 Catheter Management of Ventricular Septal Defect
Catheter Management of Ventricular Septal Defect
Introduction
Ventricular septal defect (VSD) is currently the most common congenital heart disease in the pediatric population (see Image. Ventricular Septal Defect). As the population ages, VSDs have become the second most common congenital heart disease, right behind bicuspid aortic valves. This disease is most likely due to the early spontaneous closure of VSDs.[1] While many VSDs close naturally, some do not close spontaneously. Depending on the size and flow of the VSD, hemodynamic compromise may occur. Treatment options include surveillance for small, asymptomatic VSDs in the absence of pulmonary artery hypertension; surgical repair is recommended for medium to large-sized VSDs in the presence of hemodynamic compromise. Traditionally, VSDs have been closed with an open approach, but now there is a new emerging intervention- the percutaneous transcatheter closure currently reserved for nonsurgical candidates.[2] Whereas the closure of a VSD can pose a significant risk to the rare patient, some congenital disabilities benefit from the percutaneous closure of a VSD. The very first percutaneous transcatheter VSD closure was performed in 2013 by Lin et al.[3]
Indications
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Indications
Ventricular Septal Defect Closure
According to the American Heart Association/American College of Cardiology, recommendations for VSD closure are as follows[2]:
- Patients with left ventricular volume overload and hemodynamically significant shunts (Qp: Qs ≥1.5:1) should undergo VSD closure if pulmonary artery (PA) systolic pressure is less than 50% systemic and pulmonary vascular resistance is less than one-third systemic. [COR I, LOE B]
- Surgical closure of perimembranous or supra cristal VSD is reasonable in adults in the presence of worsening aortic regurgitation (AR) secondary to the VSD. [COR IIa, LOE C]
- Surgical closure of a VSD may also be reasonable in adults with a history of infective endocarditis secondary to a VSD if not otherwise contraindicated. [COR IIb, LOE C]
- Consider VSD closure in the presence of a net left-to-right shunt (Qp: Qs ≥1.5:1) when PA systolic pressure is 50% or more than systemic and/or pulmonary vascular resistance is greater than one-third systemic. [COR IIb, LOE C]
- Surgical VSD closure is currently the recommended form of VSD closure. While percutaneous transcatheter VSD closure is recommended in patients who are non-surgical candidates. Transcatheter closure is also a viable alternative to surgical intervention if conduction abnormalities are a concern.
Contraindications
VSD Closure
According to the AHA/ACC guidelines, VSD closure should not be performed in adults with severe PAH with PA systolic pressure greater than two-thirds systemic, pulmonary vascular resistance greater than two-thirds systemic, and a net right-to-left shunt.[2]
Personnel
For successful percutaneous catheterization intervention of a VSD, an interprofessional team is needed, including but not limited to an interventional/structural cardiologist, pediatric cardiologist, anesthesiologist, cardiothoracic surgeon, radiologist, and ancillary staff.[4]
Preparation
Preparation for percutaneous transcatheter VSD closure begins with an evaluation of the patient to assess if he qualifies for catheter closure. This evaluation is most commonly done via transthoracic echocardiogram, including the sizing and location of the VSD, followed by cardiac catheterization to evaluate for the presence of pulmonary hypertension. Additional pre-operative tests may include chest X-rays, electrocardiograms, and blood tests to assess the patient's general health and kidney function. Before the procedure, all patients undergoing VSD closure should be pre-treated with antiplatelet therapy, commonly with both aspirin and clopidogrel daily. Warfarin therapy alone may be an option in patients who need chronic antithrombotic therapy followed by bridging with low-molecular-weight heparin before and after VSD closure. An intravenous antibiotic dose should be given one hour before percutaneous access, typically cefazolin or vancomycin, if they are allergic to penicillin. To avoid left atrial hypovolemia, patients should also receive IV normal saline before and during the procedure. All patients should be evaluated by anesthesia before the procedure. Typically, patients under ten years of age undergo general anesthesia, and conscious sedation is used for patients older than ten years of age.[5]
Technique or Treatment
The catheterization procedure is begun by obtaining vascular access through the right femoral vein and right femoral artery. Profiling of the VSD should be done through angiographic evaluation of the left ventricle at a 55 degrees/20degrees left anterior oblique projection/cranial. The location, size, and relationship of the VSD with the aortic valve should be carefully assessed. The diameter of the VSD should be measured at the peak of the diastolic phase. The occlude should be selected based on VSD type and measurements. If the VSD type and sizes are consistent with intervention, then an arteriovenous (AV) circuit is created via a femoral vein approach at the access site. Once the appropriate occlude is selected, a 5 Fr catheter should be advanced from the LV across the defect. Advance a long sheath (6 to 12 Fr) to the LV through the AV circuit and position it under the aortic valve. Through the long sheath, deploy the VSD occluder using fluoroscopic and echocardiographic guidance. Angiography of the LV and ascending aorta should be performed to verify complete occlusion and to identify any new-onset aortic valve regurgitation. After the intervention, patients should be monitored inpatient for 24 hours with continuous ECG monitoring as this period has the highest arrhythmia risk. All patients should receive aspirin (5 mg/kg daily) to decrease the risk of thromboembolism.[5]
Complications
Arrhythmias: The most common complication of VSD closure. Patients have a 4.6 to 17‰ risk of arrhythmia following device implantation.[6] While most arrhythmias occur within one day to one-week post-operation, transient atrioventricular block and complete heart block have been noted intraoperatively with a prevalence of about 1.6%. Common post-operative arrhythmias include: right bundle branch block (RBBB) 6.4%, left bundle branch block (LBBB) 1.6%, sinus tachycardia (ST) 3.2%, second atrioventricular block (AVB) 1.09%.[7] The risk of pacemaker dependence is approximately 3.8%, according to Carminatti et al.[8] There is evidence to support various arrhythmia risks depending on the VSD type. For example, complete heart block seems to occur more often in perimembranous VSD compared to muscular VSDs.[8]
Trivial residual shunt: A trivial residual shunt occurs when venous blood enters the bloodstream without passing through functional lung tissue without hemodynamic compromise. Approximately 5 to 6.7% of patients who undergo VSD closure will develop a trivial residual shunt.[8][9]
Aortic Regurgitation: According to one study, the rate of AR following VSD device closure is approximately 3.4‰.[6]
Tricuspid Regurgitation: TR was noted in several case studies. It is thought that post-VSD closure TR is secondary to direct trauma to the tricuspid valve.[10]
Iatrogenic embolization of VSD occluder: The rate of device embolization occurring approximately 0.82%.[11] Data suggest an association between device embolization and small sizing of devices along with incompetent aortic rims. Despite possible device embolization, most embolic phenomena are retrievable by percutaneous catheter extraction.
Endocarditis: While rare, occurrence data varies from 0.3% to 0.9%. More extensive studies may be needed to discern a more accurate prevalence.[12]
Pulmonary hypertension: According to Jorveit et al., the approximate rate of development of PH status post VSD closure is about 0.3‰.[6][13]
Clinical Significance
The development of percutaneous transcatheter interventions for VSD is a considerable advancement in the world of cardiology today. While VSD is one of the most common cardiac defects worldwide, this technique now provides a treatment option for a large population who previously would have only been offered medical management.
Enhancing Healthcare Team Outcomes
The development of catheter-based treatment for cardiac structural defects has introduced new complexity to the field of cardiology. The need for interprofessional communication has become increasingly more vital than ever to optimize patient-centered care and improve outcomes. Percutaneous transcatheter interventions require care coordination by physicians in specialties, including cardiac imaging, interventional cardiology, pediatric cardiology, anesthesia, cardiothoracic surgery, and radiology.[4] All institutions with interventional capabilities currently performing percutaneous VSD interventions or planning to complete the percutaneous interventions of the VSD in the future must have protocols in place to maximize team communication and treatment plans for each patient.
To provide the best outcomes, an interprofessional team perioperatively should include specialty-trained: clinicians, pharmacists, and nurses. Team coordination of patient and family education and monitoring prior to and after the procedure should occur. The pharmacist should assist with evaluating for potential drug-drug interactions and any drug-induced complications. The clinical team should provide open lines of communication. An interprofessional approach will lead to the best outcomes. [Level 5]
Media
(Click Image to Enlarge)
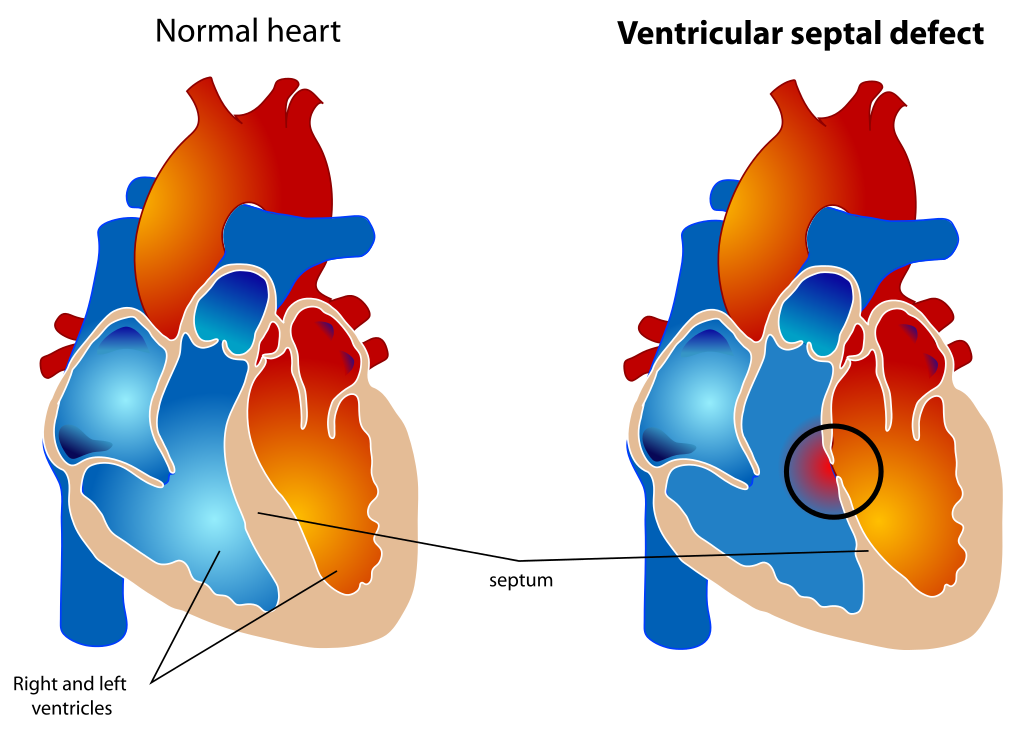
Ventricular Septal Defect. The diagram depicts a healthy heart on the left and a heart with the 4 anatomic malformations characteristic of the Tetralogy of Fallot on the right.
Mariana Ruiz, Public Domain, via Wikimedia Commons.
References
Liu F, Yang YN, Xie X, Li XM, Ma X, Fu ZY, Chen BD, Huang Y, Shan CF, Ma YT, Gao XM. Prevalence of Congenital Heart Disease in Xinjiang Multi-Ethnic Region of China. PloS one. 2015:10(8):e0133961. doi: 10.1371/journal.pone.0133961. Epub 2015 Aug 28 [PubMed PMID: 26317413]
Lock JE, Block PC, McKay RG, Baim DS, Keane JF. Transcatheter closure of ventricular septal defects. Circulation. 1988 Aug:78(2):361-8 [PubMed PMID: 3396173]
Lin CH, Huddleston C, Balzer DT. Transcatheter ventricular septal defect (VSD) creation for restrictive VSD in double-outlet right ventricle. Pediatric cardiology. 2013 Mar:34(3):743-7. doi: 10.1007/s00246-012-0337-1. Epub 2012 May 12 [PubMed PMID: 22580772]
Level 3 (low-level) evidenceKutty S,Delaney JW,Latson LA,Danford DA, Can we talk? Reflections on effective communication between imager and interventionalist in congenital heart disease. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2013 Aug; [PubMed PMID: 23768692]
Thakkar B, Patel N, Bohora S, Bhalodiya D, Singh T, Madan T, Shah S, Poptani V, Shukla A. Transcatheter device closure of perimembranous ventricular septal defect in children treated with prophylactic oral steroids: acute and mid-term results of a single-centre, prospective, observational study. Cardiology in the young. 2016 Apr:26(4):669-76. doi: 10.1017/S1047951115001018. Epub 2015 Jun 24 [PubMed PMID: 26105182]
Level 2 (mid-level) evidenceJortveit J, Leirgul E, Eskedal L, Greve G, Fomina T, Døhlen G, Tell GS, Birkeland S, Øyen N, Holmstrøm H. Mortality and complications in 3495 children with isolated ventricular septal defects. Archives of disease in childhood. 2016 Sep:101(9):808-13. doi: 10.1136/archdischild-2015-310154. Epub 2016 Apr 18 [PubMed PMID: 27091847]
Xie YM, Zhang ZW, Li YF, Qian MY, Wang HS. [Management of the arrhythmia around the procedure of transcatheter closure of ventricular septal defects in pediatric patients]. Zhonghua xin xue guan bing za zhi. 2005 Dec:33(12):1092-4 [PubMed PMID: 16563277]
Level 2 (mid-level) evidenceCarminati M, Butera G, Chessa M, De Giovanni J, Fisher G, Gewillig M, Peuster M, Piechaud JF, Santoro G, Sievert H, Spadoni I, Walsh K, Investigators of the European VSD Registry. Transcatheter closure of congenital ventricular septal defects: results of the European Registry. European heart journal. 2007 Oct:28(19):2361-8 [PubMed PMID: 17684082]
Level 2 (mid-level) evidenceChessa M, Carrozza M, Butera G, Negura D, Piazza L, Giamberti A, Feslova V, Bossone E, Vigna C, Carminati M. The impact of interventional cardiology for the management of adults with congenital heart defects. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2006 Feb:67(2):258-64 [PubMed PMID: 16416475]
Matyal R, Wang A, Mahmood F. Percutaneous ventricular septal defect closure with Amplatzer devices resulting in severe tricuspid regurgitation. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2013 Nov 15:82(6):E817-20. doi: 10.1002/ccd.24803. Epub 2013 Jul 1 [PubMed PMID: 23553968]
Level 3 (low-level) evidenceDurham JA, Scansen BA, Bonagura JD, Schober KE, Cheatham SL, Cheatham JP. Iatrogenic embolization and transcatheter retrieval of a ventricular septal defect occluder in a dog. Journal of veterinary cardiology : the official journal of the European Society of Veterinary Cardiology. 2015 Dec:17(4):304-13. doi: 10.1016/j.jvc.2015.08.003. Epub 2015 Oct 26 [PubMed PMID: 26515420]
Nguyen HL, Phan QT, Dinh LH, Tran HB, Won H, Thottian JJ, Duc DD, Quang TN, Kim SW. Nit-Occlud Lê VSD coil versus Duct Occluders for percutaneous perimembranous ventricular septal defect closure. Congenital heart disease. 2018 Jul:13(4):584-593. doi: 10.1111/chd.12613. Epub 2018 Jul 17 [PubMed PMID: 30019378]
Bambul Heck P, Eicken A, Kasnar-Samprec J, Ewert P, Hager A. Early pulmonary arterial hypertension immediately after closure of a ventricular or complete atrioventricular septal defect beyond 6months of age. International journal of cardiology. 2017 Feb 1:228():313-318. doi: 10.1016/j.ijcard.2016.11.056. Epub 2016 Nov 9 [PubMed PMID: 27866021]