Introduction
Cardioplegia is a pharmacological therapy administered during cardiac surgery to intentionally and temporarily arrest the heart. The first solution used during cardiopulmonary bypass was reported by Dr. Melrose in the early 1950s, who identified that high levels of potassium citrate induced a reversible cardiac arrest.[1] An influx of potassium depolarizes the myocardial membrane causing contraction and thus release and subsequent sequestration of calcium ions resulting in a diastolic arrest. The resting membrane potential in myocytes is about -85 mV, and with the influx of sodium ions, depolarization occurs (membrane becomes more positive -45 to -30 mV) and thus generating an action potential that is potentiated by L-type calcium channels. These voltage-gated channels are targeted with cardioplegia to induce cardiac arrest.[2] The persistence of potassium reduces the membrane potential and does not allow for adequate repolarization. This, in turn, creating a diastolic cardiac arrest. As the solution diffuses and there is a washout of its components along with products of anaerobic cellular metabolism, electrical activity begins to appear, and redosing of cardioplegia is required if clinically indicated. Potassium, however, is not the only ion commonly found in cardioplegia. Other ions such as calcium, sodium, and magnesium all participate in reducing contractility and preserving the myocardium. Additionally, components such as lidocaine, bicarb, and even glucose may be added for further protection.[3]
Cardioplegia is an essential component of cardiopulmonary bypass and with the primary goal to reduce myocardial oxygen demand by creating electrical quiescence and cooling the heart to reduce the ischemic effects of being on bypass. The use of cardioplegia, in addition to being cardioprotective, also provides a relatively bloodless and motionless surgical field. There are many forms of cardioplegia ranging from the location of administration retrograde vs. antegrade as well as varying components within the solution, temperature, indications, adverse effects, pharmacokinetics, and pharmacodynamics.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Cardioplegia administration can be anterograde, retrograde, or both. The anterograde cardioplegia is inserted in the proximal aorta and contains three lumens: one to administer the cardioplegia, another for suctioning, and the third to measure intraluminal pressure.[4] Barometric monitoring of cardioplegia administration is required to prevent potential endothelial cell damage and reperfusion injury secondary to elevated infusion pressures.[5] The patient is heparinized, cooled, and, when appropriate, is placed on cardiopulmonary bypass. The aortic cross-clamp is placed just distal to the anterograde cardioplegia cannula, and once secured, the perfusionist can begin administering cardioplegia at set doses and intervals as requested by the cardiothoracic surgeon.[4] Anterograde simply means that the solution runs down the right and left coronary arteries and supplies the myocardium in the same distribution that blood would normally.
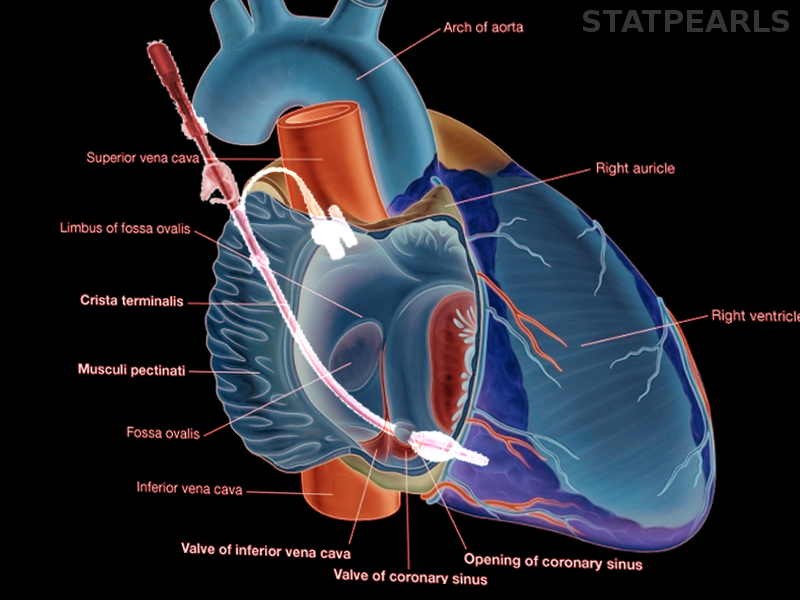
During cases with anatomical variants such as an incompetent aortic valve, severe coronary artery stenosis, previous coronary artery bypass graft to the internal mammary artery, or previous damage to coronary arterial ostia are all indications to supplement cardioplegia with a retrograde cardioplegia approach. The cardioplegia catheter is inserted via atriotomy in the right atrium into the coronary sinus, and flow is administered in a retrograde fashion. Intraoperative transesophageal echocardiography is used to confirm the position of coronary sinus cannula. Using only retrograde cardioplegia into the coronary sinus may be insufficient for adequate myocardial protection to the right ventricle due to the anterior cardiac veins draining directly into the right atrium and not to the coronary sinus.[6] Cannulating the coronary sinus carries a risk of perforation, and while rare, perforation can cause significant morbidity and mortality and is technically difficult to repair due to its posterior location. In minimally invasive robotic cardiac surgery, coronary sinus cannulation is done by the anesthesiologist via the right internal jugular vein central access and transesophageal echocardiography (TEE) guidance.[7]
Indications
The indications for cardioplegia include the need for myocardial protection and a motionless, bloodless field during on-pump cardiac surgery. Patients will receive anterograde cardioplegia; however, retrograde cardioplegia may be used as well. Indications for retrograde cardioplegia include moderate to severe aortic regurgitation, severe coronary artery stenosis, previous coronary artery bypass with a patent graft such as the internal mammary artery essentially providing an external source of perfusion, or an anticipated prolonged pump time such as in the repair of the aortic root or valve. Inadequate cardioplegia distribution may result in myocardial cell damage with ischemia following aortic cross-clamping and initiation of cardiopulmonary bypass.
Contraindications
Anatomical variants may make the placement of either the anterograde or retrograde cardioplegia catheter technically difficult, or placement of the aortic cross-clamp may be contraindicated altogether. Severe ascending aorta calcification or unstable atheromas increases the risk of dissection and stroke following the removal of the aortic cross-clamp, and alternate sites of cannulation must be used, such as the subclavian or femoral artery.[8][9] Likewise, in patients with venous anatomical anomalies must be assessed pre-operative to determine the safety of coronary sinus cannulation. While uncommon, a persistent left superior vena cava (PLSVC) may present intraoperatively on TEE as a dilated coronary sinus and contraindicate the use of retrograde cardioplegia. Of note in previous case reports, the PLSVC has been seen draining directly into the left atrium or the coronary sinus directly into the right atrium.[10][6][11] Some case reports have been published of coronary sinus orifice atresia with a PLSVC in pediatric patients; however, these are commonly associated with other congenital abnormalities and discovered during evaluation for cardiac surgery.[12]
Equipment
Cardioplegia cannula is needed to deliver infusion during cardiac surgery. Additional equipment to have readily available includes an aortic cross-clamp, cannulas for arterial and venous access, and accessory cannulas for initiation and maintenance of cardiopulmonary bypass. Monitors must be displayed at all times for all members of the team, including the anesthesiologist, perfusionist, and cardiothoracic surgeon. Monitors should visibly portray mean arterial blood pressure and intraluminal pressure of the cardioplegia delivery system.
Personnel
The perfusionist is the main individual responsible for delivering cardioplegia by keeping track of the flow rate, volume, temperature, components, and timing of each dose. There is an important interplay between the cardiothoracic surgeon and perfusionist just prior, during, and coming off of bypass. The perfusionist also assesses and controls the intraluminal pressure through which the cardioplegia solution is being administered and alters the flow rate, volume, temperature, and pressure accordingly.[13] The cardiothoracic anesthesiologist will assist in confirming the position of the retrograde cardioplegia catheter placed by the cardiothoracic surgeon on TEE and also facilitates the assessment of the atherosclerosis of the ascending aorta for clamping and cannulation.
Preparation
There are various types of cardioplegia solutions that not only vary from adults to pediatric patients, but also from institution to institution. A global survey sent to Europe, Australia/New Zealand, North and South America regarding cardioplegia use and practices during cardiac surgery and found a wide variety in responses confirming there is no clear consensus on best practices regarding cardioplegia use.[1] Cardioplegia varies by composition, delivery methods, temperature, and additives; however, all solutions must include potassium chloride (15-35 meq/L) important for inducing cardiac arrest, and other electrolytes such as Mg2+, low-dose Ca2+, Cl-. Na+.[14] Bicarbonate is added just prior to administration to ensure its integrity and to adjust the pH of the solution as needed.
Single-dose cardioplegia is becoming increasingly popular due to its use in minimally invasive cardiac surgery and basic CABG procedures. Benefits of single-dose cardioplegia include reduced aortic cross-clamp time, reduced interruptions during the procedure, and a decreased incidence in postoperative myocardial dysfunction. Two cardioplegia solutions are used for single-dose administration: Bretschneider solution and del Nido extracellular cardioplegia solution, and their characteristics are identified below.[15]
Bretschneider solution:
- Type of long-acting crystalloid cardioplegia[16]
- Low sodium and calcium concentrations resulting in reduced action potential leading to diastolic cardiac arrest
- Potassium chloride
- Magnesium sulfate
- Sodium chloride
- Tryptophan for membrane stabilization
- Ketoglutarate for increased energy production during reperfusion - a precursor to ATP
- Mannitol osmotic agent for cell membrane regulation
- Histidine buffer that supports anaerobic glycolysis and is better at maintaining pH and postoperative electrolytes and metabolic levels
Del Nido solution:
- Multiple electrolytes based which its contents make it similar to the components of extracellular fluid
- Mannitol for cell membrane regulation
- Magnesium sulfate which functions as a calcium antagonist
- Sodium bicarbonate buffer
- Potassium chloride
- Lidocaine, a class IB antiarrhythmic that functions as a Na+ channel blocker
- 1:4 blood: crystalloid composition[17]
- Initially developed for neonates and pediatric cardiac patients for the immature myocardium
Studies published comparing solutions noted no clinically significant difference between the Bretschneider solution and blood or crystalloid cardioplegia. When comparing del Nido solution, a reduction in troponin T and CK-MB levels were noted postoperatively, and spontaneous conversion to normal sinus rhythm was more frequent than in other cardioplegia solutions likely secondary to the addition of lidocaine. Additionally, there was a reduction in the insulin requirement when compared to the crystalloid solution likely due to the exclusion of glucose in del Nido cardioplegia. Del Nido provides approximately 60 minutes of myocardial protection; however, care must be taken, and redosing may be required in patients with left ventricular hypertrophy or cases of severe obstructive coronary artery disease due to the risk of maldistribution and subsequent inadequate myocardial protection.[15] Single-dose cardioplegia, while beneficial during low-risk cardiac surgeries, multi-dose cardioplegia may be indicated and safer for myocardial protection during high-risk cardiac surgery. Conventional cardioplegia used in multi-dose administration can be described as being either crystalloid cardioplegia and blood cardioplegia, and their characteristics are described below:
Crystalloid cardioplegia:
- Reduces ischemia injury by using potassium chloride concentrations <26 mEq/L
- Additives including glucose and sodium bicarbonate
- St. Thomas cardioplegia solution is a type of extracellular crystalloid based cardioplegia that requires short-interval (~20 minutes) repeat dosing due to its incidence of myocardial acidosis in between doses.[1][17] As a result, the use of del Nido cardioplegia, which provides 60 minutes of protection, is being studied as an alternative.
Blood cardioplegia (Buckerberg's):
- 4:1 blood: a crystalloid composition that was created and seen as the ideal delivery solution due to blood having:
- Natural buffering system
- Normal oncotic pressure
- Improved oxygen delivery
- Innate free radical scavenging mechanism
- A variant known as microplegia is a concentrated version using whole blood cardioplegia
- Microplegia aims to reduce the volume used and thus prevent hemodilution and edema.[18]
Calafiore cardioplegia [16]
- Another type of blood-based cardioplegic solution
- May be administered in a low or continuous flow
- Believed to provide better physiologic conditions and improved oxygen-carrying capacity
While many studies have tried to compare one to the other, some find no clinically significant difference, and others determine blood to be superior to crystalloid cardioplegia. Likewise, there is variation in the temperature at which to administer cardioplegia, and studies have failed to prove one temperature to be superior to the other. To date, there is no clear consensus. The dose of cardioplegia is also widely variable and often dosed empirically. It takes about 300 to 500 mL to arrest the heart; however, larger doses are infused during the initial dose to ensure adequate perfusion to the myocardium in cases of severe coronary artery stenosis or left ventricular hypertrophy.[19] Del Nido cardioplegia for infants and children is dosed at 20 mL/kg at a temperature of 8 to 12 degrees celsius in an anterograde fashion.[3] Redosing is required depending on the length of the procedure and cardioplegia used, and it is important to maintain cardiac arrest, pH, and the delivery of substrates, wash the byproducts of anaerobic metabolism and reduce edema.[15] The initial dose of cardioplegia is typically cold, and when preparing to come off bypass and rewarming of the patient occurs, the surgeon may request a "hot-shot," which is a dose of warm cardioplegia.
Complications
Cardioplegia following aortic cross-clamping results in many physiological changes that may hinder the recovery of the myocardium. It is essential to understand the electrolyte abnormalities, myocardial stunning, pH imbalance that may occur secondary to cardioplegia administration. Before coming off cardiopulmonary bypass, electrolytes, temperature, glucose, pH, hemoglobin, and hematocrit must be within normal limits. Excessive physiological changes may result in myocardial stunning, arrhythmias, ischemic injury leading to the inability to wean from cardiopulmonary bypass. Prophylactic measures are taken to reduce the complications of cardioplegia, such as frequent blood sampling by the perfusionist and notifying the surgeon and anesthesiologist of derangements while treating abnormalities as they present.
Clinical Significance
Cardioplegia is an essential component in myocardial protection during aortic cross-clamping and cardiopulmonary bypass. A good understand of the various compositions of cardioplegia, routes of administration, and potential side effects are necessary to reduce morbidity and mortality in patients undergoing cardiac surgery. Inadequate administration may result in permanent reperfusion injury and myocardial ischemia.
Enhancing Healthcare Team Outcomes
The safe use of cardioplegia during cardiac surgery requires a team approach and excellent interprofessional communication. With numerous moving parts encountered during open-heart surgery and the use of the cardiopulmonary bypass machine, it is of utmost importance for physicians, perfusionists, nurses, and surgical assistance to pay close attention and speak up should an issue arise. A designated heart team that practices and works together can make for improved patient safety and better patient outcomes. Close-loop feedback is used during critical times between the cardiac surgeon and perfusionist to diminish any room for error. As previously noted, cardioplegia solutions vary, and to date, there are no evidence-based guidelines regarding the administration of cardioplegia during cardiac surgery. As illustrated by the 2011 ACCF/AHA Guideline for CABG Surgery, reductions in reperfusion injury or surgically induced systemic inflammatory response by way of pharmacological agents or controlled reperfusion strategies that seek to induce myocardial preconditioning have not been proven, and their benefits remain uncertain.[20] [Class IIb, Level A evidence] Thus, further clinical studies are needed to reach a consensus of best practices regarding cardioplegia and myocardial preservation.
Media
References
Ali JM, Miles LF, Abu-Omar Y, Galhardo C, Falter F. Global Cardioplegia Practices: Results from the Global Cardiopulmonary Bypass Survey. The journal of extra-corporeal technology. 2018 Jun:50(2):83-93 [PubMed PMID: 29921986]
Level 3 (low-level) evidenceChambers DJ, Fallouh HB. Cardioplegia and cardiac surgery: pharmacological arrest and cardioprotection during global ischemia and reperfusion. Pharmacology & therapeutics. 2010 Jul:127(1):41-52. doi: 10.1016/j.pharmthera.2010.04.001. Epub 2010 Apr 14 [PubMed PMID: 20398698]
Level 3 (low-level) evidenceMatte GS, del Nido PJ. History and use of del Nido cardioplegia solution at Boston Children's Hospital. The journal of extra-corporeal technology. 2012 Sep:44(3):98-103 [PubMed PMID: 23198389]
Level 3 (low-level) evidenceLopes JB, Santos CCMD Júnior. Coronary Perfusion Pressure during Antegrade Cardioplegia in On-Pump CABG Patients. Brazilian journal of cardiovascular surgery. 2017 May-Jun:32(3):171-176. doi: 10.21470/1678-9741-2017-0035. Epub [PubMed PMID: 28832794]
Katayama O, Amrani M, Ledingham S, Jayakumar J, Smolenski RT, Severs N, Rothery S, Yacoub MH. Effect of cardioplegia infusion pressure on coronary artery endothelium and cardiac mechanical function. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 1997 Apr:11(4):751-62 [PubMed PMID: 9151049]
Level 3 (low-level) evidenceKamassai JD, Lowery DR. Retrograde Cardioplegia. StatPearls. 2023 Jan:(): [PubMed PMID: 30855873]
Watanabe G, Ishikawa N. Alternative method for cardioplegia delivery during totally endoscopic robotic intracardiac surgery. The Annals of thoracic surgery. 2014 Sep:98(3):1129-31. doi: 10.1016/j.athoracsur.2014.02.070. Epub [PubMed PMID: 25193212]
Ward AF, Loulmet DF, Neuburger PJ, Grossi EA. Outcomes of peripheral perfusion with balloon aortic clamping for totally endoscopic robotic mitral valve repair. The Journal of thoracic and cardiovascular surgery. 2014 Dec:148(6):2769-72. doi: 10.1016/j.jtcvs.2014.05.035. Epub 2014 May 21 [PubMed PMID: 24952820]
Yeung KK, Groeneveld M, Lu JJ, van Diemen P, Jongkind V, Wisselink W. Organ protection during aortic cross-clamping. Best practice & research. Clinical anaesthesiology. 2016 Sep:30(3):305-15. doi: 10.1016/j.bpa.2016.07.005. Epub 2016 Aug 20 [PubMed PMID: 27650341]
Fernando RJ, Johnson SD. Inability to Utilize Retrograde Cardioplegia due to a Persistent Left Superior Vena Cava. Case reports in anesthesiology. 2017:2017():4671856. doi: 10.1155/2017/4671856. Epub 2017 Dec 3 [PubMed PMID: 29333298]
Level 3 (low-level) evidenceGoyal SK, Punnam SR, Verma G, Ruberg FL. Persistent left superior vena cava: a case report and review of literature. Cardiovascular ultrasound. 2008 Oct 10:6():50. doi: 10.1186/1476-7120-6-50. Epub 2008 Oct 10 [PubMed PMID: 18847480]
Level 3 (low-level) evidenceSantoscoy R, Walters HL 3rd, Ross RD, Lyons JM, Hakimi M. Coronary sinus ostial atresia with persistent left superior vena cava. The Annals of thoracic surgery. 1996 Mar:61(3):879-82 [PubMed PMID: 8619710]
Level 3 (low-level) evidenceBaker RA, Bronson SL, Dickinson TA, Fitzgerald DC, Likosky DS, Mellas NB, Shann KG, International Consortium for Evidence-Based Perfusion for the American Society of ExtraCorporeal Technology. Report from AmSECT's International Consortium for Evidence-Based Perfusion: American Society of Extracorporeal Technology Standards and Guidelines for Perfusion Practice: 2013. The journal of extra-corporeal technology. 2013 Sep:45(3):156-66 [PubMed PMID: 24303597]
Mankad PS, Chester AH, Yacoub MH. Role of potassium concentration in cardioplegic solutions in mediating endothelial damage. The Annals of thoracic surgery. 1991 Jan:51(1):89-93 [PubMed PMID: 1898693]
Level 3 (low-level) evidenceSiddiqi S, Blackstone EH, Bakaeen FG. Bretschneider and del Nido solutions: Are they safe for coronary artery bypass grafting? If so, how should we use them? Journal of cardiac surgery. 2018 May:33(5):229-234. doi: 10.1111/jocs.13539. Epub 2018 Feb 14 [PubMed PMID: 29444545]
Level 2 (mid-level) evidenceComentale G, Giordano R, Palma G. Comparison of the different cardioplegic strategies in cardiac valves surgery: who wins the "arm-wrestling"? Journal of thoracic disease. 2018 Feb:10(2):714-717. doi: 10.21037/jtd.2018.01.133. Epub [PubMed PMID: 29607140]
Mishra P, Jadhav RB, Mohapatra CK, Khandekar J, Raut C, Ammannaya GK, Seth HS, Singh J, Shah V. Comparison of del Nido cardioplegia and St. Thomas Hospital solution - two types of cardioplegia in adult cardiac surgery. Kardiochirurgia i torakochirurgia polska = Polish journal of cardio-thoracic surgery. 2016 Dec:13(4):295-299. doi: 10.5114/kitp.2016.64867. Epub 2016 Dec 30 [PubMed PMID: 28096823]
Gong B, Ji B, Sun Y, Wang G, Liu J, Zheng Z. Is microplegia really superior to standard blood cardioplegia? The results from a meta-analysis. Perfusion. 2015 Jul:30(5):375-82. doi: 10.1177/0267659114530454. Epub 2014 Apr 22 [PubMed PMID: 24756305]
Level 1 (high-level) evidenceCanty DJ, Joshi P, Royse CF, McMillan J, Tayeh S, Smith JA. Transesophageal Echocardiography Guidance of Antegrade Cardioplegia Delivery for Cardiac Surgery. Journal of cardiothoracic and vascular anesthesia. 2015 Dec:29(6):1498-503. doi: 10.1053/j.jvca.2015.03.009. Epub 2015 Mar 13 [PubMed PMID: 26142365]
Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, Cigarroa JE, DiSesa VJ, Hiratzka LF, Hutter AM Jr, Jessen ME, Keeley EC, Lahey SJ, Lange RA, London MJ, Mack MJ, Patel MR, Puskas JD, Sabik JF, Selnes O, Shahian DM, Trost JC, Winniford MD, Jacobs AK, Anderson JL, Albert N, Creager MA, Ettinger SM, Guyton RA, Halperin JL, Hochman JS, Kushner FG, Ohman EM, Stevenson W, Yancy CW, American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. The Journal of thoracic and cardiovascular surgery. 2012 Jan:143(1):4-34. doi: 10.1016/j.jtcvs.2011.10.015. Epub [PubMed PMID: 22172748]
Level 1 (high-level) evidence