Introduction
Breast implants have evolved since the crisis of the 1990s and are 1 of the most frequently performed aesthetic procedures. We now have cohesive gel implants, highly cohesive gel (ie, gummy bear) implants, saline implants, and structured saline implants with different surfaces and shapes from which to choose. Recently structured saline implants were also approved and are now available.[1][2][3][4]
Enhancing breasts goes back to 1895 when Czerny attempted fat transfers. This has been followed by paraffin injections, as well as the use of glass balls and ivory. In the 1950s, commercially manufactured sponge implants were introduced, made from various chemicals. This was plagued by contractures, infection, and erosion (see Image. Capsular Contracture). Due to the complications, enhancement surgery was not popular until the 1960s when commercially manufactured silicone gel implants were introduced
Silicone use in augmentation began in the 1940s as silicone became more widespread in its use following World War II to where it was used in medical devices in the 1950s. It's been suggested that silicone was injected into women's breasts in WWII and often migrated away from where it was placed, leading to many complications, including pain and loss of the breast. In 1962, Dr. Thomas Cronin designed a shell to contain the silicone gel and, with the Dow Corning Corporation in 1963, produced the breast implant as we know it today. Saline implants were available as well once shells were used for containing the fill, but saline implants were known to ripple more and have a higher failure rate (estimated more than 75%) as well.
Implants have gone through a myriad of styles and design changes, starting with external patches and polyurethane-coated shells, to softer gel fills, to newer designed shells to diminish diffusion of the silicone oil through the shell, all in response to the problems that were encountered with the patients. Gel also was made more cohesive, where the gel tended to stick together rather than break off into particulates that could disseminate into adjacent tissues.
In 1992, a moratorium on gel implants was issued by the FDA; saline implants were the only implants available in the United States as they were thought to be safer. There were textured anatomic, textured round, and smooth round implants available that could be filled with a range of volumes, which allowed for the correction of mild asymmetries if appreciated. Smooth round implants became the most popular implant of choice once it was determined from radiographic studies that round implants assumed the same shape as shaped implants when viewed from the side in an upright position.
In the mid-2000s, cohesive gel implants were introduced and allowed by the FDA. Varying levels of cohesiveness were also being experimented with to where we now have highly cohesive, form-stable implants in the textured anatomic and smooth round designs, cohesive gel in textured or smooth round implants, and round saline implants in textured or smooth surfaces. Just recently, the structured (bi-lumen) Ideal implant has been introduced and is available for use. All companies offer comparable warranties for rupture and capsular contracture.
Upon return of gel implants to the market, the FDA arbitrarily limited gel implants for use only in women older than 22 unless used in the reconstruction. Gel implants can be used "off label" in patients younger than 22, but surgeons must caution their patients that their warranties may not be valid or honored by the manufacturer. Hopefully, the FDA lifts this age restriction someday.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The breast implant controversy peaked in the early 1990s when Connie Chung aired a segment suggesting implants were causing autoimmune diseases after anecdotal reports were linking gel implants with systemic diseases such as connective tissue disorders and even cancer. Due to safety concerns and a lack of clinical studies to support the safety of the devices, the FDA issued a moratorium on the use of gel implants. This led to a class-action lawsuit that was settled in 1994 and led to the Chapter 11 bankruptcy of Dow Corning. Saline implants were the only type available until the return of gel implants in the mid-2000s.[5][6][7][8]
Epidemiology
In 2000, the Institute of Medicine published its summary findings of all the research done on implants. They concluded that there was no evidence that silicone implants caused any systemic diseases and that implants do not last forever. Despite many attempts to refute this, no new evidence to the contrary has been found. In March 2017, the FDA issued a warning that there was a link between textured implants and anaplastic large-cell lymphoma. This is now called breast implant-associated anaplastic large cell lymphoma or BIA-ALCL, and the risk of having this occur was estimated to be 1:30,000.[9]
Toxicokinetics
To the best of our knowledge, and since publication from the Institute of Medicine in 2000, there is no evidence to suggest any relationship between silicone and systemic diseases of the whole body. Despite continued research, there is no new evidence to suggest otherwise. It is also important to understand that implants do not last forever and that having an augmentation requires more surgery in the future to maintain results or to deal with complications such as contracture or malposition of the implant. More recently, women have been claiming breast implant illness (BII), motivating them to have "en block" removals. Still, there remains no evidence that there is an identifiable link between implants and BII, except for these anecdotal reports.[10]
History and Physical
Implants must be placed in a sterile environment. Implants should be bathed in an antibiotic solution before completely exposing the implant from its packaging to minimize risks for the static attraction of foreign particulates before insertion. Saline implants should only be filled using closed systems where the saline is never exposed to the air to eliminate risks for contamination of the saline. This is accomplished using intravenous (IV) tubing from the source bag to the syringe to the fill tube.
If concerned about implant integrity, implant displacement exercises can usually determine if an implant is intact or not. With both hands on the augmented breast, the pressure is placed on 1 hand to move the implant toward the other. With intact implants, the implant displaces the other hand, confirming that the implant is intact. With gel implants, if the breasts feel completely normal without any appreciation of the implant, the implant may be compromised. With saline implants, a rupture should be obvious with a smaller, more natural breast mound, and no implant should be appreciated on the exam.
With silicone breast implants, this capsule can camouflage a breast implant rupture, as the silicone remains trapped within the capsule and shows no signs of change. This is called a silent rupture and the reason why the government recommends getting an MRI every 2 years to evaluate the integrity of the implant. This is not a mandate, and most patients do not follow this unless concerns or issues arise, as it is often not covered. Sometimes the fibrous tissue (capsule) becomes inflamed, resulting in pain, soreness, or swelling. It can also cause changes in breast or shape, lumps in the affected breast, and hardening of the affected breast.
Evaluation
Ultrasound, mammogram, and MRI may be necessary for evaluating rupture concerns as well as implant displacement mentioned prior to physical exam for gel implants. Saline implant failure is obvious as 1 mound becomes noticeably smaller. If in doubt, have the patient use a molded cup bra that does not stretch and examine her mounds weekly or monthly in the molded cup bra. If there is a leak of any kind, space in the cup increases as the implant volume diminishes from a failing saline implant.
Treatment / Management
Surgical intervention with a replacement of the ruptured implant is recommended if rupture is suspected or documented on radiographic studies or clinical exams and the patient wishes to maintain her breast volume. If she does not, explant alone may be considered, and in some cases, mastopexy may be needed to produce the best outcome possible. Deflated saline shells may be left in the breast as long as not problematic or symptomatic, and consideration must be given to repairing muscle origins of the pectoralis major if a submuscular placement was originally done in attempts to minimize or resolve the animation that resulted from submuscular placement.[11][12][13]
Differential Diagnosis
The differential diagnoses for breast implants include the following:
- Breast cancer
- Capsular contracture
- Cysts
- Fat necrosis
- Fibroadenoma
- Fibrocystic disease
- Late hematoma
- Late inflammatory/infectious process
- Papilloma
- Presence of seroma
Pearls and Other Issues
Breast implant surgery demand continues to increase annually. Implants available for implantation include saline and gel implants of differing shapes, textures, and profiles. A newer baffled, dual-lumen saline implant has just been introduced to provide the characteristics of a gel implant without any risks of having silicone gel, but their profile is currently limited to high-profile styles, and they are working on introducing a moderate profile in the future. All implant shells are still manufactured from silicone, so exposure to silicone is not eliminated. Implant choice depends on many variables, including anatomy, tissue thickness, patient desires, and surgeon preferences, and excellent outcomes have been achieved.
There is a small but vocal group advocating silicone poisoning, and this is diagnosed by sending in tissue to their lab, where the diagnosis is established. They provide treatment products that patients can pay for out of pocket. Mainstream science has not supported their contentions, but patients who believe in this need extra attention and empathy when trying to outline a treatment plan, as they often want surgeons who also believe in what they believe.
Implant companies have many statistics to show that their product is better than the competition. Still, in the end, if 1 product were truly better than the others, it would emerge as the dominant product and the implant of choice for all surgeons. That has not yet happened.
In March 2017, the FDA released a statement that confirmed a relationship between implants and the development of anaplastic large-cell lymphoma. Information released by the American Society of Plastic Surgeons, shortly after the FDA announcement, reported the following information. This is a T-cell lymphoma that is not breast cancer. The studies suggest that textured implants are the only common thread with this phenomenon, estimated to occur at 1:30,000 worldwide, but smooth-surfaced implants cannot be excluded. Anaplastic large-cell lymphoma has been seen with both saline and gel implants. This usually presents as a delayed seroma or acute expansion of the mound once the patient has recovered from the procedure. Diagnosis requires fluid aspiration and immunohistochemistry and should be discussed with the pathologist so the proper studies on the fluid are done to rule this out or confirm the diagnosis. A multidisciplinary approach is recommended if this diagnosis is made with PET/CT and MRI helpful in management. If there is no spread, a complete capsulectomy with implant removal is considered curative, as incomplete capsulectomies have been associated with recurrence and a poorer prognosis.
Enhancing Healthcare Team Outcomes
Plastic surgeons primarily insert breast implants. When patients seek a breast implant, the primary care providers should have some knowledge about the implants. Implants come in varying profiles of the same volume, which changes the shape and base width with each profile. Implants are selected based on anatomic measurements taken during consultation while accounting for the desired volume. Patients should be informed of the benefits and risks of the different cohesivities, fillers, styles, shapes, and surfaces of the implants and make a choice that is best for them. As for what brand surgeons use, it truly is personal preference, often guided by the service provided by the manufacturer or the surgeon's experiences. The outcomes for most patients who undergo implants are good to excellent.[14] Clinicians are involved in patient education and follow-up, informing the interprofessional team of important changes in patient status.
Media
(Click Image to Enlarge)
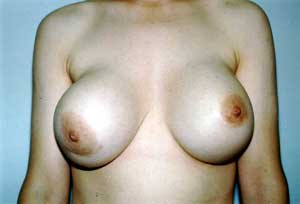
Capsular Contracture. The photograph shows grade IV capsular contracture in the right breast of a 29-year-old woman 7 years after subglandular placement of 560-cc silicone gel-filled breast implants.
Walter Peters, Public Domain, via Wikimedia Commons.
References
Chopra S, Marucci D. Cutaneous complications associated with breast augmentation: A review. International journal of women's dermatology. 2019 Feb:5(1):73-77. doi: 10.1016/j.ijwd.2018.08.005. Epub 2018 Oct 24 [PubMed PMID: 30809582]
Coombs DM, Grover R, Prassinos A, Gurunluoglu R. Breast augmentation surgery: Clinical considerations. Cleveland Clinic journal of medicine. 2019 Feb:86(2):111-122. doi: 10.3949/ccjm.86a.18017. Epub [PubMed PMID: 30742581]
Deva AK, Cuss A, Magnusson M, Cooter R. The "Game of Implants": A Perspective on the Crisis-Prone History of Breast Implants. Aesthetic surgery journal. 2019 Jan 31:39(Suppl_1):S55-S65. doi: 10.1093/asj/sjy310. Epub [PubMed PMID: 30715170]
Cheng F, Cen Y, Liu C, Liu R, Pan C, Dai S. Round versus Anatomical Implants in Primary Cosmetic Breast Augmentation: A Meta-Analysis and Systematic Review. Plastic and reconstructive surgery. 2019 Mar:143(3):711-721. doi: 10.1097/PRS.0000000000005371. Epub [PubMed PMID: 30601325]
Van Slyke AC, Carr NJ. Reply: Not All Breast Implants Are Equal: A 13-Year Review of Implant Longevity and Reasons for Explantation. Plastic and reconstructive surgery. 2019 Mar:143(3):664e-665e. doi: 10.1097/PRS.0000000000005342. Epub [PubMed PMID: 30601311]
Shin BH, Kim BH, Kim S, Lee K, Choy YB, Heo CY. Silicone breast implant modification review: overcoming capsular contracture. Biomaterials research. 2018:22():37. doi: 10.1186/s40824-018-0147-5. Epub 2018 Dec 20 [PubMed PMID: 30598837]
Prasad K, Zhou R, Zhou R, Schuessler D, Ostrikov KK, Bazaka K. Cosmetic reconstruction in breast cancer patients: Opportunities for nanocomposite materials. Acta biomaterialia. 2019 Mar 1:86():41-65. doi: 10.1016/j.actbio.2018.12.024. Epub 2018 Dec 18 [PubMed PMID: 30576863]
Hansson E, Jepsen C, Hallberg H. Breast reconstruction with a dermal sling: a systematic review of surgical modifications. Journal of plastic surgery and hand surgery. 2019 Feb:53(1):1-13. doi: 10.1080/2000656X.2018.1533840. Epub 2018 Dec 17 [PubMed PMID: 30557054]
Level 1 (high-level) evidenceEvren S, Khoury T, Neppalli V, Cappuccino H, Hernandez-Ilizaliturri FJ, Kumar P. Breast Implant-Associated Anaplastic Large Cell Lymphoma (ALCL): A Case Report. The American journal of case reports. 2017 May 31:18():605-610 [PubMed PMID: 28559535]
Level 3 (low-level) evidence. . :(): [PubMed PMID: 31045985]
Montemurro P, Fischer S, Hager S, Hedén P. Secondary Breast Augmentation: Is There a Trend for Bigger Implants? Aesthetic plastic surgery. 2019 Feb:43(1):59-69. doi: 10.1007/s00266-018-1244-5. Epub 2018 Oct 1 [PubMed PMID: 30276457]
Chang EI, Hammond DC. Clinical Results on Innovation in Breast Implant Design. Plastic and reconstructive surgery. 2018 Oct:142(4S The Science of Breast Implants):31S-38S. doi: 10.1097/PRS.0000000000005000. Epub [PubMed PMID: 30252757]
Mohebali K, Wixtrom RN. Breast Implant Engineering and Performance. Plastic and reconstructive surgery. 2018 Oct:142(4S The Science of Breast Implants):6S-11S. doi: 10.1097/PRS.0000000000004997. Epub [PubMed PMID: 30252754]
Jewell ML, Edwards MC, Murphy DK, Schumacher A. Lactation Outcomes in More Than 3500 Women Following Primary Augmentation: 5-Year Data From the Breast Implant Follow-Up Study. Aesthetic surgery journal. 2019 Jul 12:39(8):875-883. doi: 10.1093/asj/sjy221. Epub [PubMed PMID: 30165661]