Introduction
Breast cancer is a common diagnosis, with 252710 new invasive cases and 63410 in situ cases diagnosed annually, according to the American Cancer Society data in 2017. Despite its incidence, it is commonly a treatable disease, with a 90% 5-year survival rate and an 83% 10-year survival rate. Currently, 3 million women are living with the disease. Mastectomy is the common measure for both the treatment and prophylaxis of breast cancer.
Although mastectomies have been performed for many years, reconstruction has only been a consideration more recently. The first reported case of breast reconstruction was in 1887 when Aristide Verneuil used a pedicle-based off the opposite breast for reconstruction. It was closely followed by Vincent Czerny who used a lipoma to reconstruct a lumpectomy defect. Iginio Tansini first performed a latissimus dorsi flap in 1906, although most advocated against reconstruction during this period, as they felt it inhibited cancer care. It wasn’t until the 1950s when breast reconstruction became an option again, with surgeons like Dr. Gilles performing tubed pedicled flaps.[1] The invention of silicone implants in the 1960s started the age of prosthetic breast reconstruction. Free flaps were not used until the late 1970s when Holmstrom published using a “free abdominoplasty flap” for breast reconstruction. However, microsurgery was not commonplace during that time. Autologous reconstruction really took off when Dr. Hartrampf published his method for pedicled TRAM (transverse rectus abdominis myocutaneous) flap in 1982. The pedicled TRAM evolved to the free TRAM, as microsurgery was more common and the deep inferior epigastric artery had improved blood supply when compared to the superior epigastric artery (the basis of the pedicled flap). This process has further evolved into the free MS-TRAM (muscle-sparing TRAM) and deep inferior epigastric perforator (DIEP) flap, in addition to utilizing other free flaps for breast reconstruction.[1][2][3][4]
Today, breast reconstructions are common operations, with 106294 operations done annually, according to the American Society of Plastic Surgery 2018 data. However, most reconstructions are implant-based, and only 1316 were performed by autologous methods (TRAM, DIEP, latissimus dorsi, and other flaps).
Since there are so many survivors that go on to live a long life, quality of life issues are paramount. Plastic surgeons can contribute to the quality of life issues of these patients by performing breast reconstruction. In addition, all insurance carriers have to cover breast reconstruction thanks to the Women’s Health and Cancer Rights Act in 1998.[5] Patients undergoing autologous reconstruction reported a higher quality of life and improved outcomes than their implant-based counterparts.[2][6] Advantages of autologous reconstruction include high postoperative satisfaction and a long-lasting result, with natural aging, ptosis, responsiveness to change in body weight, improved aesthetic results, and body contouring at the donor site. Autologous reconstructions do not have the major disadvantages of implants, like capsular contracture and the risk of device failure. It is easier to match a unilateral autologous reconstruction to the remaining natural breast. Also, some patients will not have enough skin after mastectomy and will not be candidates for implant-based reconstruction anyway. Because autologous reconstructions are more closely able to resemble pre-operative form, they are now considered the gold standard. The DIEP flap is typically the flap of choice, if available, secondary to the improved aesthetic result at the donor site paired with minimal donor site morbidity. Other donor sites are also suitable depending on the patient’s habitus and reconstructive needs.[2][6][7][8]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
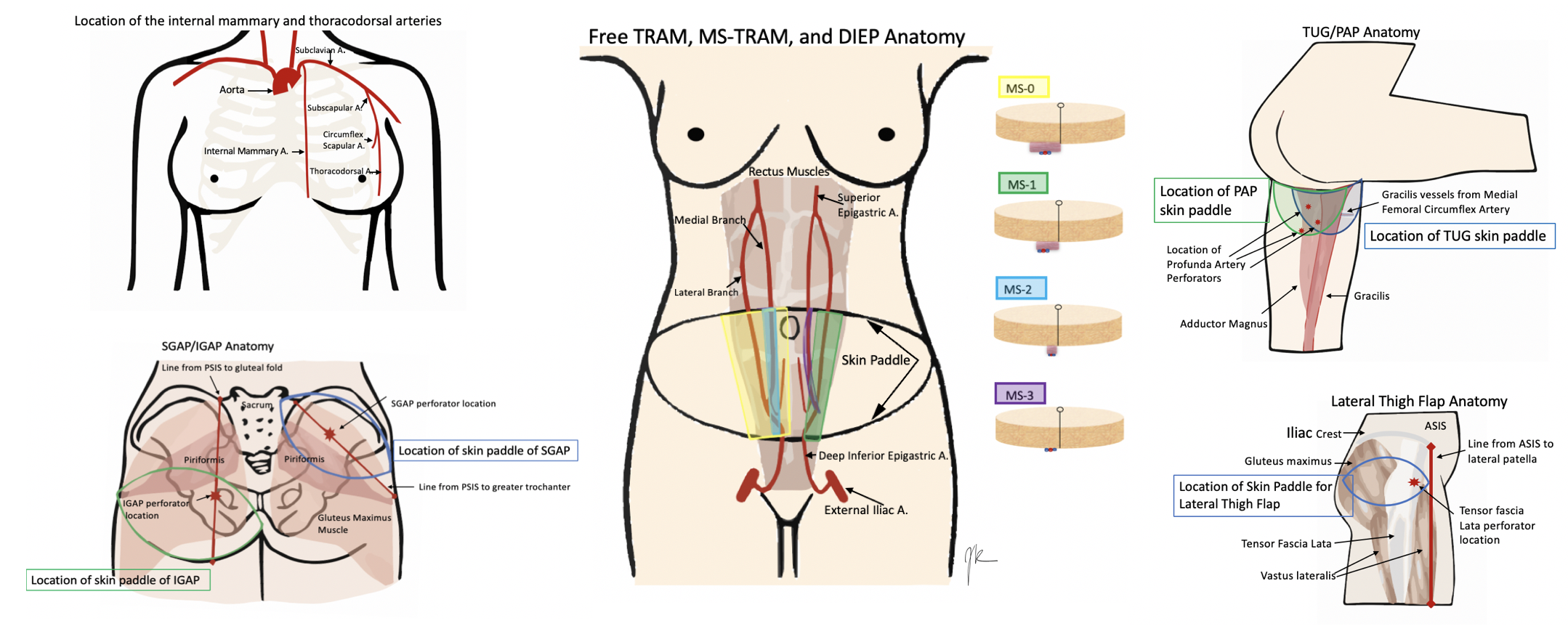
Anatomy varies a lot by the operation chosen. Therefore, this will include the most common choices for free flap breast reconstruction, although this is not all-inclusive. See the images below for the anatomy.
Starting with the recipient site. Patients who are getting free flap breast reconstruction are undergoing or have had a mastectomy. Therefore the recipient's vessels need to be located near the site of reconstruction. Historically, the thoracodorsal arteries were utilized although the internal mammary arteries are now more commonly used. The results are comparable regardless of the site chosen. The thoracodorsal arteries are similarly sized to the internal mammary arteries at 1.0 to 2.5mm in diameter. They are already exposed in a patient who underwent an axillary dissection but is prone to injury in a patient who requires a delayed axillary dissection. The internal mammary arteries have a higher flow than the thoracodorsal arteries. Also, they can be utilized for anastomosis in both the anterograde and retrograde direction and can best be used at the 3 to 5 intercostal spaces. Occasionally, perforators off of the internal mammary are large enough to be utilized. Internal mammary arteries require less pedicle length to be reached and are advantageous for small flaps with short pedicles.[2][3][6]
Below is the list of the common donor sites/flaps:
Abdomen
There are several iterations of flaps from the abdomen that can be utilized, from most invasive to least invasive, the free TRAM, ms-TRAM, DIEP, and SIEA (superficial inferior epigastric artery) flap. These flaps are beneficial to harvest, as many patients have some extra abdominal tissue and get the added benefit of abdominal contouring from the harvesting of the transverse skin island. The TRAM derives from the rectus muscle, which is a Mathes and Nahai type III flap, with two dominant pedicles. TRAM, ms-TRAM (ms-1 and 2), vs. DIEP (ms-3), all depend on how much muscle gets removed. The free TRAM takes the entirety of the muscle (ms-0), ms 1 leaves either a medial or lateral component, ms 2 leaves medial and lateral components, ms 3 (DIEP) is a true perforator flap and leaves all muscle intact. The muscle is split to free the vessels. The typical anatomy of the rectus muscle is two rows of perforators, although variants with one and three rows also exist. The advantage of taking more muscle is improved blood supply, but it sacrifices more abdominal wall morbidity. A large amount of tissue is harvestable, and the pedicle is about 12cm in length and 2.0mm in diameter. The SIEA is considered ms 4 as it only takes the skin and fat off the fascia based on the superficial vessel and does not violate the muscle or fascia. However, only a hemi-abdomen can be utilized with this flap, which often does not have a vessel sufficient in size to use.[7][8][9][10]
When the abdominal tissue is not available or sufficient, secondary flap options are a possible choice. This option varies between buttock and thigh flaps, with flaps like the SGAP (superior gluteal artery perforator), IGAP (inferior gluteal artery perforator), TUG (transverse upper gracilis), and PAP (profundal artery perforator).[9] Some surgeons prefer to use the thigh, as the tissue is more pliable, it does not require an intraoperative position change, and the donor site scar is less obtrusive.
Medial Thigh
There are two options from the medial thigh, the TUG flap, and the PAP flap. The TUG flap produces a relatively small breast but is an easy flap to elevate. It utilizes the area that otherwise discarded during a medial thigh lift, but keeps the gracilis, and thus the vascular pedicle (the ascending branch of the medial circumflex femoral artery) with it. Since the TUG flap is based on the gracilis, it is a Mathes and Nahai Type II flap. The transversely oriented skin paddle can be up to 25x10cm in size, with a small pedicle (6cm in length and 1.6mm vessels). This flap is ideal for small-breasted women who do not have an abdominal donor site or do not want an abdominal donor site.[3][6]
The profunda artery perforator flap, or PAP flap, has recently been described to improve upon the TUG flap. It has the advantages of longer pedicle length, better donor-site scar, larger skin paddle, no muscle harvest, and decreased lymphedema risk. However, pre-operative imaging is necessary to determine the location of the pedicle, to design the skin paddle properly. It is more posteriorly located than the TUG donor site and can make a flap of about 6 to 7cm by 18 to 20cm. The pedicle is about 10cm in length and usually has a diameter of 2.2mm. These flaps can be coned to re-shape the breast and tend to look more natural than gluteal tissue.[6][8][9][11]
Gluteal Flaps
The gluteal flaps were actually described before the thigh-based flaps, but are used less commonly as they require position changes for harvest and inset. Gluteal tissue is firmer and more difficult to shape and inset. They can be based on either the superior (SGAP) or inferior gluteal arteries (IGAP). The SGAP skin island is 10 to 12 x 25 to 32 cm with a 6 to 8 cm pedicle. The IGAP is usually about 8x18 cm, and the pedicle is about 8 to 11 cm. The inferiorly based flap avoids the divot created by the SGAP donor site but risks sciatic exposure if taking the muscle.[2][6][12][13]
Lateral Thigh Flap
A more recently described flap is the lateral thigh or subcutaneous tensor fascia lata perforator flap, based off perforators originating from the ascending branch of the lateral circumflex artery. The perforators are in the septum between tensor fascia lata and gluteus medius muscles. Flap dimensions range between 6 to 9 cm x 18 to 22 cm with a 6 to 8 cm pedicle length and 2.0mm in diameter.[7]
Indications
The main indication of free flap breast reconstruction is acquired absence of the breast(s), typically from a mastectomy for breast cancer or prophylactic mastectomy for BRCA or another genetic proclivity for breast cancer, but also congenital breast deformity. Although breast-sparing options exist, many women still choose or need a mastectomy to treat their cancer. The goals being to restore the breast dimensions, contour, and consistency.[2][6][7]
Contraindications
There are not specifically true contraindications for breast reconstruction, presuming the patient is medically able to tolerate a general anesthetic for the duration of the chosen case. However, there are risk factors that might make it unsafe or undesirable to pursue reconstruction. Age is not a risk for these procedures. However, age over 65 is an increased risk for a hernia after abdominally based surgery and thrombosis. Obesity with a BMI over 30 is associated with increased overall complications, donor site complications, partial flap loss and fat necrosis, and recipient site complications. BMI over 40 is at high risk for flap failure and should prompt caution before performing the reconstruction. ASA class III and increased operative times are also risk factors for complications. Certain flaps are contraindicated on individual patients depending on their habitus and anatomy; for example, contraindications to DIEP include someone who has had a prior abdominoplasty. Collagen vascular diseases and thrombotic diseases increase the risk of free flap failure. Patients on chemotherapy and steroids have an increased risk of delayed wound healing. It is contraindicated to do an elective free flap during pregnancy.[6][10] Smoking is a relative contraindication, with some surgeons refusing to perform the procedure until the patient stops smoking to prevent wound complications and healing issues.[14]
Radiation needs to be taken into consideration as well. While autologous methods are the ideal reconstruction in patients who require post-mastectomy radiotherapy, radiating the flap can decrease the aesthetics and risk partial flap loss and fibrosis.[6]
Equipment
The equipment required is similar to any standard operation with the addition of specific microsurgical equipment. Surgeons require some magnification, either with loupes or a microscope or both. Microsurgical equipment includes micro sutures (8-0 - 10-0), jeweler’s forceps, microscissors, a vessel dilator, a microneedle holder, microvascular clamps, microsurgical hemoclips, and a micro-instrument wipe. Medications including heparin saline (100u/mL), papaverine, and possibly TPA. A venous coupler is often necessary for vein anastomosis. Also, standard equipment for monitoring the flap is mandatory, typically this is a Doppler probe, but an internal doppler and other devices can be used depending on surgeon preferences.
Personnel
The personnel needed for free flap breast reconstruction is similar to other surgeries. A microsurgeon and an assistant are required, as is an anesthesiologist, circulating nurse, and scrub tech. Also, the floor nurses need to be trained to monitor the flap appropriately.
Preparation
A thorough history and physical is requisite. Risks and comorbidities are reviewed and modified when appropriate; this requires discussing the plan with anesthesia, especially avoiding bands, IVs, and blood pressure cuffs on donor extremities, and the avoidance of pressors and vasoactive drugs during the case, and the need to have anticoagulants available.
Technique or Treatment
Surgical techniques depend on the flap chosen. In brief, there are two parts of the operation, flap elevation and preparation of the recipient's vessels, which two surgeons can perform simultaneously. In all instances, the patient gets a usual surgical prep and sterile drape in the supine position unless otherwise stated. In all cases, surgical staff will mark the patient in the pre-op holding area. Standard markings include sternal midline and standard breast markings for the mastectomy (ellipse, Wise pattern, vertical reduction pattern, nipple-sparing incisions) depending on patient habitus, tumor characteristics, and surgeon preference. The donor site is also marked: an elliptical abdominoplasty style incision for DIEP, TRAM, and ms-TRAM flaps, medial thigh lift for TUG (which can be modified based on where the imaging shows the perforator for a PAP), near the superior or inferior gluteal creases for SGAP/IGAP, and an ellipse around the lateral thigh perforator for the LTP flap. The location of these is in the Figure. The flap is elevated on its perforator for all flaps, dissected down the pedicle, and kept in situ until the recipient site is prepared. Once the vessels are ready (internal mammary in most cases), the pedicle is ligated distally, and the flap removed from the site. The flap is flushed with heparinized saline, and the vessels are cleaned, separated, and prepared for anastomosis. The recipient vessels are also prepared, distally ligated, and flushed with heparinized saline. Arterial inflow is checked, and the vessels are temporarily clamped with Acklund clamps to keep a dry surgical field. The flap is temporarily fixated in a position to allow inset while keeping the flap secure so there is no motion or tension on the vessels during the anastomosis. It is also crucial to look at the vessel lie to make sure the vessels are straight and that nothing will kink the vessels after anastomosis. There are many techniques to anastomose vessels. One preferred technique is a coupler for the vein and an interrupted hand-sewn anastomosis with 8-0 or 9-0 nylon sutures for the artery. The flap is inset, and the breast gets shaped with sutures, and the cavity drained.
Elevation of the Flap
Abdominally based flaps: The abdomen is marked as described for the use of either the whole abdomen or a hemi-abdomen, depending on the size of flap required. One preferred method is to make the superior incision first, down to the fascia, and elevate the upper abdominal skin, which is transposed, so one knows where to make the lower incision to be able to close the abdomen. The inferior incision is then made, with care to find and dissect free the superficial inferior epigastric vein. Next, (if needed) the midline incision and umbilical incisions are made. The flap is elevated off the abdominal wall from lateral to medial. Depending on the type of flap chosen, the whole rectus muscle, part of it, or just the perforator(s) chosen are dissected free. These are isolated on the deep inferior epigastric artery and vein and taken as described above. The abdomen is closed over drains by transposing the superior abdominal skin down and closing in layers. The umbilicus is re-sited through the abdominal flap.
TUG: The skin flap marking is as above, and the patient is in the supine frog-leg position. The flap should center over the gracilis and the pedicle, which is about 10cm from the pubis. The skin is incised, leaving it attached to the gracilis muscle and staying superficial over the lymphatics. Once the flap is isolated on the gracilis, the adductor longus is retracted, and the pedicle dissected out to the origin. When the pedicle is ready, the gracilis is divided, the vessels are taken, and the flap transferred.
PAP: Again, the patient is marked as described and centered on the perforator identified by imaging. The patients are in a supine, frog-leg position. The incision is started medially down to the deep fascia. The flap is elevated in a subfascial plane posterior to the adductor longus and proceeding until the perforators are located (usually at the level of the adductor magnus fascia. The perforator dissection proceeds through the adductor magnus up until its origin at the profunda vessels. The flap is taken and transferred as above.
SGAP/IGAP: The patient is pre-operatively marked for the flap chosen. The patient's position is either in the prone or lateral decubitus position for flap harvest. The flap is marked based on anatomic landmarks (SGAP perforators are 1/3 of the distance on a line from the ASIS to the greater trochanter, and the IGAP are 2/3 of the distance on a line from ASIS to gluteal fold). The incision is made with a bevel so that extra gluteal fat can be included superiorly and inferiorly. The dissection is taken down to the level of the muscle, and dissection proceeds distally to proximally until the perforator(s) is(are) found. A perforator of at least 1mm is chosen and dissected down through the muscle to the pedicle off either the superior or inferior gluteal artery. It is taken at a level with sufficient length and caliber for a microsurgical anastomosis.
Lateral thigh: Again, the patient is in the supine position, and the flap markings get drawn pre-operatively. The dissection through the skin occurs, and then the flap is elevated medially to laterally until reaching the perforators. A line is drawn extending from the ASIS to the lateral patella; this is the anterior border of the flap, with another line drawn from the pubic bone perpendicular to that line. Perforators are usually located along the horizontal line posterior to the vertical line. During the dissection, the lateral femoral cutaneous nerve is identified and preserved. The perforators are found and dissected into the septum between tensor fascia lata and gluteus medius up to the ascending branch of the lateral circumflex femoral artery. The pedicle is taken, and the flap transferred.
Preparation of the recipient's vessels: Many surgeons use the internal mammary vessels as the preferred recipient vessels for our anastomosis. Either through the old scar or the mastectomy incision, the space between the 3rd and 4th ribs is palpated. If space is large, a rib sparing approach is possible. If not, the cartilage of the 3rd rib is removed. The pectoralis muscle is split, leaving a groove for the vessels and the intercostal muscles to be excised. The vessels are dissected free for the length of the space.[3][7][12][13][11]
Complications
All surgeries have complications, and this is no exception. The highest complications are wound-related and include infections, seromas, hematomas, skin flap necrosis, and delayed healing. In some series, wound complications can be up to 30% to 50%. Problems with the microsurgery occur commonly as well, with flap loss rates from venous or arterial thrombosis 1 to 4% and fat necrosis of 5% to 40%. If choosing the mammary arteries, there is a small risk of pneumothorax. Donor site complications depend on the flap chosen. For abdominally based flaps, complications include abdominal bulge, hernia, and weakness. Thigh flaps have a high rate of breakdown, sensory disturbance to the thigh, and risk of lymphedema. Gluteal flaps can risk sciatic exposure or lack of padding and also have wound healing complications.[2][3][6][8][15]
Clinical Significance
Autologous breast reconstruction is an important addition to the reconstructive armamentarium. Since there are more options for donor sites and patients can avoid an internal or external prosthesis, it may allow more patients to choose mastectomy, allowing them to have clinical screening and avoid mammograms. There is a significant psychological impact of the loss of a breast, and performing reconstruction can help by enabling patients to have improved self-confidence and mental health.[2][6]
Enhancing Healthcare Team Outcomes
Plastic surgeons are always involved in breast reconstruction. However, they must work very closely with the oncologic breast surgeon to determine the best treatment path for the patient. For free flaps, the skin incision/excision is typically planned by both teams so that the resection can be oncologic, but also as cosmetically sensitive as possible. Other specialists including radiation oncologists and medical oncologists are also part of the patient's care team and plans for chemotherapy and radiation impact reconstruction timing and choice. Patients with very complex diagnoses are frequently discussed at an interprofessional tumor board to determine their best options. It is also necessary to have an excellent nursing team who are specialty trained to monitor free flaps and can alert the plastic surgeon immediately if there is a problem. The interprofessional approach is the best means by which to increase optimal patient care and satisfaction, as well as minimizing risks and complications. [Level V]
Media
References
Rozen WM, Rajkomar AK, Anavekar NS, Ashton MW. Post-mastectomy breast reconstruction: a history in evolution. Clinical breast cancer. 2009 Aug:9(3):145-54. doi: 10.3816/CBC.2009.n.024. Epub [PubMed PMID: 19661037]
Serletti JM, Fosnot J, Nelson JA, Disa JJ, Bucky LP. Breast reconstruction after breast cancer. Plastic and reconstructive surgery. 2011 Jun:127(6):124e-135e. doi: 10.1097/PRS.0b013e318213a2e6. Epub [PubMed PMID: 21617423]
Arnez ZM, Pogorelec D, Planinsek F, Ahcan U. Breast reconstruction by the free transverse gracilis (TUG) flap. British journal of plastic surgery. 2004 Jan:57(1):20-6 [PubMed PMID: 14672674]
Level 3 (low-level) evidenceHartrampf CR, Scheflan M, Black PW. Breast reconstruction with a transverse abdominal island flap. Plastic and reconstructive surgery. 1982 Feb:69(2):216-25 [PubMed PMID: 6459602]
Level 3 (low-level) evidenceWilkins EG, Alderman AK. Breast reconstruction practices in north america: current trends and future priorities. Seminars in plastic surgery. 2004 May:18(2):149-55. doi: 10.1055/s-2004-829049. Epub [PubMed PMID: 20574493]
Macadam SA, Bovill ES, Buchel EW, Lennox PA. Evidence-Based Medicine: Autologous Breast Reconstruction. Plastic and reconstructive surgery. 2017 Jan:139(1):204e-229e. doi: 10.1097/PRS.0000000000002855. Epub [PubMed PMID: 28027256]
Tuinder SMH, Beugels J, Lataster A, de Haan MW, Piatkowski A, Saint-Cyr M, van der Hulst RRWJ, Allen RJ. The Lateral Thigh Perforator Flap for Autologous Breast Reconstruction: A Prospective Analysis of 138 Flaps. Plastic and reconstructive surgery. 2018 Feb:141(2):257-268. doi: 10.1097/PRS.0000000000004072. Epub [PubMed PMID: 29019861]
Patel NG, Ramakrishnan V. Microsurgical Tissue Transfer in Breast Reconstruction. Clinics in plastic surgery. 2017 Apr:44(2):345-359. doi: 10.1016/j.cps.2016.12.002. Epub 2017 Feb 13 [PubMed PMID: 28340667]
Healy C, Allen RJ Sr. The evolution of perforator flap breast reconstruction: twenty years after the first DIEP flap. Journal of reconstructive microsurgery. 2014 Feb:30(2):121-5. doi: 10.1055/s-0033-1357272. Epub 2013 Oct 25 [PubMed PMID: 24163223]
Howard MA, Mehrara B. Emerging trends in microsurgical breast reconstruction: deep inferior epigastric artery perforator (DIEP) and the superior gluteal artery perforator (SGAP) flaps. International journal of surgery (London, England). 2005:3(1):53-60 [PubMed PMID: 17462259]
Allen RJ Jr, Lee ZH, Mayo JL, Levine J, Ahn C, Allen RJ Sr. The Profunda Artery Perforator Flap Experience for Breast Reconstruction. Plastic and reconstructive surgery. 2016 Nov:138(5):968-975. doi: 10.1097/PRS.0000000000002619. Epub [PubMed PMID: 27391834]
Allen RJ, Tucker C Jr. Superior gluteal artery perforator free flap for breast reconstruction. Plastic and reconstructive surgery. 1995 Jun:95(7):1207-12 [PubMed PMID: 7761507]
Level 3 (low-level) evidenceAllen RJ, Levine JL, Granzow JW. The in-the-crease inferior gluteal artery perforator flap for breast reconstruction. Plastic and reconstructive surgery. 2006 Aug:118(2):333-9 [PubMed PMID: 16874198]
Muller-Sloof E, de Laat HEW, Hummelink SLM, Peters JWB, Ulrich DJO. The effect of postoperative closed incision negative pressure therapy on the incidence of donor site wound dehiscence in breast reconstruction patients: DEhiscence PREvention Study (DEPRES), pilot randomized controlled trial. Journal of tissue viability. 2018 Nov:27(4):262-266. doi: 10.1016/j.jtv.2018.08.005. Epub 2018 Aug 14 [PubMed PMID: 30126630]
Level 3 (low-level) evidenceVega S, Smartt JM Jr, Jiang S, Selber JC, Brooks CJM, Herrera HR, Serletti JM. 500 Consecutive patients with free TRAM flap breast reconstruction: a single surgeon's experience. Plastic and reconstructive surgery. 2008 Aug:122(2):329-339. doi: 10.1097/PRS.0b013e31817f45cb. Epub [PubMed PMID: 18626347]
Level 2 (mid-level) evidence