Introduction
Cardiac surgery is the specialty of medicine concerning the surgical treatment of pathologies related to the heart and thoracic aorta (see Image. Cardiac Surgery). The spectrum of modern cardiac surgery can be understood by its history beginning at the end of the 19th century.[1][2] Since then, cardiac surgery developed through the work of numerous dedicated surgeons offering more and more treatments for diverse cardiac pathology. This development is still ongoing today.[3][4]
In 1882, Billroth performed the first pericardiectomy. The first successful treatment of cardiac trauma was done by Ludwig Rehn when he operated on a cardiac stab wound in 1896 against the widely held belief that the heart is not an organ on which surgeons should operate.[5] The development of cardiopulmonary bypass was necessary to reach the structures of interest and was pushed by the high mortality of early cardiac operations like embolectomy (first completed by Trendelenburg).[6]
Surgical revascularization is 1 option to relieve ischemic heart disease with complicated atherosclerosis.[7] Vineberg implanted the left internal mammary artery (LIMA) into the anterior free wall forming no direct anastomoses to the coronary vessels.[8] In earlier experiments, he has observed that collaterals develop when ischemia is present. During the 1960s, several surgeons in different locations pioneered the first coronary artery bypass grafting (CABG) operations.[9] The era of reversing coronary artery disease started with the invention of cardiac catheterization by Forssman in 1929 and the injection of contrast media to visualize coronary vessels and locate stenosis by Shirey in 1962. Bypass grafting and interventional revascularisation form the 2 main possibilities to treat ischemic heart disease besides drug treatment.
Surgical treatment of valvulopathies started closed mitral commissurotomy by passing a finger or instrument through the narrow orifice of the mitral stenosis to dilate or cut it as did Cutler in 1923 for the first time. The Hufnagel cage and ball valve was the first artificial valve introduced in 1952. It was placed in the descending thoracic aorta to prohibit blood flow reversal in aortic regurgitation. In 1967 a similarly structured valve, the Edwards cage and ball valve, had been implanted 1000 times for mitral valve disease.[10] Surgical techniques improved from early single-valve procedures to 4-valve replacement in 1992. Special techniques were introduced, for example, the Ross procedure replacing the aortic valve with pulmonic valve autograft. To treat proximal aortic dissection or aneurysm, Bentall implanted an artificial aortic valve combined with ascending aortic vessel prosthesis.
In 1944, cardiac surgeons Blalock, Taussig, and Thomas first forayed into the field of congenital heart lesions when they operated on the tetralogy of Fallot, 1 of the cyanotic heart lesions.[11]There are also acyanotic heart lesions such as pulmonary stenosis.[12]
Regarding cardiac arrhythmias, the Cox-Maze procedure offers surgical treatment of atrial fibrillation. The evolution of cardiac pacemakers started by applying external electrodes to stimulate the heart. Lillehei placed electrodes directly into the heart during open-heart surgery. The first implanted pacemaker lasted only 8 hours. Modern aggregates offer long-lasting solutions to diverse rhythm abnormalities.[13]
In 1967, several surgical teams around the world performed the first heart transplantations: Barnard in South Africa, Shumway in Stanford (offering increased post-transplant survival by adding immunosuppressive treatment), and Kantrowitz with pediatric transplantation in New York.[14]
Some devices can supply mechanical circulatory support. Since 1963, the intra-aortic balloon pump (IABP) enhanced left ventricular function through counterpulsation. Open heart surgery requires a cardiopulmonary bypass (CPB) to temporarily replace the human heart and lung with an external circuit consisting of pumps and an oxygenation membrane. Artificial hearts were first applied extracorporal in 1982. Later devices allowed for implantation.
Cardiac surgery represents high operative and perioperative risk requiring professional staff and advanced equipment. Besides the diseases that require cardiac surgery, the perioperative period shows a variety of characteristic pathologies: systemic inflammatory response following CBP, myocardial stunning and low cardiac output syndrome, arrhythmias, massive transfusion requirements, and multiorgan involvement with kidney injury, stroke, and respiratory distress.
With the surge of interventional and minimally invasive methods to treat cardiac pathologies, cardiology and cardiac surgery must adapt to these changes.[15] As Lytle and Mack described in their 2005 editorial, "The times they are changing," the field of cardiac surgery is undergoing a fundamental transformation. In his presidential address, Guyton said: "If we do not embrace innovation, we will become its victims." Recent developments include the upcoming cardiac arrest centers, broader and simpler application of extracorporeal membrane oxygenation (ECMO), organizational changes such as fast-track hospital stay, interprofessional decision-making by heart teams, and challenges posed by an aging patient population).[16][17][18][19]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
The rib cage surrounds the chest organs. This allows additional protection against environmental influences but confers a higher difficulty for surgeons to reach the structures of interest. Surrounded by the ribs laterally, the sternum anteriorly, and the thoracic spine posteriorly, 2 compartments can be divided: the bilateral pleural and the mediastinal compartment. The pleural spaces contain both wings of the lung. The mediastinum encloses many structures, such as the esophagus, the trachea, the vena cava superior and inferior, thoracic arteries, the vagus nerve, the azygos vein, lymphatic vessels, the thymic remnant, and the pericardial sac containing the heart. The confined space of the pericardial sac poses the heart at risk for tamponade physiology. This occurs when hematoma compresses the heart, obstructing normal cardiac blood flow.[20]
As blood flows through the 4 chambers of the heart, it passes 4 valves. Reaching the right atrium via the superior and inferior vena cava, blood passes through the tricuspid entering the right ventricle and pulmonary valve to be released into pulmonary circulation. The right heart is a low-pressure system compared to the left heart, which supplies the systemic circulation that requires more myocardial tissue to produce higher pressure. Blood is collected from the 4 pulmonary veins to enter the left atrium and passes through the mitral valve, the left ventricle, and the aortic valve into the systemic circulation.[21] The aortic root comprises the sinuses of Valsalva, the interleaflet triangles, the sinotubular junction, and the aortic valve annulus with leaflet attachment; the 3 leaflets named according to the coronary arteries right, left, and noncoronary cusp (RCC, LCC, and NCC respectively). Since the parts of the valve apparatus are interdependent, surgeons might need to address connected structures to relieve pathology.
All cardiac valves are surrounded by an annulus that poses difficulties in creating artificial grafts without leakage. To illustrate the importance of anatomy and physiology, the example of mitral valve replacement and the negative consequence of left ventricular outflow tract obstruction can be named. While the aortic valve annulus is circular, the mitral valve annulus is more crescent-shaped, requiring more sophisticated prosthesis and implantation technique. A plentitude of artificial valve models expresses this, each having advantages and disadvantages regarding anatomic, physiologic, technical, and procedural characteristics. Having a closer look at the mitral valve, the annulus and the anterior and posterior valve each consist of 3 segments (A1 to A3 and P1 to P3) and are connected to the subvalvular apparatus, which consists of chordae tendineae connecting the valve leaflet to the papillary muscles.
The heart has an intrinsic rhythmic activity that can be slowed or quickened through the influence of the autonomic nervous system, with sympathetic reaction leading to accelerated heart rate and parasympathetic reaction leading to decelerated heart rate. The cardiac electrical conduction is structured hierarchically and runs from the sinus node through the right atrium to the atrioventricular node, the His bundle, and via the Purkinje fibers to the ventricles. The anatomic and physiologic role of cardiac electrical activity is important to understand its clinical implications. Following cardiac surgery, atrial fibrillation is common. The Maze procedure applies anatomical knowledge to interrupt uncontrolled atrial activity to solve atrial fibrillation. The implantation of artificial heart valves can lead to atrioventricular blocking due to proximity to the valve area.
The quality of vessel grafts is important for the outcome of CABG surgery. Surgeons can harvest a variety of vessels. Arteries are of superior, long-term durability compared to veins.[22] The quality of vessel grafts can be assessed preoperatively. Commonly used vessels include the left and right internal mammary arteries (RIMA), radial arteries, and saphenous veins (SVG). Due to vessel length, the RIMA is usually connected to the right coronary artery (RCA) and the LIMA to the left anterior descending artery (LAD). The posterior descending artery (PDA) and left circumflex artery (LCX) are commonly supplied with a venous bypass graft. Venous grafts can be used to create bypasses to smaller branches, such as the diagonal branches of the LAD and marginal branches of the LCX.[23]
Indications
Current guidelines describe indications for cardiac surgery.[24][25] Although some recommendations differ, the general indications are the same. Decision-making is done in consensus, with cardiologists meeting as a heart team. Prior imaging with echocardiography, computed tomography (CT), or magnet resonance imaging (MRI) is routinely necessary.
In valvular heart disease, stenotic lesions and regurgitation can be differentiated. In general classification of valvulopathies is a 3-step approach, mild, moderate, and severe, in contrast to 4-step angiographic grading done in the catheterization laboratory. Severe valve regurgitation or stenosis requires intervention. Depending on the valve affected, surgeons offer replacement or reconstruction.[24]
With the upcoming transcatheter aortic valve replacement (TAVR) introduced by Cribrier in 2002, the heart team decides on the surgical or interventional treatment of severe aortic stenosis.[25][26] Aortic stenosis is severe when the opening area becomes less than 1 square centimeter. The operative risk is an important consideration. According to EUROScore or STS score, people with high operative risk should be treated with TAVR.[27] Lower risk scores allow for surgical aortic valve replacement (SAVR). High operative risk includes patients with porcelain aorta, liver cirrhosis, child-pugh B and C, previous CABG with lima, and frailty. Aortic valve endocarditis requires SAVR. The 2010 PARTNER Trial compared outcomes of TAVR and SAVR, thus influencing patient selection.
Decision-making in aortic regurgitation requires pathophysiologic consideration. Aortic regurgitation due to enlargement of the ascending aorta requires operative treatment. Patients with symptoms due to severe aortic regurgitation also need surgery. Asymptomatic patients with decreased left ventricular ejection fraction (LVEF) or increased left ventricular residual volumes should also receive operation. (LVEF less than 50%, LVEDD greater than 70 mm, or LVESD greater than 50 mm). Echocardiographic characteristics of severe aortic stenosis are vena contracta of more than 6 mm, pressure half-time of less than 200 ms, effective regurgitant office area of more than 30, and regurgitant volume of more than 60 ml.
Mitral stenosis is mainly caused by rheumatic heart disease, which is rare in industrialized countries. Mitral regurgitation (MR) is treated depending on the etiology.[28] Primary MR describes the structural pathology of the mitral valve apparatus itself, including leaflets, annulus, chords, and papillary muscles. Primary MR is classified according to Carpentier. Secondary, functional MR is caused by left ventricular dilatation, ischemia, and tethering. In contrast to aortic valve pathology, lesions affecting the mitral valve are primarily treated with repairing techniques, including Alfieri edge-to-edge, resection, annuloplasty, and notochordal. Severe MR is defined by echocardiographic criteria with vena contracta more than 7 mm, a regurgitant volume of more than 60 ml, and a regurgitant fraction of more than 50%.[29]
Multivalvular disease comprises an especially difficult decision-making since studies are rare.[30] When an operation for left heart disease is considered, secondary tricuspid regurgitation can be corrected concomitantly. Criteria for assessing the right ventricle and the tricuspid valve to decide the operation are unclear. A tricuspid area diameter of more than 40 mm indicates tricuspid valve repair when mitral valve surgery is planned.[31][32][33][34][35][36]
Cardiac surgery offers treatment for a wide range of cardiac rhythm disturbances through the implantation of pacemakers, such as dual chamber devices for atrioventricular blocks, defibrillators for ventricular arrhythmia, and cardiac synchronization therapy for advanced heart failure.
Congenital heart disease is divided into cyanotic and non-cyanotic lesions. Ventricular and atrial septal defects can be surgically closed. Special operation techniques have been developed for diseases such as Ebstein anomaly, tetralogy of Fallot, and transposition of the great vessels.
The most common benign tumor of the heart is an atrial myxoma. The most frequent malign cardiac tumor is a sarcoma. Secondary metastatic tumors to the heart are more frequent than primary cardiac tumors. Tumors may cause obstructive or embolic symptoms.
Pulmonary thrombendarterectomy may be necessary as a final treatment option for severe pulmonary embolism. With a certain extension of the thoracic aorta, operation of dissection and aneurysm and replacement with a vessel graft are indicated.
Surgical revascularization can be a preferred option for treating coronary artery disease (CAD).[39] Management decisions can be made by the heart team, where cardiologists and cardiac surgeons meet. Decision aids are the syntax score describing the complexity of CAD to decide between treatment by CABG or percutaneous coronary intervention (PCI). The left main stem and triple vessel disease are common indications for CABG. High-risk PCI patients with increased operative risk may be treated percutaneously despite difficult stenotic lesions.
Terminal heart failure, despite the best medical therapy, can be treated with cardiac resynchronization, implantation of assist devices or heart transplantation.[37] Resynchronization therapy is indicated in patients with a severe reduction in left ventricular function (LVEF less than 35%), symptoms described as NYHA III to IV, and an electrocardiogram showing the QRS complex to be longer than 130 ms. In times of organ shortage, assist devices are becoming increasingly popular.[38][39] Studies showed comparable outcomes between these 2 treatment options.[40] The REMATCH trial showed a 2-year survival advantage in patients with an assist device of 23% versus 8% with medical therapy. Slaughter and colleagues compared continuous flow assist devices to pulsatile flow LVADs. The median duration of support is 1.7 years for continuous flow and 0.6 years for pulsatile flow, with 88% and 79% of time spent outside the hospital, respectively.[41] Timing and selection of patients for assisted device implantation or heart transplantation is challenging. The INTERMACS classification and the Lietz-Mmiller destination risk score are useful tools in patient selection.
Contraindications
During operation preparation, risk factors and contraindications are evaluated. Since cardiac surgery is limited to advanced cardiac diseases, the benefits of operation as the last treatment option often outweigh the risks. To assess the operative risk the EuroScore risk stratification tool has been developed. There are many other risk tools, such as the Parsonnet score or the Society of Thoracic Surgeons (STS) score, to identify patients who are eligible for surgery.[42][43][44]
The instability of the patient may cause the operation to be postponed. In a patient with myocardial infarction planned for CABG, the time point of operation may be difficult to decide. Valve replacement in cases of endocarditis may require operating on a septic patient to control the infectious source.
Equipment
Cardiac surgery requires a lot of sophisticated equipment. For diagnostic purposes, pulmonary artery catheters, thermodilution techniques, pulse contour analysis, and ultrasound, among others, can be used to assess cardiac performance and disease. Critical issues related to cardiac output, volume responsiveness, and tissue oxygenation should be addressed.[45]
Equipment for treatment includes pacemakers, assist devices, extracorporeal membrane oxygenation (ECMO), and CPB. The application of CPB started in the 1930s when Gibbon used an external pump circuit to maintain life in Boston. The development was interrupted due to early drawbacks. Since then, modifications have been made. Dogliotti presented a partial heart and lung machine with a 1 liter per minute flow in 1951. Lewis first used the Hypothermic technique to reduce metabolic demand and injury to the heart in 1952 when closing an atrial septal defect. Lillehei came up with the idea of cross circulation. He temporarily replaced 1 dog's circulation by sharing it with another dog's circulation. A broader application was possible with the mechanical heart-lung machine by Kirklin, who modified the IBM Gibbon machine, increasing survivors' numbers. CPB allows for venting the heart and clearing the operating area.[46]
However, CPB comes with side effects. Exposure to the circuit elicits a systemic inflammatory response.[47] Changes to the operation technique, such as decreasing the time and size of CPB and changing the surface of the tubes, have an effect. The inflammatory response to CPB appears similar to infection triggered by an inflammatory reaction with increased inflammatory markers and signs of shock, but the time course is different, peaking on the first postoperative day and decreasing after that.[48] Cuthbertson described SIRS due to CPB as the ebb and flow process with initial reduced metabolic activity lasting 2 to 3 days, followed by a hypermetabolic phase lasting over a week. Several solutions have been proposed, such as aprotinin, heparin-coated CPB circuits, hemofiltration, leukofiltration, and off-pump coronary artery bypass (OPCAB).[49][50]
The use of CPB and assist devices requires frequent coagulation tests. Together with cases of postoperative hemorrhage, usual coagulation diagnostics such as international normalized ratio (INR) and partial thromboplastin time (PTT) and more detailed laboratory assessment, for example, rotational thrombelastography, activated clotting time (ACT) or certain coagulation factors (antithrombin III, fibrinogen, factor VII) may be necessary.[51]
Veno-venous ECMO includes an oxygenator and thus supports the respiratory system in adding oxygen and removing carbon dioxide. Veno-arterial ECMO not only allows for oxygenation and decarboxylation but also partially replaces cardiac output. Cannulation for VA-ECMO is either placed centrally in the ascending aorta at sternotomy, producing antegrade flow, or peripherally with cannulas placed in the external iliac artery and vein. ECMO can be used as a bridge to recovery, bridge to bridge, and bridge to transplant modality.
The use of intra-aortic balloon pumps has decreased since studies could not show any benefit in mortality. Initial physiologic consideration was improved diastolic coronary perfusion of the coronaries and pull effect during systole via counterpulsation of a balloon placed in the descending thoracic aorta.
Decision-making on which prosthetic heart valve to use is necessary for valve replacement. Mechanical heart valves offer higher resistance to structural valve degeneration (SVD) but require lifelong anticoagulation. Mechanical heart valves are recommended for younger patients. Bioprosthetic heart valves from porcine or pericardial tissue do not require anticoagulation but may suffer from earlier SVD and reoperation.[52] Bioprosthetic heart valves are indicated in women who wish to have children and older patients. Another option for aortic valve replacement is the Ross procedure, a pulmonic autograft placed in the aortic position.[53]
Personnel
After finishing medical school, graduates can apply for cardiac surgery training. The length and content of the postgraduate curriculum differ. With prior general surgical experience, cardiac surgery training can be completed with an additional 2 years of specialization. On the other hand, 6-year training programs include subspecialty training in minimally invasive surgery, adult cardiac surgery, pediatric and congenital cardiac surgery, vascular surgery, endovascular interventions, general thoracic surgery, or heart failure surgery.
Training is highly competitive and demanding due to the specialty's changing nature, with an increasingly elderly and multimorbid population, working hour restrictions, and new interventional methods. Recent evaluations have predicted shortages of professionals in different countries during the next decades.
The cardiac perioperative team includes additional disciplines besides cardiac surgeons. These include extracorporeal technologists, cardiac anesthesiologists, cardiac intensive care providers, surgical nurses, cardiologists, and radiologists, each with special training.
Preparation
When sending a patient for cardiac surgery, the following preparations should be taken:
- Blood tests to assess a broad range of body systems function (kidney and liver function tests, coagulation, complete blood count [CBC], electrolytes)
- ECG to check for normal cardiac rhythmic activity
- Echocardiography and cardiac catheterization to detect coronary artery disease and valvulopathies
- Chest x-ray or chest computed tomography to visualize thoracic comorbidities and plan operative technique
- Ultrasound of the neck vessels to evaluate stroke risk
- Ultrasound of lower extremity veins as possible grafts
Carotid doppler ultrasound examination should be done in patients with left main disease, peripheral vascular disease, carotid bruits, history of CVA, history of heavy tobacco use, and age older than 65 years. Further tests might be necessary when significant stenosis is detected, and an endarterectomy is performed.
Anticoagulation in the perioperative period needs special consideration. Platelet-inhibiting drugs should be stopped before operation, depending on the kind of medication. For example, clopidogrel should be stopped 5 days before surgery, and ASA and heparin can be continued until the operation. The benefit of revascularization should be balanced with bleeding risk. Strategies to reduce bleeding complications in patients requiring urgent surgery under the impact of anticoagulation include OPCAB, coagulation diagnostic and adapted management, coagulation factors and platelet transfusion, and antifibrinolytic agents.[54][55][56][55][57][58]
Technique or Treatment
Open heart surgery is the traditional approach to reach the heart via opening the thorax by sternotomy or upper hemisternotomy. CABG is 1 of the most frequent operations in the world. Once the pericardial sac is opened (with an inverted T incision sparing both side phrenic nerves), cannulation, CPB, and cardioplegia are applied, and the heart can be moved to identify the arteries of interest. Epiaortic ultrasound is used to assess atherosclerosis of the ascending aorta. When grafts have been harvested, they can be connected directly, y or t manner to the coronaries and the aorta. Following anastomis, the success is proven by checking flow rates through the grafts. After finishing CPB, the thorax and the sternum with wires are closed stepwise. Pleural and mediastinal suction drains are left in place for the following postoperative days. Intraoperatively placed epicardial electrodes connected to an external pacemaker help to treat rhythmic complications but are removed before hospital discharge.
OPCAB is especially useful in patients with high operative risk[59] and significant atherosclerosis of the ascending aorta because clamping during CPB may release thrombogenic material and cause a stroke. The no-touch technique avoids manipulating the potentially thromboembolic endothelial surface of the ascending aorta by using the internal mammary or innominate artery. It requires stabilizers and positioners for accurate anastomosis to the coronary vessels and is especially useful in patients with high operative risk. For these patients, surgical LIMA to LAD anastomosis combined with later PCI to other affected arteries as hybrid coronary staged revascularization may be advantageous compared to usual CABG.[60][61] The inflammatory response is the same with on-pump versus off-pump surgery, but other positive effects have been found. The transfusion requirements, for example, are reduced. The off-pump bypass may be feasible in up to 95% of CABG patients, but recently, only 20% of surgical revascularization has been done through this technique.
Minimally invasive surgery (MIDCAB) and endoscopic coronary artery bypass grafting (TECAB) are done through smaller incisions, video-guided, and with the help of special instruments. It requires additional training; hence, it is offered only in some centers. Patients may profit from less surgical trauma and faster postoperative convalescence. Many procedures can be done through a minimally invasive technique. Since the invention of robotic surgery, these systems have also found their way into the cardiac operating room.
The myocardium can be protected by using cardioplegia and hypothermia.[62] Different cardioplegic solutions are available. Cardioplegia can be applied to anterograde aortic root or retrograde through the coronary sinus.[63][64][65] Brain protection during cardiac surgery can be achieved via improving brain perfusion, decreasing thrombogenicity through changes to blood constituents, and anticoagulation.[66][67][66][68]
There are certain valve-repairing techniques such as bicuspidization, DeVega, and clover technique for the tricuspid valve and Alfieri edge-to-edge, foldoplasty, neochordae, and sliding plasty for the mitral valve. Depending on the requirement to replace or spare the aortic valve, aneurysms and dissections of the ascending aorta may be operated using David, Yacoub, or Bentall techniques.[69][70]
Complications
The overall mortality in cardiac surgery is between 2% and 3%. Major complications include postoperative bleeding, stroke, [71][72] renal failure, [73] mesenteric ischemia, atrial fibrillation, [74] cardiogenic shock,[75] and respiratory distress. Postoperative bleeding and hemorrhagic shock coagulation disorders such as heparin-induced thrombocytopenia are reasons why 10% to 20% of national blood products are consumed in cardiac surgery. Acute kidney injury occurs in up to 18% of patients undergoing cardiac surgery. Two percent of all require renal replacement therapy. The number of complications may serve as a quality indicator and influence reimbursement and patient choice.
Myocardial infarction following cardiac surgery is classified as type 5 myocardial infarction according to the universal classification of myocardial infarction. The incidence is between 5% to 10%. Diagnosing postoperative myocardial infarction is challenging since cardiac enzymes are routinely elevated due to manipulation during operation, and symptoms are influenced by postoperative status. Therefore, other diagnostic modalities should be emphasized, for example, electrocardiogram (ECG) echocardiography, and coronary angiography to assess bypass patency. Echocardiography may show septal wall motion abnormalities not related to myocardial ischemia. Signs of refractory schock, arrhythmias are highly suggestive of myocardial infarction. Myocardial infarction following CABG can be divided into graft-related and non-graft-related. Early graft dysfunction is observed in up to 3%. Non-graft-related causes include insufficient myocardial protection and embolization. Treatment strategies for type 5 myocardial infarction include conservative medical treatment, PCI, and redo cabg.[76][77][78]
Following mitral valve replacement (MVR), the left ventricular outflow tract (LVOT) obstruction can occur. This is described as systolic anterior motion (SAM) of the anterior mitral leaflet. Treatment is proposed in a step-wise manner. Starting with beta-blockers, increasing afterload with fluids, and allowing hypertension, and finally reoperation with different surgical techniques: edge-to-edge, posterior leaflet (PL) shortening, short neochord, sliding plasty, and ellipsoid excision of anterior leaflet (AL).[79] Preoperative risk factors include thick basal interventricular septum (IVS), small LV, a short distance between the interventricular septum and the mitral leaflet coaptation point, tall posterior leaflet, and an aorto-mitral angle of less than 120 degrees.[80][81][80]
Fever, edema, and increased inflammatory markers can be routinely observed in patients in the postoperative ward. Thus, it might be challenging to differentiate patients with true infection and evolving sepsis.[82] The time course can give additional information. Signs and symptoms of infection after the second to the third day of operation should prompt investigation for infection.[83]
Perioperative antibiotic prophylaxis is used to reduce postoperative infections. Guidelines recommend cephalosporine prophylaxis during the 24 to 48-hour perioperative period. Deep sternal wound infection (DSWI) is a unique postoperative complication of cardiac surgery occurring with a frequency of 0.4% to 4%. It may progress to mediastinitis resulting in great mortality. Treatment of DSWI consists of pathogen-specific antibiotics (frequently cultured strains include Staphylococcus aureus or Staphylococcus epidermidis treated with clindamycin or according to resistance pattern), surgical exploration, and application of negative-pressure wound therapy (NPWT).
Clinical Significance
Cardiac surgery plays an important role in cardiovascular health. The prevalence of cardiovascular diseases is increasing continuously due to the epidemiologic transition implicating atherosclerosis, hypertension, and associated lifestyle risk factors. Regarding costs, cardiac surgery represents 1% to 2% of the healthcare budget in the United States, with an average inpatient cost of $40,000, summing up to $20 billion in total. There is an increased demand for healthcare professionals specializing in cardiology and cardiac surgery.[84]
Enhancing Healthcare Team Outcomes
The heart team illustrates an excellent example of patient-centered care. Professionals in different fields of medicine, cardiologists, interventionalists, cardiac surgeons, radiologists, and other health care providers come together to find the best solution for the individual patient.[85]
Healthcare professionals caring for patients with assistive devices should be aware of unique device complications. Due to the necessity of anticoagulation, regular coagulation testing should be performed. If coagulation is not within target levels, thrombosis or bleeding can occur. High power output with increased LDH indicates pump thrombosis. Hemolysis and deranged coagulation leads to mucosal bleeding, hematuria, melena, and hemoptysis.[86] Studies have shown near equal outcomes comparing terminal heart failure patients treated with assist devices to heart transplantation. Nevertheless, 1-year survival without any adverse event might only be 30%.
Media
(Click Image to Enlarge)
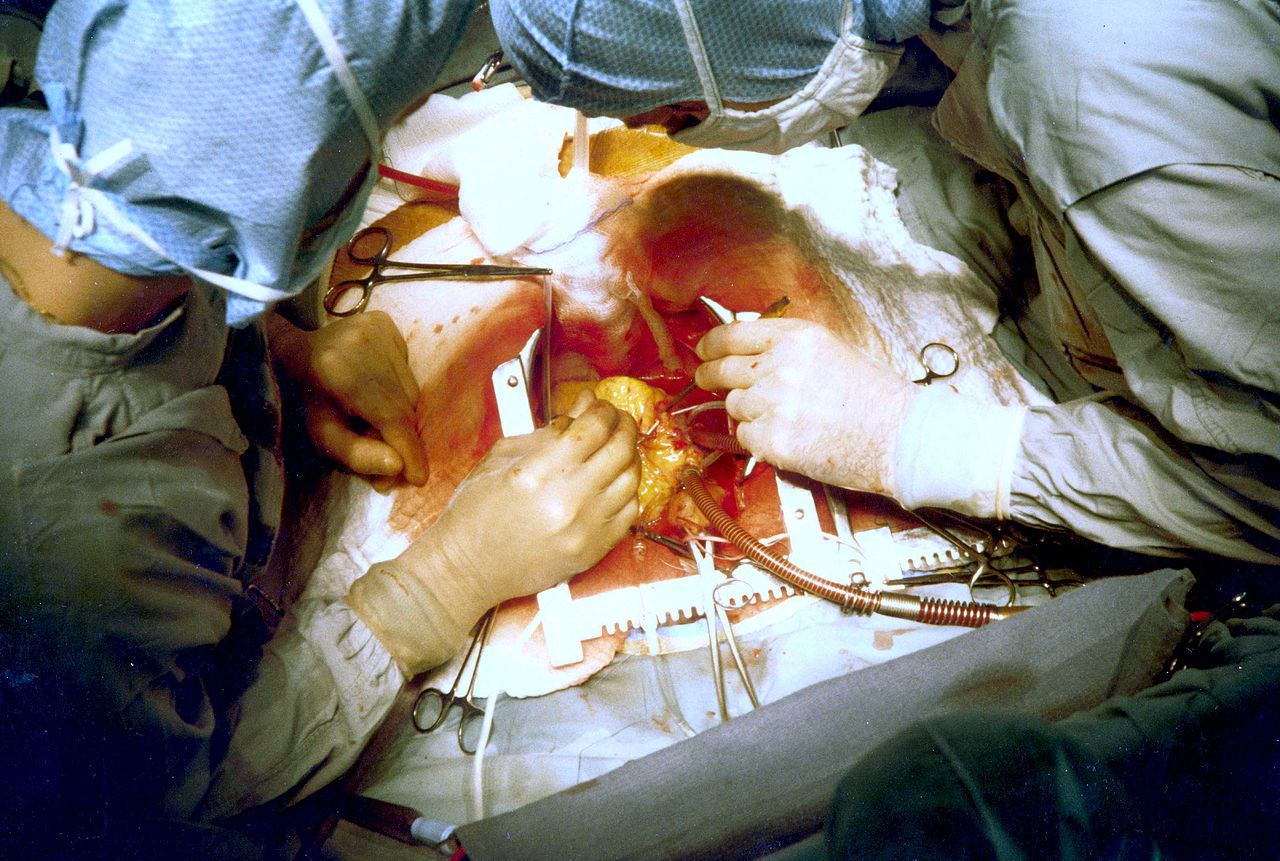
Cardiac Surgery. Two surgeons are performing coronary artery bypass surgery.
National Institutes of Health, Public Domain, via Wikimedia Commons
References
Aris A. Francisco Romero, the first heart surgeon. The Annals of thoracic surgery. 1997 Sep:64(3):870-1 [PubMed PMID: 9307502]
Braile DM, Godoy MF. History of heart surgery in the world. 1996. Revista brasileira de cirurgia cardiovascular : orgao oficial da Sociedade Brasileira de Cirurgia Cardiovascular. 2012 Jan-Mar:27(1):125-36 [PubMed PMID: 22729311]
Weisse AB. Cardiac surgery: a century of progress. Texas Heart Institute journal. 2011:38(5):486-90 [PubMed PMID: 22163121]
Richenbacher WE, Myers JL, Waldhausen JA. Current status of cardiac surgery: a 40 year review. Journal of the American College of Cardiology. 1989 Sep:14(3):535-44 [PubMed PMID: 2671092]
Blatchford JW 3rd. Ludwig Rehn: the first successful cardiorrhaphy. The Annals of thoracic surgery. 1985 May:39(5):492-5 [PubMed PMID: 3888132]
Hessel EA 2nd. A Brief History of Cardiopulmonary Bypass. Seminars in cardiothoracic and vascular anesthesia. 2014 Jun:18(2):87-100. doi: 10.1177/1089253214530045. Epub 2014 Apr 10 [PubMed PMID: 24728884]
Beck CS. THE DEVELOPMENT OF A NEW BLOOD SUPPLY TO THE HEART BY OPERATION. Annals of surgery. 1935 Nov:102(5):801-13 [PubMed PMID: 17856670]
VINEBERG A. Coronary vascular anastomoses by internal mammary arter implantation. Canadian Medical Association journal. 1958 Jun 1:78(11):871-9 [PubMed PMID: 13536944]
Konstantinov IE. Robert H. Goetz: the surgeon who performed the first successful clinical coronary artery bypass operation. The Annals of thoracic surgery. 2000 Jun:69(6):1966-72 [PubMed PMID: 10892969]
Level 3 (low-level) evidenceSTARR A, EDWARDS ML. Mitral replacement: clinical experience with a ball-valve prosthesis. Annals of surgery. 1961 Oct:154(4):726-40 [PubMed PMID: 13916361]
Lillehei CW, Varco RL, Cohen M, Warden HE, Gott VL, DeWall RA, Patton C, Moller JH. The first open heart corrections of tetralogy of Fallot. A 26-31 year follow-up of 106 patients. Annals of surgery. 1986 Oct:204(4):490-502 [PubMed PMID: 3767482]
Level 3 (low-level) evidenceBROCK RC. Pulmonary valvulotomy for the relief of congenital pulmonary stenosis; report of three cases. British medical journal. 1948 Jun 12:1(4562):1121-6 [PubMed PMID: 18865959]
Level 3 (low-level) evidenceAquilina O. A brief history of cardiac pacing. Images in paediatric cardiology. 2006 Apr:8(2):17-81 [PubMed PMID: 22368662]
DiBardino DJ. The history and development of cardiac transplantation. Texas Heart Institute journal. 1999:26(3):198-205 [PubMed PMID: 10524743]
Level 3 (low-level) evidenceNguyen TC, George I. Beyond the hammer: the future of cardiothoracic surgery. The Journal of thoracic and cardiovascular surgery. 2015 Mar:149(3):675-7. doi: 10.1016/j.jtcvs.2014.11.091. Epub 2014 Dec 4 [PubMed PMID: 25623909]
Mullany D, Shekar K, Platts D, Fraser J. The rapidly evolving use of extracorporeal life support (ECLS) in adults. Heart, lung & circulation. 2014 Nov:23(11):1091-2. doi: 10.1016/j.hlc.2014.04.009. Epub 2014 Apr 24 [PubMed PMID: 25070684]
Level 3 (low-level) evidenceParissis H. Cardiac surgery: what the future holds? Journal of cardiothoracic surgery. 2011 Jul 27:6():93. doi: 10.1186/1749-8090-6-93. Epub 2011 Jul 27 [PubMed PMID: 21794111]
Level 3 (low-level) evidenceDrury NE, Nashef SA. Outcomes of cardiac surgery in the elderly. Expert review of cardiovascular therapy. 2006 Jul:4(4):535-42 [PubMed PMID: 16918272]
Pierri MD, Capestro F, Zingaro C, Torracca L. The changing face of cardiac surgery patients: an insight into a Mediterranean region. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2010 Oct:38(4):407-13. doi: 10.1016/j.ejcts.2010.02.040. Epub [PubMed PMID: 20399675]
Level 2 (mid-level) evidenceLeiva EH, Carreño M, Bucheli FR, Bonfanti AC, Umaña JP, Dennis RJ. Factors associated with delayed cardiac tamponade after cardiac surgery. Annals of cardiac anaesthesia. 2018 Apr-Jun:21(2):158-166. doi: 10.4103/aca.ACA_147_17. Epub [PubMed PMID: 29652277]
Sievers HH, Hemmer W, Beyersdorf F, Moritz A, Moosdorf R, Lichtenberg A, Misfeld M, Charitos EI, Working Group for Aortic Valve Surgery of German Society of Thoracic and Cardiovascular Surgery. The everyday used nomenclature of the aortic root components: the tower of Babel? European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2012 Mar:41(3):478-82. doi: 10.1093/ejcts/ezr093. Epub 2011 Dec 1 [PubMed PMID: 22345173]
Parsa CJ, Shaw LK, Rankin JS, Daneshmand MA, Gaca JG, Milano CA, Glower DD, Smith PK. Twenty-five-year outcomes after multiple internal thoracic artery bypass. The Journal of thoracic and cardiovascular surgery. 2013 Apr:145(4):970-975. doi: 10.1016/j.jtcvs.2012.11.093. Epub 2013 Feb 10 [PubMed PMID: 23402687]
Puskas JD, Yanagawa B, Taggart DP. Advancing the State of the Art in Surgical Coronary Revascularization. The Annals of thoracic surgery. 2016 Feb:101(2):419-21. doi: 10.1016/j.athoracsur.2015.10.046. Epub [PubMed PMID: 26777919]
Maganti K, Rigolin VH, Sarano ME, Bonow RO. Valvular heart disease: diagnosis and management. Mayo Clinic proceedings. 2010 May:85(5):483-500. doi: 10.4065/mcp.2009.0706. Epub [PubMed PMID: 20435842]
Thourani VH, Ailawadi G, Szeto WY, Dewey TM, Guyton RA, Mack MJ, Kron IL, Kilgo P, Bavaria JE. Outcomes of surgical aortic valve replacement in high-risk patients: a multiinstitutional study. The Annals of thoracic surgery. 2011 Jan:91(1):49-55; discussion 55-6. doi: 10.1016/j.athoracsur.2010.09.040. Epub [PubMed PMID: 21172485]
Level 2 (mid-level) evidenceHolmes DR Jr, Nishimura RA, Grover FL, Brindis RG, Carroll JD, Edwards FH, Peterson ED, Rumsfeld JS, Shahian DM, Thourani VH, Tuzcu EM, Vemulapalli S, Hewitt K, Michaels J, Fitzgerald S, Mack MJ, STS/ACC TVT Registry. Annual Outcomes With Transcatheter Valve Therapy: From the STS/ACC TVT Registry. Journal of the American College of Cardiology. 2015 Dec 29:66(25):2813-2823. doi: 10.1016/j.jacc.2015.10.021. Epub 2015 Nov 30 [PubMed PMID: 26652232]
Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S, PARTNER Trial Investigators. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. The New England journal of medicine. 2010 Oct 21:363(17):1597-607. doi: 10.1056/NEJMoa1008232. Epub 2010 Sep 22 [PubMed PMID: 20961243]
Level 1 (high-level) evidenceO'Gara PT, Grayburn PA, Badhwar V, Afonso LC, Carroll JD, Elmariah S, Kithcart AP, Nishimura RA, Ryan TJ, Schwartz A, Stevenson LW. 2017 ACC Expert Consensus Decision Pathway on the Management of Mitral Regurgitation: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. Journal of the American College of Cardiology. 2017 Nov 7:70(19):2421-2449. doi: 10.1016/j.jacc.2017.09.019. Epub 2017 Oct 18 [PubMed PMID: 29055505]
Level 3 (low-level) evidenceArgulian E, Borer JS, Messerli FH. Misconceptions and Facts About Mitral Regurgitation. The American journal of medicine. 2016 Sep:129(9):919-23. doi: 10.1016/j.amjmed.2016.03.010. Epub 2016 Apr 5 [PubMed PMID: 27059381]
Unger P, Clavel MA, Lindman BR, Mathieu P, Pibarot P. Pathophysiology and management of multivalvular disease. Nature reviews. Cardiology. 2016 Jul:13(7):429-40. doi: 10.1038/nrcardio.2016.57. Epub 2016 Apr 28 [PubMed PMID: 27121305]
Huttin O, Voilliot D, Mandry D, Venner C, Juillière Y, Selton-Suty C. All you need to know about the tricuspid valve: Tricuspid valve imaging and tricuspid regurgitation analysis. Archives of cardiovascular diseases. 2016 Jan:109(1):67-80. doi: 10.1016/j.acvd.2015.08.007. Epub 2015 Dec 23 [PubMed PMID: 26711544]
Dreyfus GD. Functional tricuspid pathology: To treat or not to treat? That is the question. The Journal of thoracic and cardiovascular surgery. 2017 Jul:154(1):123-124. doi: 10.1016/j.jtcvs.2017.03.015. Epub 2017 Mar 10 [PubMed PMID: 28365014]
Calafiore AM, Gallina S, Iacò AL, Contini M, Bivona A, Gagliardi M, Bosco P, Di Mauro M. Mitral valve surgery for functional mitral regurgitation: should moderate-or-more tricuspid regurgitation be treated? a propensity score analysis. The Annals of thoracic surgery. 2009 Mar:87(3):698-703. doi: 10.1016/j.athoracsur.2008.11.028. Epub [PubMed PMID: 19231373]
Level 2 (mid-level) evidenceTaramasso M, Vanermen H, Maisano F, Guidotti A, La Canna G, Alfieri O. The growing clinical importance of secondary tricuspid regurgitation. Journal of the American College of Cardiology. 2012 Feb 21:59(8):703-10. doi: 10.1016/j.jacc.2011.09.069. Epub [PubMed PMID: 22340261]
McCarthy PM. Evolving Approaches to Tricuspid Valve Surgery: Moving To Europe? Journal of the American College of Cardiology. 2015 May 12:65(18):1939-40 [PubMed PMID: 25936266]
Unger P, Rosenhek R, Dedobbeleer C, Berrebi A, Lancellotti P. Management of multiple valve disease. Heart (British Cardiac Society). 2011 Feb:97(4):272-7. doi: 10.1136/hrt.2010.212282. Epub 2010 Dec 13 [PubMed PMID: 21156677]
Rousse N, Juthier F, Pinçon C, Hysi I, Banfi C, Robin E, Fayad G, Jegou B, Prat A, Vincentelli A. ECMO as a bridge to decision: Recovery, VAD, or heart transplantation? International journal of cardiology. 2015:187():620-7. doi: 10.1016/j.ijcard.2015.03.283. Epub 2015 Mar 20 [PubMed PMID: 25863737]
Fang JC. Rise of the machines--left ventricular assist devices as permanent therapy for advanced heart failure. The New England journal of medicine. 2009 Dec 3:361(23):2282-5. doi: 10.1056/NEJMe0910394. Epub 2009 Nov 17 [PubMed PMID: 19920052]
Stone ME, Pawale A, Ramakrishna H, Weiner MM. Implantable Left Ventricular Assist Device Therapy-Recent Advances and Outcomes. Journal of cardiothoracic and vascular anesthesia. 2018 Aug:32(4):2019-2028. doi: 10.1053/j.jvca.2017.11.003. Epub 2017 Nov 4 [PubMed PMID: 29338999]
Level 3 (low-level) evidencePinney SP, Anyanwu AC, Lala A, Teuteberg JJ, Uriel N, Mehra MR. Left Ventricular Assist Devices for Lifelong Support. Journal of the American College of Cardiology. 2017 Jun 13:69(23):2845-2861. doi: 10.1016/j.jacc.2017.04.031. Epub [PubMed PMID: 28595702]
Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH, HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. The New England journal of medicine. 2009 Dec 3:361(23):2241-51. doi: 10.1056/NEJMoa0909938. Epub 2009 Nov 17 [PubMed PMID: 19920051]
Level 1 (high-level) evidenceHein OV, Birnbaum J, Wernecke K, England M, Konertz W, Spies C. Prolonged intensive care unit stay in cardiac surgery: risk factors and long-term-survival. The Annals of thoracic surgery. 2006 Mar:81(3):880-5 [PubMed PMID: 16488688]
Level 2 (mid-level) evidenceAzarfarin R, Ashouri N, Totonchi Z, Bakhshandeh H, Yaghoubi A. Factors influencing prolonged ICU stay after open heart surgery. Research in cardiovascular medicine. 2014 Nov:3(4):e20159. doi: 10.5812/cardiovascmed.20159. Epub 2014 Oct 14 [PubMed PMID: 25785249]
Shahian DM, O'Brien SM, Filardo G, Ferraris VA, Haan CK, Rich JB, Normand SL, DeLong ER, Shewan CM, Dokholyan RS, Peterson ED, Edwards FH, Anderson RP, Society of Thoracic Surgeons Quality Measurement Task Force. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1--coronary artery bypass grafting surgery. The Annals of thoracic surgery. 2009 Jul:88(1 Suppl):S2-22. doi: 10.1016/j.athoracsur.2009.05.053. Epub [PubMed PMID: 19559822]
Carl M, Alms A, Braun J, Dongas A, Erb J, Goetz A, Goepfert M, Gogarten W, Grosse J, Heller AR, Heringlake M, Kastrup M, Kroener A, Loer SA, Marggraf G, Markewitz A, Reuter D, Schmitt DV, Schirmer U, Wiesenack C, Zwissler B, Spies C. S3 guidelines for intensive care in cardiac surgery patients: hemodynamic monitoring and cardiocirculary system. German medical science : GMS e-journal. 2010 Jun 15:8():Doc12. doi: 10.3205/000101. Epub 2010 Jun 15 [PubMed PMID: 20577643]
KIRKLIN JW, DONALD DE, HARSHBARGER HG, HETZEL PS, PATRICK RT, SWAN HJ, WOOD EH. Studies in extracorporeal circulation. I. Applicability of Gibbon-type pump-oxygenator to human intracardiac surgery: 40 cases. Annals of surgery. 1956 Jul:144(1):2-8 [PubMed PMID: 13327835]
Level 3 (low-level) evidenceSugita J, Fujiu K. Systemic Inflammatory Stress Response During Cardiac Surgery. International heart journal. 2018:59(3):457-459. doi: 10.1536/ihj.18-210. Epub [PubMed PMID: 29848891]
Asimakopoulos G. Systemic inflammation and cardiac surgery: an update. Perfusion. 2001 Sep:16(5):353-60 [PubMed PMID: 11565890]
Level 3 (low-level) evidenceTatoulis J, Rice S, Davis P, Goldblatt JC, Marasco S. Patterns of postoperative systemic vascular resistance in a randomized trial of conventional on-pump versus off-pump coronary artery bypass graft surgery. The Annals of thoracic surgery. 2006 Oct:82(4):1436-44 [PubMed PMID: 16996948]
Level 1 (high-level) evidenceRossaint J, Berger C, Van Aken H, Scheld HH, Zahn PK, Rukosujew A, Zarbock A. Cardiopulmonary bypass during cardiac surgery modulates systemic inflammation by affecting different steps of the leukocyte recruitment cascade. PloS one. 2012:7(9):e45738. doi: 10.1371/journal.pone.0045738. Epub 2012 Sep 19 [PubMed PMID: 23029213]
Murphy DA, Hockings LE, Andrews RK, Aubron C, Gardiner EE, Pellegrino VA, Davis AK. Extracorporeal membrane oxygenation-hemostatic complications. Transfusion medicine reviews. 2015 Apr:29(2):90-101. doi: 10.1016/j.tmrv.2014.12.001. Epub 2014 Dec 18 [PubMed PMID: 25595476]
Chaikof EL. The development of prosthetic heart valves--lessons in form and function. The New England journal of medicine. 2007 Oct 4:357(14):1368-71 [PubMed PMID: 17914037]
Rahimtoola SH. Choice of prosthetic heart valve in adults an update. Journal of the American College of Cardiology. 2010 Jun 1:55(22):2413-26. doi: 10.1016/j.jacc.2009.10.085. Epub [PubMed PMID: 20510209]
Level 3 (low-level) evidenceFitchett D, Eikelboom J, Fremes S, Mazer D, Singh S, Bittira B, Brister S, Graham J, Gupta M, Karkouti K, Lee A, Love M, McArthur R, Peterson M, Verma S, Yau T. Dual antiplatelet therapy in patients requiring urgent coronary artery bypass grafting surgery: a position statement of the Canadian Cardiovascular Society. The Canadian journal of cardiology. 2009 Dec:25(12):683-9 [PubMed PMID: 19960127]
Sousa-Uva M, Storey R, Huber K, Falk V, Leite-Moreira AF, Amour J, Al-Attar N, Ascione R, Taggart D, Collet JP, ESC Working Group on Cardiovascular Surgery and ESC Working Group on Thrombosis. Expert position paper on the management of antiplatelet therapy in patients undergoing coronary artery bypass graft surgery. European heart journal. 2014 Jun 14:35(23):1510-4. doi: 10.1093/eurheartj/ehu158. Epub 2014 Apr 18 [PubMed PMID: 24748565]
Capodanno D, Angiolillo DJ. Management of antiplatelet therapy in patients with coronary artery disease requiring cardiac and noncardiac surgery. Circulation. 2013 Dec 24:128(25):2785-98. doi: 10.1161/CIRCULATIONAHA.113.003675. Epub [PubMed PMID: 24366588]
Nagashima Z, Tsukahara K, Uchida K, Hibi K, Karube N, Ebina T, Imoto K, Kimura K, Umemura S. Impact of preoperative dual antiplatelet therapy on bleeding complications in patients with acute coronary syndromes who undergo urgent coronary artery bypass grafting. Journal of cardiology. 2017 Jan:69(1):156-161. doi: 10.1016/j.jjcc.2016.02.013. Epub 2016 Mar 15 [PubMed PMID: 26987791]
Dalén M, Ivert T, Holzmann MJ, Sartipy U. Long-term survival after off-pump coronary artery bypass surgery: a Swedish nationwide cohort study. The Annals of thoracic surgery. 2013 Dec:96(6):2054-60. doi: 10.1016/j.athoracsur.2013.07.014. Epub 2013 Sep 25 [PubMed PMID: 24075498]
Level 2 (mid-level) evidencePuskas JD, Thourani VH, Kilgo P, Cooper W, Vassiliades T, Vega JD, Morris C, Chen E, Schmotzer BJ, Guyton RA, Lattouf OM. Off-pump coronary artery bypass disproportionately benefits high-risk patients. The Annals of thoracic surgery. 2009 Oct:88(4):1142-7. doi: 10.1016/j.athoracsur.2009.04.135. Epub [PubMed PMID: 19766798]
Level 2 (mid-level) evidenceBonaros N, Schachner T, Wiedemann D, Weidinger F, Lehr E, Zimrin D, Friedrich G, Bonatti J. Closed chest hybrid coronary revascularization for multivessel disease - current concepts and techniques from a two-center experience. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2011 Oct:40(4):783-7. doi: 10.1016/j.ejcts.2011.01.055. Epub 2011 Apr 3 [PubMed PMID: 21459599]
Verhaegh AJ, Accord RE, van Garsse L, Maessen JG. Hybrid coronary revascularization as a safe, feasible, and viable alternative to conventional coronary artery bypass grafting: what is the current evidence? Minimally invasive surgery. 2013:2013():142616. doi: 10.1155/2013/142616. Epub 2013 Apr 3 [PubMed PMID: 23691303]
Mentzer RM Jr. Myocardial protection in heart surgery. Journal of cardiovascular pharmacology and therapeutics. 2011 Sep-Dec:16(3-4):290-7. doi: 10.1177/1074248411410318. Epub [PubMed PMID: 21821531]
Level 3 (low-level) evidenceLi Y, Lin H, Zhao Y, Li Z, Liu D, Wu X, Ji B, Gao B. Del Nido Cardioplegia for Myocardial Protection in Adult Cardiac Surgery: A Systematic Review and Meta-Analysis. ASAIO journal (American Society for Artificial Internal Organs : 1992). 2018 May/Jun:64(3):360-367. doi: 10.1097/MAT.0000000000000652. Epub [PubMed PMID: 28863040]
Level 1 (high-level) evidenceVaage J. Retrograde cardioplegia: when and how. A review. Scandinavian journal of thoracic and cardiovascular surgery. Supplementum. 1993:41():59-66 [PubMed PMID: 8184295]
Yamamoto H, Yamamoto F. Myocardial protection in cardiac surgery: a historical review from the beginning to the current topics. General thoracic and cardiovascular surgery. 2013 Sep:61(9):485-96. doi: 10.1007/s11748-013-0279-4. Epub 2013 Jul 23 [PubMed PMID: 23877427]
Abah U, Large S. Stroke prevention in cardiac surgery. Interactive cardiovascular and thoracic surgery. 2012 Jul:15(1):155-7. doi: 10.1093/icvts/ivs012. Epub 2012 Apr 21 [PubMed PMID: 22523135]
Grogan K, Stearns J, Hogue CW. Brain protection in cardiac surgery. Anesthesiology clinics. 2008 Sep:26(3):521-38. doi: 10.1016/j.anclin.2008.03.003. Epub [PubMed PMID: 18765221]
Lelis RG, Auler Júnior JO. [Pathophysiology of neurological injuries during heart surgery: aspectos fisiopatológicos.]. Revista brasileira de anestesiologia. 2004 Aug:54(4):607-17 [PubMed PMID: 19471768]
DeBakey ME. The development of vascular surgery. American journal of surgery. 1979 Jun:137(6):697-738 [PubMed PMID: 313164]
Livesay JJ, Messner GN, Vaughn WK. Milestones in the treatment of aortic aneurysm: Denton A. Cooley, MD, and the Texas Heart Institute. Texas Heart Institute journal. 2005:32(2):130-4 [PubMed PMID: 16107099]
Salazar JD, Wityk RJ, Grega MA, Borowicz LM, Doty JR, Petrofski JA, Baumgartner WA. Stroke after cardiac surgery: short- and long-term outcomes. The Annals of thoracic surgery. 2001 Oct:72(4):1195-201; discussion 1201-2 [PubMed PMID: 11603436]
McKhann GM, Grega MA, Borowicz LM Jr, Baumgartner WA, Selnes OA. Stroke and encephalopathy after cardiac surgery: an update. Stroke. 2006 Feb:37(2):562-71 [PubMed PMID: 16373636]
Crosina J, Lerner J, Ho J, Tangri N, Komenda P, Hiebert B, Choi N, Arora RC, Rigatto C. Improving the Prediction of Cardiac Surgery-Associated Acute Kidney Injury. Kidney international reports. 2017 Mar:2(2):172-179. doi: 10.1016/j.ekir.2016.10.003. Epub 2016 Oct 21 [PubMed PMID: 29142955]
Lomivorotov VV, Efremov SM, Karaskov AM. Pharmacokinetics of Magnesium in Cardiac Surgery: Implications for Prophylaxis Against Atrial Fibrillation. Journal of cardiothoracic and vascular anesthesia. 2018 Jun:32(3):1295-1296. doi: 10.1053/j.jvca.2017.09.023. Epub 2017 Sep 20 [PubMed PMID: 29217237]
Werdan K, Ruß M, Buerke M, Delle-Karth G, Geppert A, Schöndube FA, German Cardiac Society, German Society of Intensive Care and Emergency Medicine, German Society for Thoracic and Cardiovascular Surgery, (Austrian Society of Internal and General Intensive Care Medicine, German Interdisciplinary Association of Intensive Care and Emergency Medicine, Austrian Society of Cardiology, German Society of Anaesthesiology and Intensive Care Medicine, German Society of Preventive Medicine and Rehabilitation. Cardiogenic shock due to myocardial infarction: diagnosis, monitoring and treatment: a German-Austrian S3 Guideline. Deutsches Arzteblatt international. 2012 May:109(19):343-51. doi: 10.3238/arztebl.2012.0343. Epub 2012 May 11 [PubMed PMID: 22675405]
Laflamme M, DeMey N, Bouchard D, Carrier M, Demers P, Pellerin M, Couture P, Perrault LP. Management of early postoperative coronary artery bypass graft failure. Interactive cardiovascular and thoracic surgery. 2012 Apr:14(4):452-6. doi: 10.1093/icvts/ivr127. Epub 2012 Jan 5 [PubMed PMID: 22223760]
Level 2 (mid-level) evidenceRedfors B, Généreux P, Witzenbichler B, McAndrew T, Diamond J, Huang X, Maehara A, Weisz G, Mehran R, Kirtane AJ, Stone GW. Percutaneous Coronary Intervention of Saphenous Vein Graft. Circulation. Cardiovascular interventions. 2017 May:10(5):. pii: e004953. doi: 10.1161/CIRCINTERVENTIONS.117.004953. Epub [PubMed PMID: 28495896]
Welsh RC, Granger CB, Westerhout CM, Blankenship JC, Holmes DR Jr, O'Neill WW, Hamm CW, Van de Werf F, Armstrong PW, APEX AMI Investigators. Prior coronary artery bypass graft patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. JACC. Cardiovascular interventions. 2010 Mar:3(3):343-51. doi: 10.1016/j.jcin.2009.12.008. Epub [PubMed PMID: 20298996]
Level 1 (high-level) evidenceVarghese R, Anyanwu AC, Itagaki S, Milla F, Castillo J, Adams DH. Management of systolic anterior motion after mitral valve repair: an algorithm. The Journal of thoracic and cardiovascular surgery. 2012 Apr:143(4 Suppl):S2-7. doi: 10.1016/j.jtcvs.2012.01.063. Epub [PubMed PMID: 22423603]
Level 2 (mid-level) evidenceMiura T, Eishi K, Yamachika S, Hashizume K, Hazama S, Ariyoshi T, Taniguchi S, Izumi K, Hashimoto W, Odate T. Systolic anterior motion after mitral valve repair: predicting factors and management. General thoracic and cardiovascular surgery. 2011 Nov:59(11):737-42. doi: 10.1007/s11748-011-0833-x. Epub 2011 Nov 15 [PubMed PMID: 22083691]
Level 2 (mid-level) evidenceAlfieri O, Lapenna E. Systolic anterior motion after mitral valve repair: where do we stand in 2015? European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2015 Sep:48(3):344-6. doi: 10.1093/ejcts/ezv230. Epub 2015 Jul 4 [PubMed PMID: 26142473]
Howitt SH, Herring M, Malagon I, McCollum CN, Grant SW. Incidence and outcomes of sepsis after cardiac surgery as defined by the Sepsis-3 guidelines. British journal of anaesthesia. 2018 Mar:120(3):509-516. doi: 10.1016/j.bja.2017.10.018. Epub 2017 Nov 24 [PubMed PMID: 29452807]
Paternoster G, Guarracino F. Sepsis After Cardiac Surgery: From Pathophysiology to Management. Journal of cardiothoracic and vascular anesthesia. 2016 Jun:30(3):773-80. doi: 10.1053/j.jvca.2015.11.009. Epub 2015 Nov 10 [PubMed PMID: 26947713]
Lee JJ, Park NH, Lee KS, Chee HK, Sim SB, Kim MJ, Choi JS, Kim M, Park CS. Projections of Demand for Cardiovascular Surgery and Supply of Surgeons. The Korean journal of thoracic and cardiovascular surgery. 2016 Dec:49(Suppl 1):S37-S43. doi: 10.5090/kjtcs.2016.49.S1.S37. Epub 2016 Dec 5 [PubMed PMID: 28035296]
Farooq V, Di Mario C, Serruys PW. Balancing idealism with realism to safeguard the welfare of patients: The importance of Heart Team led decision-making in patients with complex coronary artery disease. Indian heart journal. 2016 Jan-Feb:68(1):1-5. doi: 10.1016/j.ihj.2015.10.385. Epub 2016 Jan 12 [PubMed PMID: 26896257]
Görlinger K, Bergmann L, Dirkmann D. Coagulation management in patients undergoing mechanical circulatory support. Best practice & research. Clinical anaesthesiology. 2012 Jun:26(2):179-98. doi: 10.1016/j.bpa.2012.04.003. Epub [PubMed PMID: 22910089]