Introduction
Cauda equina and conus medullaris syndromes have overlap in anatomy and clinical presentation. Therefore, for the purpose of this discussion, they will be grouped, and notable differences highlighted. The conus medullaris is the terminal end of the spinal cord, which typically occurs at the L1 vertebral level in the average adult.[1] Conus medullaris syndrome (CMS) results when there is compressive damage to the spinal cord from T12-L2.[1] The cauda equina is a group of nerves and nerve roots stemming from the distal end of the spinal cord, typically levels L1-L5 and contains axons of nerves that give both motor and sensory innervation to the legs, bladder, anus, and perineum.[2] Cauda equina syndrome (CES) results from compression and disruption of the function of these nerves and can be inclusive of the conus medullaris or distal to it, and most often occurs when damage occurs to the L3-L5 nerve roots.[1] Both syndromes are neurosurgical emergencies as they can present with back pain radiating to the legs, motor and sensory dysfunction of the lower extremities, bladder and/or bowel dysfunction, sexual dysfunction and saddle anesthesia.[3] CMS and CES also carry a high risk of litigation as delays in diagnosis and management can lead to devastating life-long impairment.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Cauda equina syndrome and conus medullaris syndrome result from compression of the spinal cord and nerves/nerve roots arising from L1-L5 levels. The most common cause of compression in 45% of CES is a herniated lumbar intervertebral disc.[3] Other causes include epidural abscess, spinal epidural hematoma, diskitis, tumor (either metastatic or a primary CNS cancer), trauma (particularly when there is retropulsion of bone fracture fragments), spinal stenosis and aortic obstruction.[4] Rare reported cases exist in which CES was associated with chiropractic manipulation, placement of interspinous devices, and thrombosis of the inferior vena cava.[4]
Epidemiology
Cauda equina syndrome and conus medullaris syndrome are rare, with an estimated prevalence of 1 in 30,000 to 100,000 people per year.[5] Estimates of annual incidence are between 1.5 to 3.4 per million people.[5] It occurs in 3% of all disc herniations.[5] Cauda equina syndrome and conus medullaris syndrome are most common in young men, possibly due to this population group being more likely to experience compressive thoracolumbar trauma.[5] One study estimated that in the U.S. we would expect to see 1016 new causes of cauda equina syndrome and 449 new cases of conus medullaris per year.[5]
History and Physical
A thorough history is necessary, with detailed questions regarding recent falls, trauma or injuries, use of anticoagulation, presented spinal instrumentation, intravenous drug use, history of malignancy, chiropractic manipulation, and constitutional symptoms like fevers/chills.[3]
Patients can present with:
- Back pain and sciatica (seen in as many as 97% of patients)
- Weakness and changes in sensation in the lower extremities
- Bladder dysfunction (disruption of autonomic fibers results in either retention or incontinence in up to 92% of patients)
- Bowel dysfunction (retention or incontinence in up to 72% of patients)
- Saddle anesthesia or decreased sensation in the perineum (in up to 93% of patients)
- Sexual dysfunction (impotence in men)[3]
The symptoms above when presenting in isolation, are neither specific nor sensitive for CMS/CES. However, several of the signs and symptoms mentioned above taken as a constellation should raise clinical suspicion.[2] These symptoms also lack significant positive predictive value for the syndromes, especially early on.[6] However, the onset of perineal anesthesia associated with bladder dysfunction is typical of the start of CES and the time at which the clock starts on diagnosis and management.[2] It is important also to note that painless urinary retention often has the greatest predictive value as a stand-alone symptom, but it is unfortunately indicative of late, often irreversible CMS/CES.[7]
Once clinical suspicion is established based on history, a thorough neurological examination is paramount. Findings to watch for include:
- Motor or sensory deficits in the legs – usually bilateral but can also be unilateral and asymmetrical (particularly in cases with an incomplete injury)
- Lower motor neuron signs in the legs – areflexia, hypotonia, atrophy (in cases of chronic cord compression resulting in CES)
- Saddle anesthesia
- Absent or decreased rectal tone
- Absent or decreased bulbocavernosus reflex
- Palpable bladder indicating urinary retention[3]
It is important to note that in the case of isolated conus medullaris syndrome, deficits of the lower extremities are more often bilateral and symmetric. Also, upper motor neuron signs can be present, such as spasticity and hyperreflexia.[1]
Evaluation
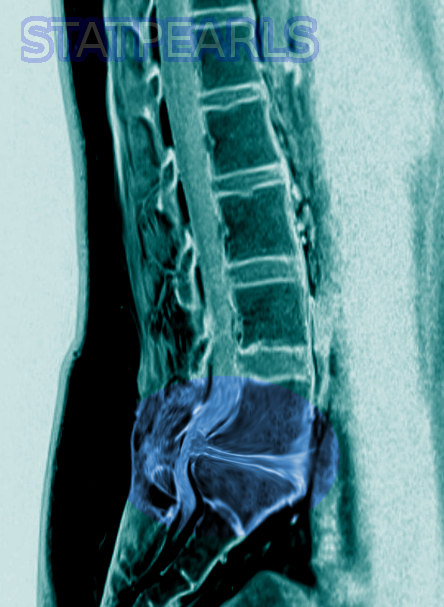
The gold standard method of evaluation for CMS/CES is obtaining urgent MRI imaging with sagittal and axial T1 and T2 sequences.[2] There has not been a specific timeframe established for “door to MRI time” in the ED, but early MRI and neurosurgical or orthopedic consultation is imperative. An ideal goal for MRI is one hour from the patient presentation.[2] For patients with contraindications to MRI, such as those with metal implants, a CT myelogram is a viable option.[2] This imaging modality has limited utility as it requires injecting contrast through a spinal tap to visualize the spinal cord and its associated structures. A bladder scan checking for a post-void residual volume should also be obtained to evaluate for urinary retention.[2]
Treatment / Management
Prompt neurosurgical or orthopedic consultation is necessary in cases of cauda equina and conus medullaris syndromes, as the treatment is surgical decompression via laminectomy with or without subsequent discectomy, or via sequestrectomy.[2]
Differential Diagnosis
The differential diagnosis centers on non-compressible causes of spinal cord dysfunction such as:
- Spinal cord infarct
- HIV-related myelopathy
- Transverse myelitis
- Multiple sclerosis
- Syringomyelia
- Spinal arteriovenous malformation
Prognosis
Several studies have looked at prognosis and outcomes based on the timing of surgical decompression. Early intervention by surgical decompression in patients with conus medullaris and cauda equina syndromes is associated with a better prognosis, particularly when surgery occurs within 48 hours of initial presentation.[6] The longer the compression continues, the worse the permanent structural and functional impairment, and the poorer the prognosis.[2] It is important to note that the presence of bladder dysfunction prior to surgery has been linked to poorer outcomes regardless of the timing of decompression, although early decompression is still the recommendation for a better prognosis irrespective of clinical status at initial presentation.[2]
Complications
Complications in cauda equina syndrome and conus medullaris syndrome occur in a large percentage of those diagnosed. One study looked at 63-day outcomes on micturition, defecation, saddle anesthesia, sexual function, and sciatica in cauda equina syndrome. The data indicate that a large percentage of patients still experience residual symptoms irrespective of their time to surgical decompression.[3] Micturition deficits such as retention requiring self-catheterization or presence of suprapubic or indwelling catheters and incontinence still presented in 47.7% of patients.[3] Dysfunction with defecation decreased post-operatively significantly, but 41.8% of patients still had problems at 63 days post-operatively.[3] Sexual dysfunction persisted in 53.3% of patients, and saddle anesthesia in 56.6%.[3] Sciatica was present in 47.5% of patients.[3] The best predictors of outcome are neurological status at presentation and degree of injury. Incomplete injuries tend to have better outcomes.[3]
Deterrence and Patient Education
Patients presenting with sciatica and no other evidence of CES/CMS in the history or exam should receive counsel on the possible development of other related symptoms such as bladder or bowel dysfunction, impotence, and saddle anesthesia. These patients must be given strict return precautions in the event they develop other symptoms pointing to CES/CMS. Patients who are suspected to have CES/CMS and are undergoing evaluation for these syndromes need to remain informed and updated on their investigations (MRI, bladder scan, etc.) and pending surgical consultations. Patients who receive a definitive diagnosis need counseling on complication rates and must receive a realistic prognosis based on their degree of injury. Due to the nature of sensitive lifelong sequelae resulting from these syndromes, cases with CES/CMS have a high involvement in medicolegal litigation.[8] For physicians, it is vital to document the history and physical exam thoroughly and accurately and reach the diagnosis as promptly as possible. In court cases involving CES, a positive association exists between time to surgical decompression greater than 48 hours and an adverse decision for the physician involved.[8] The degree of functional loss did not affect court rulings in the cases studies.[8]
Enhancing Healthcare Team Outcomes
The involvement and effective collaboration of the entire healthcare team is essential in any patient encounter, but even more so in cases where the presentation requires urgency in proper diagnosis and management. Due to the high degree of functional impairment arising from CES/CMS, speedy diagnosis and subsequent treatment via surgical decompression are important. The efficiency of the medical system must begin with the person responsible for triaging appropriately in the emergency department, where a significant proportion of patients with CES/CMS initially present for evaluation. After the initial triage, nurses and physicians must work together to keep the patient updated on the suspected diagnosis, workup, and test results. The radiologist must know the reason for ordering the MRI these patients and given the clinical history and suspected diagnosis early.
MRI imaging is considered the gold standard imaging modality to asses of CES/CMS, Class I evidence. Many studies recommend obtaining MRI within an hour from presentation. Furthermore, it is paramount that prompt neurosurgical consultation take palce promptly, as surgical decompression is required. Many studies have been done that show decompression within 48 hours is best, although some studies recommend the timing be closer to under 24 hours, Class II evidence. It is challenging to design large RCTs to obtain Class I evidence for the timing of surgical decompression for obvious ethical reasons.[9] Despite prompt surgical intervention, patients will experience residual symptoms. It is vital to counsel patients on this possibility. The primary care provider also has a vital role in further outpatient management postoperatively.
Media
References
Brouwers E, van de Meent H, Curt A, Starremans B, Hosman A, Bartels R. Definitions of traumatic conus medullaris and cauda equina syndrome: a systematic literature review. Spinal cord. 2017 Oct:55(10):886-890. doi: 10.1038/sc.2017.54. Epub 2017 May 23 [PubMed PMID: 28534496]
Level 1 (high-level) evidenceQuaile A. Cauda equina syndrome-the questions. International orthopaedics. 2019 Apr:43(4):957-961. doi: 10.1007/s00264-018-4208-0. Epub 2018 Oct 29 [PubMed PMID: 30374638]
Korse NS, Pijpers JA, van Zwet E, Elzevier HW, Vleggeert-Lankamp CLA. Cauda Equina Syndrome: presentation, outcome, and predictors with focus on micturition, defecation, and sexual dysfunction. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2017 Mar:26(3):894-904. doi: 10.1007/s00586-017-4943-8. Epub 2017 Jan 19 [PubMed PMID: 28102451]
Lavy C, James A, Wilson-MacDonald J, Fairbank J. Cauda equina syndrome. BMJ (Clinical research ed.). 2009 Mar 31:338():b936. doi: 10.1136/bmj.b936. Epub 2009 Mar 31 [PubMed PMID: 19336488]
Podnar S. Epidemiology of cauda equina and conus medullaris lesions. Muscle & nerve. 2007 Apr:35(4):529-31 [PubMed PMID: 17143890]
Thakur JD, Storey C, Kalakoti P, Ahmed O, Dossani RH, Menger RP, Sharma K, Sun H, Nanda A. Early intervention in cauda equina syndrome associated with better outcomes: a myth or reality? Insights from the Nationwide Inpatient Sample database (2005-2011). The spine journal : official journal of the North American Spine Society. 2017 Oct:17(10):1435-1448. doi: 10.1016/j.spinee.2017.04.023. Epub 2017 Apr 26 [PubMed PMID: 28456676]
Todd NV. Guidelines for cauda equina syndrome. Red flags and white flags. Systematic review and implications for triage. British journal of neurosurgery. 2017 Jun:31(3):336-339. doi: 10.1080/02688697.2017.1297364. Epub 2017 Mar 2 [PubMed PMID: 28637110]
Level 1 (high-level) evidenceDaniels EW, Gordon Z, French K, Ahn UM, Ahn NU. Review of medicolegal cases for cauda equina syndrome: what factors lead to an adverse outcome for the provider? Orthopedics. 2012 Mar 7:35(3):e414-9. doi: 10.3928/01477447-20120222-15. Epub 2012 Mar 7 [PubMed PMID: 22385455]
Level 1 (high-level) evidenceTodd NV. Cauda equina syndrome: the timing of surgery probably does influence outcome. British journal of neurosurgery. 2005 Aug:19(4):301-6; discussion 307-8 [PubMed PMID: 16455534]
Level 1 (high-level) evidence