-conjugated bilirubin is markedly elevated in such cases.[6][7][8][9][2]
Media
(Click Image to Enlarge)
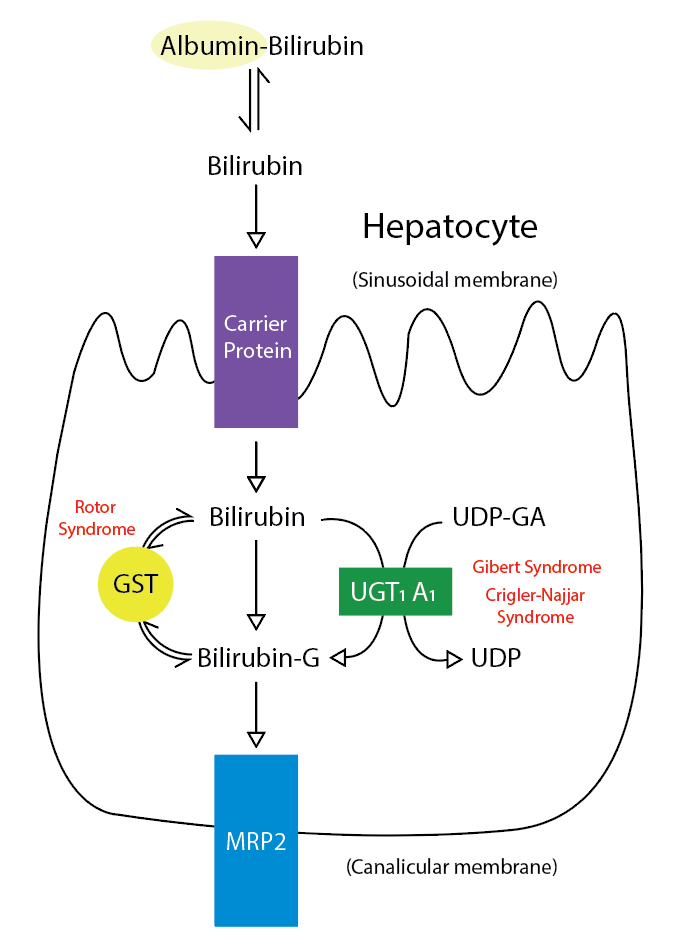
Metabolic Pathway for Bilirubin in the Hepatocyte. Bilirubin-G corresponds to bilirubin glucuronate; the donor is uridine diphosphate glucuronic acid (UDP-GA). This is catalyzed by the enzyme uridine diphosphate-glucuronyltransferase (UGT1A1). Gilbert and Crigler-Najjar syndrome is associated with decreases in UGT1A1 activity. Glutathione-S-transferase (GST) is a carrier protein that assists bilirubin uptake into the cytosol and may be implicated in Rotor syndrome.
Contributed by R Kabir, MD
References
Kosmachevskaya OV, Topunov AF. Alternate and Additional Functions of Erythrocyte Hemoglobin. Biochemistry. Biokhimiia. 2018 Dec:83(12):1575-1593. doi: 10.1134/S0006297918120155. Epub [PubMed PMID: 30878032]
Dosch AR, Imagawa DK, Jutric Z. Bile Metabolism and Lithogenesis: An Update. The Surgical clinics of North America. 2019 Apr:99(2):215-229. doi: 10.1016/j.suc.2018.12.003. Epub [PubMed PMID: 30846031]
Shen H, Zeng C, Wu X, Liu S, Chen X. Prognostic value of total bilirubin in patients with acute myocardial infarction: A meta-analysis. Medicine. 2019 Jan:98(3):e13920. doi: 10.1097/MD.0000000000013920. Epub [PubMed PMID: 30653097]
Level 1 (high-level) evidenceHinds TD Jr, Stec DE. Bilirubin, a Cardiometabolic Signaling Molecule. Hypertension (Dallas, Tex. : 1979). 2018 Oct:72(4):788-795. doi: 10.1161/HYPERTENSIONAHA.118.11130. Epub [PubMed PMID: 30354722]
Ngashangva L, Bachu V, Goswami P. Development of new methods for determination of bilirubin. Journal of pharmaceutical and biomedical analysis. 2019 Jan 5:162():272-285. doi: 10.1016/j.jpba.2018.09.034. Epub 2018 Sep 18 [PubMed PMID: 30273817]
Benesic A. [Drug-induced liver injury (DILI)]. MMW Fortschritte der Medizin. 2019 May:161(8):57-62. doi: 10.1007/s15006-019-0458-z. Epub [PubMed PMID: 31037662]
Patel SP, Vasavda C, Ho B, Meixiong J, Dong X, Kwatra SG. Cholestatic pruritus: Emerging mechanisms and therapeutics. Journal of the American Academy of Dermatology. 2019 Dec:81(6):1371-1378. doi: 10.1016/j.jaad.2019.04.035. Epub 2019 Apr 19 [PubMed PMID: 31009666]
Coucke EM, Akbar H, Kahloon A, Lopez PP. Biliary Obstruction. StatPearls. 2024 Jan:(): [PubMed PMID: 30969520]
Snyder E, Kashyap S, Lopez PP. Hepatobiliary Iminodiacetic Acid Scan. StatPearls. 2024 Jan:(): [PubMed PMID: 30969603]