Introduction
Cervical cancer continues to rank among the top gynecologic cancers worldwide. According to current data, it is ranked 14th among all cancers and is the 4th most common cancer among women worldwide.[1] Cervical cancer intervention focuses on primary and secondary prevention.[2] Primary prevention and screening are the best methods to decrease the burden of cervical cancer and mortality.
In the United States and other developed countries, most screening and diagnostic efforts are directed toward the early identification of high-risk human papillomavirus (HPV) lesions through HPV testing and Papanicolaou (Pap) smears. Although HPV testing is not recommended in women younger than 30 years, low-risk younger women should begin screening with Pap tests at age 21 and continue until age 65, per the United States Preventive Services Task Force (USPSTF) recommendations. Newer recommendations offer 3- to 5-year intervals between screenings based on a patient's prior results and the use of Pap and HPV cotesting.[3][4]
Like many diseases and cancers, disparities exist in screening, early diagnosis, and timely treatment rates. Screening rates are lower in low socioeconomic and low-resource areas with racial, ethnic, and age variations. Studies show women with obesity and chronic disease may have lower cervical and breast cancer screening rates. A study of ethnic minority women in the United Kingdom reports several barriers to screening, including lack of awareness, fear, embarrassment, shame, and low perceived risk.[5] Another study reviewing the barriers for Haitian women revealed socioeconomic barriers, language barriers, and a limited understanding of health and disease.[6] In the United States, cervical cancer mortality is disproportionately higher in black women.
As cervical cancer is a sexually transmitted infection (STI), it is preventable, and the global incidence can be reduced through targeted education, screening, and intervention. Since 2006, vaccination has been available for the prevention of cervical cancer. Vaccination can improve cancer death rates in populations with higher mortality rates and in developing countries where resources may not be available for routine screening.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Current literature reports that HPV is found in most sexually active people at some point during their lifetime. There are more than 130 types of known HPV, with 20 HPV types identified as cancer-related. HPV exposure rates are only known in women since men are not screened outside of research protocols. HPV types 16 and 18 are the most common HPV types identified in invasive cervical cancer. Population-based HPV prevalence studies show the greatest prevalence of high-risk HPV occurs in adults younger than 25 years, and cervical cancer deaths peak in middle-aged women between 40 and 50 years. Studies have shown that HPV-related cervical disease in women younger than 25 years is largely self-limiting. However, those with coinfection of multiple HPV types may be less likely to have spontaneous clearance and, thus, progress to cancer.
HPV is transmitted by skin-to-skin contact, including during sexual intercourse, hand-to-genital contact, and oral sex. Risk factors for HPV and cervical cancer include young age at sexual initiation, multiple sexual partners, high parity, smoking, herpes simplex, HIV, coinfection with other genital infections, and oral contraceptive use.[7][8]
Epidemiology
Persistent HPV infection causes more than 99% of all cervical cancers. Every year, there are more than 500,000 new cases of cervical cancer and approximately 250,000 deaths due to cervical cancer worldwide. Eighty percent of cases occur in developing countries.[9] In the United States, about 4000 women die yearly from cervical cancer. Blacks, Hispanics, and women in low-resource areas have more disparity in evidenced-based care and a significantly higher mortality rate.[10][11] Mortality is higher among women not screened in the past 5 years and those without consistent follow-up after identifying a precancerous cervical lesion. Trends show that women with the highest-mortality risk may be less likely to receive HPV vaccination.
Pathophysiology
More than 75% of cervical cancer cases are due to high-risk HPV types 16 and 18.[12] Other HPV types also can cause malignancy. Some low-risk HPV types, specifically types 6 and 11, cause condylomata acuminate, commonly referred to as anogenital warts. Although there are more than half a million cases of HPV identified annually, most are low-grade infections and will spontaneously resolve within 2 years. The progression of high-grade lesions and cancer is seen in the presence of other carcinogenic risk factors, as previously described.
Within HPV DNA, the oncoproteins E6 and E7 interfere with the critical host cell cycle; specifically, E6 interferes with suppressive tumor protein p53, whereas E7 interferes with retinoblastoma protein (pRB). Additionally, the E5 protein may play a role in immune evasion. These are significant factors in HPV-related neoplasia, including primary vagina cancer.[13] Oxidative stress and microRNAs are believed to play a role in cervical carcinogenesis. Future research to elucidate the proposed interplay is needed.[12][14]
Histopathology
Squamous cell carcinoma and adenocarcinoma are the most common histological subtypes of cervical cancer, with squamous cell carcinoma being vastly more frequent. Adenocarcinoma constitutes approximately 5% of invasive cervical cancers worldwide, although this percentage is increasing in some countries.[15] Both subtypes result from precursor lesions, cervical intraepithelial neoplasia (CIN), or carcinoma in situ (CIS). Squamous CIS and adenocarcinoma in situ (AIS) are the most immediate precursors to invasive cervical cancer. Adenocarcinoma of the cervix should be carefully distinguished from endometrial adenocarcinoma with immunohistochemistry and HPV in situ hybridization.[16]
Most malignancies arise from the sqamocolumnar junction of the cervix. Microscopically, anastomosing irregular nests or single tumor cells with stromal inflammation or desmoplasia are present. Lymphovascular invasion (LVI) may also be present. Grading is predicated on nuclear pleomorphism, nucleoli size, mitotic activity, and necrosis, and does not correlate with prognosis.[17]
History and Physical
Patients with cervical cancer are usually asymptomatic during the early stages. A complete medical history must include a sexual history, including the patient's age at first sexual encounter. Sexual history also includes questions about postcoital bleeding and pain during intercourse. Questions about previous STIs, including HPV and HIV, the number of lifetime sexual partners, tobacco use, and prior vaccination against HPV are all vitally important.[18] Women should also be asked about menstrual patterns, abnormal bleeding, persistent vaginal discharges, irritations, and known cervical lesions.[19]
The physical exam must include a complete evaluation of the external and internal genitalia. Positive exam findings in women with cervical cancer might include a friable cervix, visible cervical lesions, erosions, masses, bleeding with the examination, and fixed adnexa.[20] Many patients will have no positive findings on physical examination. Screening by Pap and/or HPV testing is essential in the workup and diagnosis of patients with cervical cancer and its precursor lesions.
Evaluation
According to the United States Preventative Services Task Force (USPTF), Pap screening is recommended beginning at age 21; at age 30, HPV testing starts in conjunction with Pap smear cytology. Screening is recommended every 3 years for women with continued negative screening results and those at low risk for cervical cancer. For women older than 30 years, cytology can be done every 5 years with HPV testing. One Level A recommendation for women with low-risk status and consistently negative screenings is discontinuing cervical cytology and HPV testing at age 65. Women who have had a total abdominal hysterectomy, including removal of the cervix for benign disease, do not require subsequent screening.[4]
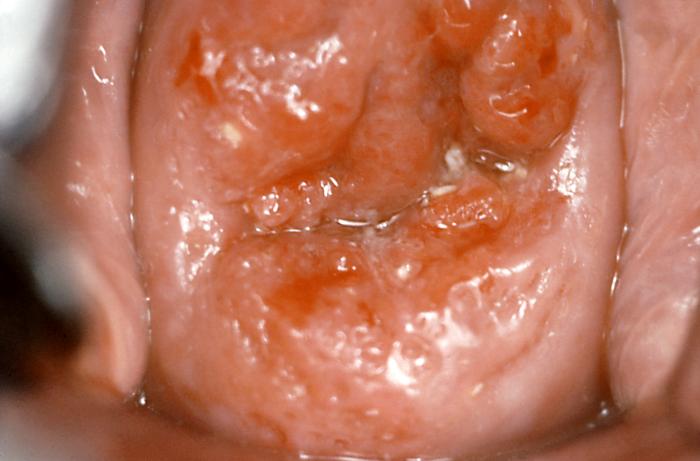
Colposcopy is the diagnostic procedure of choice for evaluating abnormal cytology and/or persistent high-risk HPV infection. The American Society for Colposcopy and Cervical Pathology (ASCCP) has issued guidance on procedural indications, and their recommended algorithms are considered standard of care. Multiple colposcopic-guided biopsies and endocervical sampling are often indicated, except during pregnancy.[21] Abnormal colposcopic findings may include acetowhite color change with the application of acetic acid, rich vascularity, atypical vessels, mosaicism, and punctation (see Image. Invasive Cervical Cancer).
Patients diagnosed with invasive disease require a comprehensive staging workup. The International Federation of Gynecology and Obstetrics (FIGO) staging system employs several methods to stage a patient's disease. Classically, staging was based on the local extent of the tumor, which could be determined with a combination of pelvic examination, cystoscopy, proctoscopy, chest x-ray, intravenous pyrography, and basic labs. More recently, advanced imaging modalities such as MRI and PET scans have been utilized for staging. A pelvic MRI is excellent for detecting local tumor extension and can also be used for monitoring tumor response. PET scans are more sensitive than CT scans for detecting nodal and visceral metastases. This is critical as nodal disease can significantly influence prognosis.[22]
Treatment / Management
Precancerous lesions are managed conservatively for women younger than 25 years. Most positive findings in women younger than 25 years are low-risk cervical dysplasia and will resolve spontaneously. Colposcopy evaluates persistent, abnormal cytology or lesions suspected to be moderate or high risk. These are managed according to findings.
Low-risk lesions may be observed and reevaluated more frequently, and high-risk lesions are treated based on size, depth, and location. Cryotherapy or excision is performed to manage precancerous lesions limited in size and depth. Conization, laser, or loop electrosurgical excision procedure (LEEP) are used to manage lesions that include the endocervical canal and are more extensive. LEEP may provide better visualization of the squamocolumnar junction and provide the benefit of less bleeding in the outpatient setting.[23]
If invasive cancer is diagnosed, the next step in management is staging to determine further treatment. Staging is based on findings and results from reported signs and symptoms, examination, tissue pathology, and imaging. Grading is based on the size and depth of the cancer and signs of spread to other organs. Treatment of early-stage disease is typically surgical resection, ranging from a conization to a modified radical hysterectomy. However, women with high-risk pathology postresection may require adjuvant treatment with chemotherapy and radiation. Conization or trachelectomy may be an option for women with early-stage disease who desire future fertility. For patients with more advanced disease, concurrent chemoradiation is the standard of care.
Differential Diagnosis
Evaluating visible cervical lesions for invasive cancer is essential. However, as discussed above, most cervical cancer is asymptomatic and will not present with an overt mass in the early stages. Other possible causes of cervical lesions and/or abnormal bleeding include STIs, cervical polyps or fibroids, and endometriosis. Diagnosis may require further evaluation of symptoms and testing to determine whether the disease is cervical cancer. A diagnostic biopsy is needed to finalize the diagnosis.
Other pathology-determined conditions in the differential diagnosis include carcinosarcoma, epithelioid trophoblastic tumor, placental site nodule, immature squamous metaplasia, and metastatic disease from a noncervical primary tumor. Rarely, a routine Pap smear may identify metastatic cancer on the uterine cervix.[24]
Surgical Oncology
Surgical resection is offered to patients with early-stage disease confined to the cervix; it can range from relatively noninvasive procedures such as cervical conization to more extensive operations such as radical hysterectomy. Although surgery is the preferred treatment modality for early-stage cervical cancer, it is especially important in younger patients for whom preservation of ovarian function and/or fertility is desired. Surgery is also indicated in select patients with recurrent disease.
Types of Surgery
Cervical conization
Cervical conization is typically indicated in patients with CIS or stage IA1 invasive cervical cancer. Using a scalpel or laser, a cold knife cone (CKC) removes the cervical transformation zone and a portion of the cervix with at least a 3-mm margin. Pathologic evaluation of the margins and assessment of the presence or absence of lymphovascular invasion (LVI) are critical. If a positive margin or LVI is present, reexcision or more invasive surgical treatment may be required.
If no LVI exists on the specimen, lymph node involvement is exceedingly rare, so nodal evaluation is unnecessary. Patients without any adverse pathologic findings may be observed. Recurrence rates are typically <10%, but patients must be followed closely with periodic colposcopy and cytology. Five-year survival rates exceed 95%. Complications include hemorrhage, infection, cervical incompetence, cervical stenosis, and infertility. The complication rate ranges from 2% to 12%.[25][26]
Radical trachelectomy
Patients who are not candidates for conization due to adverse pathological features or more advanced disease but who desire future fertility are candidates for a radical trachelectomy. The procedure consists of removing most of the cervix, resecting the parametria, and mobilizing the ureters, bladder, and rectum. A 5-mm section of the cervix is preserved for the placement of a cerclage, allowing for future pregnancy. Due to the increased risk of nodal involvement, a lymph node evaluation with a sentinel node biopsy or pelvic lymphadenectomy typically accompanies a radical trachelectomy.
Adverse pathologic features such as positive margins, parametria involvement, lymph node involvement, or meeting Sedlis criteria would necessitate adjuvant treatment with radiotherapy with or without chemotherapy. A vaginal approach or laparotomy can be used, but there are insufficient data on minimally invasive techniques. The 5-year recurrence rate is approximately 5%, and the overall survival rate is 97%. The pregnancy rate postprocedure is 24%, with a live birth occurring in 75% of patients.[27] Complications include cervical suture problems, dysmenorrhea, isthmic stenosis, and vaginal discharge.
Extrafascial hysterectomy
Extrafascial hysterectomy, also known as a Type A radical hysterectomy, has a narrow range of clinical indications.[28] Typically, this surgery is offered to patients with stage IA1 disease who are not interested in future fertility; it involves the removal of the entire cervix and uterus. As ovarian removal is optional, ovarian function can be preserved. The parametria are not resected, and a vaginal approach or laparotomy may be utilized. Lymph node evaluations are not usually performed unless adverse pathologic features are discovered postoperatively. Patients with adverse pathologic features may require a complete parametrectomy or external beam radiotherapy with or without chemotherapy.
Radical hysterectomy
A radical hysterectomy may be considered in almost all early-stage cervical cancer cases when fertility preservation is not desired. The older Piver-Rutledge-Smith classification has been replaced by the Querleu–Morrow system, which simplifies the classification process based solely on the extent of lateral parametria resection. Four types of radical hysterectomy are described (Types A through D). Type A includes a minimal parametrial resection. In contrast, Type D completely resects the paracervical region to the pelvic sidewall. The most commonly performed radical hysterectomies fall in the Type B and C categories, which differ in the transection of the paracervical region at the level of the ureters or internal iliac vessels, respectively.[29]
Minimally invasive approaches have been shown to provide inferior oncologic outcomes compared with more established open approaches in terms of disease-free survival (91% vs 97%) and overall survival (93% vs 99%), with most patients having stage IB1 disease.[30] Complications include bleeding, infection, venous thromboembolism, pulmonary embolus, small bowel obstruction, vesicovaginal fistula, hydronephrosis, ureteral injury, urinary stress incontinence, and lower extremity edema.
Laparoscopic radical hysterectomy
Although this procedure offers a quicker recovery for the patient, it has been largely abandoned due to poor oncologic outcomes and increased recurrence compared with open surgery. The mechanism for this difference is unknown, but 2 large independent studies have confirmed this finding.[31][32][33]
Lymph node evaluation
Detecting lymph node involvement is essential, as it yields important prognostic information and guides therapeutic decision-making. The risk of nodal involvement should guide the decision to evaluate the lymph nodes and is a function of the disease stage (see Table. Pelvic Nodal Risk by Stage). Para-aortic (PA) nodal risk typically carries one-half the pelvic nodal risk by stage. Although pelvic lymphadenectomies are considered the gold standard, sentinel node biopsies may also be pursued for select early stage I cervical cancer. Generally, sentinel node biopsies are safe and effective. They have been investigated in stage IA1 to stage IIA1 patients with a sensitivity of 92%, a negative predictive value of 98%, less lymphatic morbidity, and no differences in recurrence-free survival.[34][35] The sentinel node biopsy approach continues to be investigated in large-scale international trials called SENTICOL III (NCT03386734).
Table. Pelvic Nodal Risk by Stage
| Stage | Pelvic Nodal Risk |
| IA1 | <1% |
| IA2 | 6% |
| IB | 15% |
| IIB | 30% |
| III | 45% |
Pelvic exenteration
Pelvic exenteration is the most radical surgical procedure for cervical cancer. Indications are confined to patients with central pelvic recurrence after radiotherapy or patients with stage IVA disease who cannot receive radiotherapy. Classically, a total pelvic exenteration includes the removal of the uterus, fallopian tubes, ovaries, vagina, bladder, urethra, and rectum. Reconstruction consists of an ileal conduit, or continent diversion, for the urinary system and an end colostomy for the GI system. Continent diversions include the Indiana and Miami pouch techniques.[36][37]
Total exenteration is generally performed for cervical cancer. Variations of this technique include anterior and posterior exenteration, which spare the rectum or the bladder, respectively. Exenteration can be further classified into supralevator and infralevator, depending on whether the urogenital diaphragm and levator muscles are removed. It is important to ensure that the central tumor can be completely resected and there is no metastatic disease. Minimally invasive pelvic exenteration has been described in the literature, although further evaluation of this technique is warranted.[38][39]
A neovagina can be formed with a myocutaneuous flap or split-thickness skin graft with an omental J-flap. The posterior supralevator exenteration approach allows for the possibility of maintaining not only urinary function but fecal continence if a colonic anastomosis can be formed. The 5-year survival rate for patients treated with exenteration ranges from 40% to 50% in the recurrent setting.[40] At 3 and 5 years, local recurrence rates are 84% and 75%, respectively.[41] Survival is not impacted by the type of exenteration performed.[42] Mortality from the operation has fallen dramatically over the last 70 years to less than 5%, but surgical morbidity is over 50%.[41] Early complications include infection, abscess formation, thromboembolism, fistulas, urinary leakage, peritonitis, stoma necrosis, flap necrosis, and stump dehiscence. Late complications include stoma stenosis, incontinence, hydronephrosis, stone formation, chronic pain, and abdominal wall hernia.
Cervical Cancer in Pregnancy
Although cervical cancer is one of the most common malignancies diagnosed in pregnancy, it poses unique staging and treatment challenges in pregnant patients. A maternal-fetal medicine specialist should evaluate patients to discuss fetal risks and potential pregnancy loss. Women must weigh the risk of delaying treatment until after delivery versus proceeding immediately with treatment.
The determination of gestational age is crucial. Minimum fetal viability is approximately 24 weeks gestation, but there is a significant risk of neonatal death or long-term disability in those who survive. Disability-free survival at 25 years increases substantially with advancing gestational age, with 4% of infants born at 22 weeks versus 78% for those born at 28 weeks gestation. In contrast, full-term infants have a disability-free survival of 97%.[43]
While the radiation delivered during a PET or CT scan remains far below the threshold for the development of congenital malformations and pregnancy loss, ionizing radiation in imaging studies should be kept to a minimum in this population.[44] Other imaging modalities, such as ultrasound and MRI, are preferred when appropriate to determine local tumor extent and distant metastatic involvement. However, MRI and ultrasound have a low sensitivity for small nodal metastases. Consequently, more invasive staging techniques may need to be employed.
In patients with a high risk of nodal metastasis, laparoscopic pelvic lymphadenectomy may be performed to establish the disease stage.[45] The safety of this procedure has only been studied in limited case series, but it can be performed in any trimester (although earlier in the pregnancy is preferred). These techniques may still be employed when advanced imaging is unavailable or contraindicated. In addition, the FIGO staging system continues to allow the use of proctosigmoidoscopy and cystoscopy for local staging of cervical cancer.
Treatment strategies must be individualized and discussed in a multidisciplinary fashion with medical oncologists, radiation oncologists, obstetrician/gynecologists, maternal-fetal medicine specialists, and gynecologic oncologists. Generally, patients with pregnancies at gestational ages under 22 weeks and stage IA1 disease can be treated with conization, but this carries a 15% risk of significant bleeding and spontaneous abortion. Patients with pregnancies at gestational ages above 22 weeks may be able to delay treatment until after delivery if they have early-stage disease. Patients with more advanced disease (>IB1) may receive platinum-based neoadjuvant chemotherapy (cisplatin/paclitaxel) until delivery, followed by a cesarean radical hysterectomy. Limited data suggest this is a safe regimen for both mother and fetus.[46] Small series indicate approximately a 75% tumor response rate, with 15% of patients experiencing a local recurrence. The risk of fetal toxicity with these regimens is unknown, but complications may include respiratory distress syndrome, malformations, and childhood malignancies.[46] International guidelines for treatment and fetal preservation have been published.[47]
Immediate treatment is recommended for patients with documented lymph node metastasis, disease progression, or pregnancy termination. If the patient opts to terminate the pregnancy before treatment, definitive treatment recommendations are identical to those of a nonpregnant patient.
Radiation Oncology
Radiotherapy remains a crucial component in the treatment of cervical cancer. Randomized evidence from the 1990s and early 2000s has established radiotherapy in almost every facet of treatment; it may be utilized as a definitive or adjuvant treatment with or without platinum-based chemotherapy.
Definitive Radiotherapy
Early-stage cervical cancer
Radiotherapy may be utilized as the sole treatment modality in early-stage cervical cancer (stages IA1 to IIA1). External beam radiotherapy (EBRT) with a brachytherapy (BT) boost has less morbidity and equivalent 5- and 20-year overall survival rates (83% and 75%, respectively) compared with radical hysterectomy.[48][49] Some centers may perform an adjuvant hysterectomy for bulky tumors after chemoradiation therapy. However, most guidelines do not recommend this due to significant complication rates. Recent data suggest that survival rates after chemoradiation therapy and adjuvant hysterectomy are suboptimal.[50]
Advanced cervical cancer
Locally aggressive and/or node-positive diseases are typically treated with definitive concurrent platinum-based chemoradiotherapy followed by a BT boost. The addition of chemotherapy to definitive radiotherapy has resulted in considerable improvement in overall survival compared with radiotherapy alone, with an 8-year overall survival of 67% versus 41%. Improvements in local recurrence and distant metastasis have also been noted.[51]
Postoperative radiotherapy
Radiotherapy with or without chemotherapy is recommended in the postoperative setting when specific pathologic findings are present. These findings are thought to represent an increased risk of recurrence.
Conventionally, the Sedlis criteria guided the use of adjuvant radiotherapy without chemotherapy in postradical hysterectomy patients with at least 2 of the following 3 features: tumor size greater than 4 cm, LVI, or more than one-third stromal invasion.[52] These criteria were meant to identify patients with at least a 30% risk of relapse at 3 years.[53] Patients who met these criteria and were treated with pelvic radiotherapy were noted to have improved progression-free survival (78% vs 65%) and local recurrence (21% vs 14%).[52] More recently, there have been concerns that these criteria may overlook women who might benefit from adjuvant radiotherapy due to such a high threshold. A nomogram incorporating the original Sedlis criteria and tumor histology has been developed to provide a more linear and continuous risk assessment rather than a simple threshold.[53]
Classically, the addition of chemotherapy to radiotherapy was guided by the Peters trial, which randomized patients with positive nodes, involved parametria, or positive surgical margins to radiotherapy alone or radiotherapy with concurrent platinum-based chemotherapy. The addition of chemotherapy resulted in a 10% improvement in overall survival at 4 years and almost a 20% improvement in progression-free survival over the same time frame.[54] More contemporary studies such as the STARS trial have sought to expand the use of chemotherapy in the adjuvant setting in patients meeting either the original Sedlis or Peters criteria.[55]
Delivery Techniques
The 2 major delivery methods include ERBT, directed at the primary tumor and pelvic lymphatics, and BT, in which a sealed radiation source is placed in close proximity to the tumor.
EBRT
EBRT techniques include 3-dimensional conformal radiation therapy (3D-CRT) or intensity-modulated radiotherapy (IMRT). Intact cervical cancer patients’ plans can be employed. A reduction in gastrointestinal and hematological adverse effects has been documented with IMRT in both adjuvant and definitive settings.[56][57]
ERBT simulation
Patients undergoing EBRT can be placed in the supine position. Accounting for cervical motion is essential when using IMRT. This is accomplished by taking 2 separate scans of patients, first with a full bladder followed by an empty bladder. Prone positioning may also be accomplished using a belly board; however, if there are large daily fraction shifts, IMRT may not be reproducible. Prone positioning may allow for a reduction in the small bowel dose when using IMRT.[58]
ERBT target delineation
Traditionally, pelvic fields were drawn on 2-D x-rays consisting of anterior-posterior (AP)/posterior-anterior (PA) and opposed lateral fields comprising the 4-field box. The superior edge of the field was the bottom of L4 and inferiorly drawn to the bottom of the obturator foramen (or at least 3 cm below the lowest extent of disease). The lateral fields have the same superior/inferior borders, with the anterior border being the anterior pubic symphysis and the posterior border being the sacral hollow, including S2.
More precise delineation of the gross disease and elective volumes can be accomplished in the era of CT-based planning, PET/CT fusions, and IMRT. A gross tumor volume (GTV) consists of gross disease seen on a CT, PET, and physical examination. An internal target volume (ITV) accounts for variation in bladder filling and can also be employed, but this requires the patient to have 2 CT simulations (empty and full bladder). The clinical tumor volume (CTV) 1 expansion includes the entire cervix and uterus (if intact). Planning target volume (PTV) 1 on primary disease is typically 1.5 cm. The CTV2 includes the parametrial tissue, paravaginal tissues, and at least one-half of the upper vagina. If there is vaginal involvement, consideration should be given to covering the entire vagina. This PTV2 expansion should be 1.0 cm. The elective nodal volumes in the CTV3 should include obturator nodes, external iliac nodes, internal iliac nodes, and presacral nodes. If there is lower vaginal involvement, consideration should be given to coverage of the inguinal nodes. PTV expansion on the elective nodes is typically 0.7 cm.
Coverage of the PA nodes may be necessary in cases with evidence of disease in the nodal chain, or in cases in which the patient has a positive pelvic node and will not receive systemic therapy. In those instances, the superior boundary would become the T12/L1 interspace, with the nodal strip ending at the top of the pelvic field L5/S1.[59][60]
ERBT dosing and dose constraints
The standard whole pelvic dose is 45 to 50.4 Gy, specifically 1.8 to 2.0 Gy per fraction. Any gross nodal disease may be boosted to 60 Gy if possible, given that organs-at-risk (OAR) constraints are not exceeded.
EBRT has dramatically improved with the adoption of IMRT, reducing acute toxicity while maintaining oncologic outcomes. Typical OAR include the rectum, bladder, bowel, femoral heads, and bone marrow. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC) dose constraints can be an excellent guide. Typically, with EBRT, doses of 45 to 50 Gy alone will not lead to significant rates of acute bladder or bowel toxicity.[57]
If BT is planned, minimizing these doses during the external beam phase of treatment will allow higher doses to be delivered during the boost phase of treatment. Bone marrow suppression resulting in grades 3 to 4 neutropenia has been demonstrably lower (8.6% vs 27.1%) with image-guided radiotherapy and IMRT.[56] The most common constraints are for bone marrow in the pelvis.[56][57][61]
BT
BT can be used alone in early-stage cervical cancer or as a boost after EBRT in more advanced disease (stages IB2 to IVA), as it allows for highly conformal dose delivery to the tumor while minimizing exposure to normal tissues. The dose delivery is controlled by adjusting the dwell times within the delivery device. High dose-rate (HDR) BT (>12 Gy/hr) is the most commonly used technique; however, it requires a radioactive source, usually iridium-192.
Typically, BT is started after the external beam portion of the treatment or interdigitated with EBRT in the last week of treatment. EBRT and BT should not be administered on the same day. Total treatment time (EBRT and BT) should not exceed 8 weeks. Excessive treatment times can result in a 1% per day decline in local control and overall survival.
Before undergoing BT, a review of the patient’s history, physical exam, pathology, and imaging should be conducted. A complete blood count and metabolic panel should be obtained within a week of the procedure. Metabolic derangements should be investigated and corrected before the procedure. Patients with an absolute neutrophil count (ANC) less than 500 mm should not undergo the procedure until their count has recovered. A review of medications, especially anticoagulants, should be completed, and a prothrombin time/international normalized ratio result should be obtained. Careful consideration should be given to holding anticoagulant medications before the procedure. Inpatients confined to bed should be given thromboprophylaxis and sequential compression devices. Bowel preparation should also be reviewed with the patient before the procedure.
BT applicators
There are several applicators that may be used in various clinical scenarios. The ring and tandem is the most common device for intact cervical cancer. The tandem is placed in the cervical canal while the ring is placed inside the vaginal fornices. Tandem and ovoids are utilized similarly but are preferred in patients with a barrel-shaped cervix. Interstitial applicators such as the Syed template can be used in patients with extensive parametria involvement, pelvic sidewall involvement, lower vaginal involvement, or vaginal cuff recurrence. Tandem and cylinder applicators can be used in cases of vaginal stenosis, inability to place a ring or ovoid, or lower vaginal involvement less than 5 mm thick. Modifications to the tandem and ring or vaginal cylinder can also be made to accommodate interstitial needles.[62][63]
Patient discomfort experienced during the procedure can lead to suboptimal placement of the applicator, longer procedure times, and distress for the patient. An anesthetic is typically required to help optimize patient comfort and procedure mechanics. The types of anesthesia administered vary but may include general anesthesia, spinal anesthesia, epidural anesthesia, intravenous conscious sedation, and oral pain medication.
Placement of an intracavitary applicator for an intact cervix
The patient is placed in the dorsal lithotomy position in stirrups. After adequate sterile preparation of the area, a Foley catheter is inserted, and the balloon is inflated with dilute contrast to allow for detection with CT scan. A speculum is inserted to visualize the cervix, and a uterine sound is used to determine the length of the cavity. This will aid in determining the length and angle of the tandem. A Smit sleeve is occasionally used to maintain the patency of the cervical os but, if not available, serial dilations may be required. Once this is complete, the tandem is inserted, followed by the ring or ovoid. Fiducial markers may also be placed during the procedure to outline the extent of the disease or the opening of the cervical os so it is visible on CT scan. These applicators can be locked so their relative positions do not change.
Displacement of the bladder and rectum is critical to reduce the excess dose and limit toxicity. The packing material can consist of gauze soaked in a radiopaque solution. Applicators fitted with inflatable balloons or separate rectal blades are also available. Anterior packing displaces the bladder, whereas posterior packing displaces the rectum. It is essential to ensure no packing is placed in front of the ring or ovoids, as this will significantly reduce the intended dose.
Once complete, the patient will undergo a CT simulation for 3-D BT planning. Three-dimensional CT-based planning has been shown to have improved overall survival (65% vs 74%) and lower rates of grades 3 to 4 toxicity (23% vs 3%) compared with 2-D planning.[64] A 1- to 5-mm slice thickness is recommended.[62][63] More recently, MRI-guided BT has been incorporated into planning for improved tissue delineation, best seen on the T2-weighted fat-suppressed sequences (T2–fast-spin echo) imaging.[65]
Paraxial and para-coronal images should be obtained concerning the cervix-uteri and sagittal images.[66] These images can also allow for the assessment of chemoradiation response. Technically, low- and high-intensity magnetic field MRI machines can be used. Patients can be given glucagon before MRI to reduce bowel motion. A pelvic coil is recommended to increase the signal-to-noise ratio. Ideally, a 3-mm slice thickness should be obtained, which helps improve the detection of parametrial involvement, but a slice thickness of less than 5mm is acceptable. The disadvantages of this approach are the increased costs, longer procedure times, and the need for strict compatibility of the materials utilized.
Proper applicator placement should be confirmed with plain radiography or more advanced imaging such as a CT scan or MRI. Adequate placement includes the tandem bisecting the ring/ovoids on AP and lateral imaging, the tandem being one-third to one-half the distance between the sacral promontory and the pubic symphysis, the tip of the tandem being below the sacral promontory, no packing located superior to the ring/ovoids, and no inferior displacement of the ring/ovoids relative to the flange.
With HDR BT, a remote afterloading technology allows a small iridium source attached to the end of a cable to be robotically driven through multiple channels, stopping at predetermined points (dwell positions) for varying lengths of time.[67] A Cochrane review failed to show differences between HDR and low dose-rate (LDR) intracavitary brachytherapy (ICBT). Oncologic outcomes included overall survival, disease-free survival, and recurrence-free survival, as well as local control rate, recurrence, and metastasis. There was no difference in treatment-related complications. Regardless, this review recommends HDR ICBT for all clinical stages of cervix cancer due to the advantages of HDR ICBT, including accuracy of the source, applicator positioning, outpatient treatment, and patient convenience.[68]
BT target delineation
As part of 3-D–based planning, contouring the targets is essential. The Groupe Européen de Curiethérapie and the European Society for Radiotherapy and Oncology (GEC-ESTRO) have established standardized terminology regarding target delineation.[66] The high-risk clinical target volume (HR-CTV) consists of the entire cervix and all gross disease at the beginning of BT treatment; the intermediate-risk clinical target volume (IR-CTV) incorporates the HR-CTV and gross disease before any treatment; and the low-risk clinical target volume (LR-CTV), which includes the IR-CTV as well as the entire uterus, upper vagina, entire parametria, and spaces between the bladder and rectum.[69] Normal OAR should be contoured, including the bladder, rectum, sigmoid colon, and vagina.
BT dose and dose constraints
Ensuring adequate dose to the target is critical and has been shown to lead to improved local control and survival. Dose and fractionation schemes may vary by institutional preference, but they must result in a total equivalent dose in 2 Gy fractions (EQD2) of 85 Gy or higher, assuming 45 Gy was initially delivered to the pelvis. There are several calculators to determine EQD2. One of these, colloquially known as the “Vienna Spreadsheet,” was used by the Effects of a Multifaceted Intervention on Blood Pressure Actions in the Primary Care Environment (EMBRACE) trial group; it provides EQD2 calculations to the target structures and OAR. The most common dose fractionation schemes include 4 x 7 Gy, 5 x 6 Gy, and 6 x 5 Gy, with an EQD2 of approximately 90.1, 88.6, and 83.7 Gy, respectively.
For 3D-based planning, the critical dosimetric parameter is the D90 greater than or equal to 90%. The goal is for 90% of the HR-CTV to be covered by at least 90% of the prescription dose. The EQD2 should be calculated to ensure the total dose received is approximately 85 Gy or higher, although there are instances in which it is acceptable for the EQD2 to be slightly lower. In patients with a complete response before BT or partial response with less than 4 cm in residual disease, an EQD2 above 80 Gy may be used, whereas for those patients with more than 4 cm residual disease, an EQD2 greater than 85 Gy is recommended.[62][63]
Despite the transition to 3D-based volumetric planning and the continued evolution of BT treatment, 2-D dosimetric reporting systems persist and should be understood. Point A, where the uterine artery crosses the ureter, is located 2 cm up the tandem and 2 cm lateral to the tandem and was originally part of the Manchester system. This point was traditionally where the prescription dose was prescribed, but more recently has become a starting point where dose coverage can be further manipulated in 3-D to ensure coverage of the HR-CTV. Point B, also part of the original Manchester system, is located 2 cm up the tandem and 5 cm from the patient’s midline. This location represents the pelvic side wall lymphatics and typically receives one-third of the prescription dose. It has fallen out of favor and is no longer reported. The isodose lines for typical tandem and ring/ovoid implant should appear pear-shaped. In addition, the International Commission on Radiation Units and Measurements (ICRU 38) specified a bladder and rectal point. The bladder point is located posterior to the Foley balloon that has been pulled down to the neck of the bladder. The rectal point is 5 mm posterior to the vaginal wall.
Doses to OAR must also be carefully documented. Again, it is the cumulative dose of EQD2 that is critical. The dosimetric parameter D2 cc, which represents the highest dose received by 2 ccs of tissue, is commonly used for evaluating a BT plan. The American Brachytherapy Society (ABS) guidelines allow for the D2 cc of the bladder to receive 90 Gy or less EQD2, whereas the D2 cc of the rectum and sigmoid should be 75 Gy or less EQD2. However, recent data suggest that late rectal morbidity may be substantially lower even using a D2 cc of 65 Gy or less.[70]
Radiation Therapy Complications
Long-term toxicity is a concern with any patient receiving radiation therapy. Bowel, rectal, and urinary complications are the most frequent. There does not appear to be a difference in the frequency or severity of complications with respect to age. The greatest risk of late sequelae typically occurs within 3 years of treatment.
Proctitis
Radiation proctitis can result in tenesmus and intermittent bleeding with bowel movements. Postradiation proctitis typically occurs 3 months after treatment at the earliest but may take years to develop. Treatments can include mesalamine or steroid-based suppositories to relieve pain and stop bleeding. Randomized trials have suggested that mesalamine may be slightly more efficacious than steroid-based suppositories.[71] Other treatments can include a 4% formaldehyde application to control rectal bleeding. In refractory cases, argon plasma laser coagulation may be performed on the offending vessels.
Cystitis
Radiation cystitis can lead to dysuria, urinary frequency, and, in some cases, hematuria. This complication can occur acutely within 2 to 3 weeks of starting radiotherapy and up to 3 years posttreatment.
For acute cystitis, a urinary tract infection must be ruled out; therefore, a urinalysis is an appropriate first step in the diagnosis. A short course of phenazopyridine can be prescribed for dysuria relief, although the urine color change may be alarming for some patients. Patients should be made aware of this change before starting the medication. Urinary frequency can be treated with anticholinergic drugs such as oxybutynin or mirabegron, although caution is advised in elderly patients.
Chronic cystitis has an incidence of 5% to 10%.[72] These patients are more likely to experience hematuria in addition to frequency and dysuria. The severity can range from mild to severe and life-threatening. Mildly symptomatic patients may be managed conservatively. Any antiplatelet or anticoagulation medications the patient takes should be reviewed, and the necessity questioned. A cystoscopy with clot evacuation and irrigation is necessary in more severe cases. During a cystoscopy, formalin instillation can be used to manage intractable hematuria. In refractory cases, hyperbaric oxygen therapy has been utilized and shown to relieve and control bleeding in 92% of patients; however, recurrences may occur.[73] Barotrauma is a risk associated with this procedure.
Secondary malignancy
Radiation-induced cancers tend to appear several decades after treatment. The overall risk of secondary malignancy is increased with pelvic radiotherapy compared to those treated with surgery alone. Women younger than 50 years had a 40-year cumulative risk of 22% versus 16% for those older than 50 years.[74] Secondary cancers tend to be confined to the rectum, bladder, lungs, and genitals. Other retrospective studies have shown an increase in leukemia risk, which peaks around 5 to 10 years after treatment.[75] Limiting normal organ doses may help to reduce the risk.
Ovarian failure
Radiation-induced ovarian failure typically occurs within 6 to 12 months after radiation. Minimum doses for ovarian failure are inversely related to age ranging from 20.3 Gy at birth to 14.3 Gy at the age of 30 years.[76] The doses used for cervical cancer can easily precipitate ovarian failure. Premenopausal patients have several options for preserving ovarian function, namely definitive surgery or ovarian transposition. Laparoscopic ovarian transposition allows the ovaries to be placed outside the radiation field, usually 3 cm away. This procedure is also useful for fertility preservation. The functional preservation rate is approximately 80%. Ovarian cryopreservation is another option. Exogenous hormonal supplementation with estrogen should be considered to prevent osteoporosis and osteopenia and to maintain libido.
Vaginal stenosis
Vaginal stenosis and vaginal canal shortening can develop over months to years after treatment. Stenosis can make it difficult for intercourse and gynecological exams. Consistent use of a vaginal dilator is typically recommended; however, compliance is highly variable. Sexual dysfunction is quite common, ranging from a lack of desire for sexual activity to a lack of adequate vaginal lubrication.
Bone fracture
Pelvic radiotherapy can increase the risk of pelvic fractures, with most fractures occurring at dose levels of 45 to 63 Gy. Bone health is another concern in patients who undergo radiation to the pelvis. Approximately 10% of patients radiated for cervical cancer experience a pelvic fracture. The most common fracture site is the sacrum, most occurring within 2 years after treatment. Bone mineral density scans and appropriate intervention to maintain bone health may help to reduce this risk.
Uterine perforation
Uterine perforation is a complication related to BT applicator insertion and results in excessive doses to normal tissues, poor target coverage, bleeding, and infection. Rates of perforation range from 2% to 18%.[77][78][79] Management is controversial, ranging from postponement of BT to allow for healing to outright abandonment of the procedure. However, treatment delays are known to adversely affect patient outcomes. The risk of vascular and organ injury is low when a blunt instrument causes perforation. Stable patients without signs of infection may be discharged and observed. Prophylactic antibiotics may be prescribed. Signs of hemodynamic instability and infection warrant more aggressive approaches with IV fluids, antibiotics, and surgical exploration.
Medical Oncology
Definitive Therapy
The Radiation Therapy Oncology Group (RTOG) trials established the utility of platinum-based chemotherapy regimens. Compared with radiotherapy alone, there are consistent overall survival, disease-free survival, and local control advantages.[51] It is postulated that chemotherapy acts as a radiosensitizer. The most common agent used is weekly cisplatin. Single-agent platinum-based regimens have the best progression-free survival and overall survival compared to non-platinum-based regimens and a better toxicity profile than combinations of platinum-based regimens such as cisplatin/5-FU/hydroxyurea.[80] Carboplatin may also be used in patients who cannot tolerate cisplatin. Some practices continue to use a combination of cisplatin/5-FU. This regimen is delivered concurrently with radiotherapy.
Adjuvant Therapy Chemoradiotherapy may be added after surgical resection should the patient have high-risk features. Overall survival and progression-free survival benefits have been demonstrated with the addition of chemotherapy to radiotherapy in certain high-risk postoperative patients.[54]
The use of adjuvant chemotherapy after definitive chemoradiation continues to be investigated in clinical trials. The results have been mixed. Adding adjuvant carboplatin/taxol to chemoradiation for locally advanced cervical cancer resulted in higher rates of grades 3 to 5 toxicity and no difference in overall survival or progression-free survival. Another trial using concurrent and adjuvant cisplatin/gemcitabine demonstrated improved 3-year progression-free survival but markedly higher rates of grades 3 to 4 toxicity and hospitalizations.[81] RTOG 0724 is an ongoing trial investigating the use of adjuvant cisplatin/paclitaxel after definitive chemoradiation in high-risk postoperative patients (NCT00980954).
Recurrence and Metastasis
In recurrent and metastatic disease settings, patients who are not candidates for exenterative surgery or radiotherapy may receive systemic therapy alone. Many patients have previously received single-agent cisplatin-based therapy; therefore, multidrug regimens are typically used. Cisplatin/paclitaxel has been shown to improve progression-free survival in patients with recurrent cervical cancer but yields no difference in median overall survival.[82] Although other drug combinations such as cisplatin/topotecan, cisplatin/gemcitabine, and cisplatin/vinorelbine are potential options, the Gynecologic Oncology Group (GOG) has suggested that these regimens are not superior to cisplatin/paclitaxel (protocol 204).[83] Incorporating biological agents such as vascular endothelial growth factor receptor antagonists like bevacizumab into standard chemotherapeutic regimens has improved overall survival.[84] Immunotherapy with PD-1 inhibition has also been incorporated into chemotherapeutic regimens. Single-agent pembrolizumab has an objective response rate of 12% to 14% in patients with recurrent or metastatic cervical cancer.[85] Keynote-826, a randomized, double-blinded phase 3 study, demonstrated the addition of pembrolizumab to multidrug chemotherapy improved overall and progression-free survival.[86] Follow-up subgroup analysis confirmed this finding in a Japanese population.[87]
Complications
The most commonly used platinum-based drugs are cisplatin and carboplatin. Common adverse effects include neutropenia, thrombocytopenia, anemia, febrile neutropenia, nephrotoxicity, neurotoxicity, and infection. Although cisplatin is the drug of choice, carboplatin can be used in patients who may not tolerate cisplatin, particularly if they already have underlying renal disease. Carboplatin is thought to have lower efficacy than cisplatin; however, prospective data suggest noninferiority in effectiveness and a statistically significant lower incidence of febrile and nonfebrile neutropenia and creatinine elevation.[88]
Bevacizumab carries the risk of hypertension, hemorrhage, thromboembolic events, renal injury, and ovarian failure in premenopausal women. Pembrolizumab is known for precipitating autoimmune phenomena such as pneumonitis, colitis, hepatitis, nephritis, and endocrinopathies.
Staging
The International Federation of Gynecology and Obstetrics (FIGO) staging system was updated in 2018 and remains the dominant staging methodology. The 8th edition of the American Joint Committee on Cancer (AJCC) Staging Manual also has a tumor-node-metastasis classification system in which the tumor stages correspond with the FIGO stages; however, it is not regularly used.
FIGO Staging
Classically, FIGO staging would rely on clinical examination, cystoscopy, proctoscopy, hysteroscopy, urography, and plain film x-ray. These relatively basic tests were utilized so developing countries with fewer healthcare resources could adequately stage patients. More recently, advanced imaging techniques such as MRI and PET scans have become part of the staging workup. MRI is preferred for establishing the tumor stage, given superior tissue delineation compared with CT scan with contrast.[89][90]
Stage I
- The disease is strictly confined to the cervix, with the A/B designation indicating the depth of invasion (≤5 or >5 mm).
Stage II
- The disease invades beyond the uterus but has not extended into the lower vagina.
- This stage also has an A/B designation based on involvement of the parametria.
Stage III
- The disease has extended to the lower one-third of the vagina (IIIA) or to the pelvic side wall and/or hydronephrosis (IIIB).
Classically, nodal disease did not influence the FIGO staging system; however, it has been shown that nodal disease is one of the most important prognostic indicators for reduced 5-year overall survival.[91] As a result, new stages (IIIC1 and IIIC2) were added to reflect the involvement of pelvic or PA nodes, respectively.
Stage IVA
- The disease is locally aggressive, involving adjacent organs such as the bladder or rectum.
Stage IVB
- The disease has spread to other solid distant organs; this stage can also be indicative of nonregional nodal disease.
Prognosis
Multiple factors contribute to differences in patient outcomes. Potential prognostic factors for cervical cancer patients include disease stage, number of retrieved lymph nodes, use of a uterine manipulator in laparoscopic treatment, age, race, ethnicity, general health before diagnosis, and access to evidence-based care. These prognostic factors highlight opportunities to enhance care and improve overall mortality.
When diagnosed early, the 5-year relative survival rate for cervical cancer is approximately 92%. About 44% of cervical cancer patients are diagnosed at an early stage. Inconsistent screening is an independent risk factor for late diagnosis.[92] A higher stage at presentation decreases survival and increases the chance of recurrence. The 5-year relative survival rate drops to 60% when cervical cancer is diagnosed after it has spread locally or spread to regional lymph nodes. With distant metastasis at diagnosis, the 5-year relative survival rate is 19%.
Studies show certain surgical factors affect survival. It is believed that the number of retrieved lymph nodes corresponds to the thoroughness of surgical treatment. Research indicates that a higher retrieved lymph node count has a statistically significant positive effect on progression-free survival. For women having laparoscopic surgery, the use of a uterine manipulator is associated with a better prognosis.[93]
Survival rates also differ based on race. The 5-year relative survival rate is 67% for women of all races. Black women tend to have the highest mortality and lowest survival rate, with a 5-year relative survival rate of approximately 56%.[11][94]
Patient age at diagnosis also contributes to differences in prognosis and survival, independent of disease stage and histology. Older age is associated with lower survival rates. Patients younger than 50 years, aged 50 to 64 years, and 65 years and older have relative 5-year survival rates of 77%, 61%, and 46%, respectively. Studies indicate older women are a high-risk subset, often receive suboptimal treatment, and die more frequently. Even with BT alone, older women with cervical cancer obtain significant survival benefits.[95]
Complications
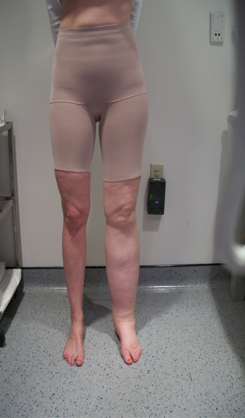
Complications of advanced disease and associated treatments are similar to other cancers. These late complications may include renal failure, hydronephrosis, pain, lymphedema, bleeding disorders, and fistulas (see Image. Secondary Lymphedema).[96]
Deterrence and Patient Education
Traditional and innovative patient education methods can increase overall awareness of cervical cancer and the need for prevention and early screening.[97][98] Literature shows that healthcare professionals may not consistently recommend or discuss HPV vaccination with all target patients. Some women and parents of young daughters may also have reservations about vaccinations that prevent them from being vaccinated. Additional medical education for clinicians serving high-risk populations may increase awareness, prevention, and screening of those patients at risk for the highest mortality.[99]
Although a patient may prefer counseling directly from the healthcare professional, additional community outreach efforts are necessary. Culturally sensitive information, appropriate language to reach lower health literacy populations, and targeted efforts to reach women not yet sexually active are needed to expand patient awareness and education and to initiate screening beyond the clinical setting.[100][101]
Pearls and Other Issues
Primary prevention of cervical cancer involves HPV vaccination to prevent cervical cancer. The estimated effectiveness of HPV vaccination is 90%.[102] A quadrivalent vaccine that prevents cervical cancer and genital warts caused by low-risk HPV types is widely available in the United States. The recommended and approved age for vaccination is 9 to 45 years for both females and males. Vaccination can significantly impact cervical cancer mortality in women in low-resource areas and those in high-risk racial and ethnic groups. A vaccine covering only HPV-16 and -18 is no longer marketed in the United States. However, its use may continue in areas outside the United States.
Enhancing Healthcare Team Outcomes
The interprofessional team can provide public health education and multidisciplinary care to improve cervical cancer awareness, prevention, screening, and management.[103][104][105] Primary care clinicians performing cervical cancer screening, colposcopies, and LEEP procedures, must have ongoing dialogues with gynecologists about findings of suspicious cervical lesions, management, and treatment. Appropriate protocols and guidelines across healthcare systems can improve outcomes by optimizing treatment and follow-up. Developing a culturally sensitive system directed at increasing patient-centered education will require the input of diverse healthcare professionals and staff with multilingual skills and cross-cultural competency.
The interprofessional team can optimize the treatment of patients with cervical cancer through communication and coordination of care. Primary care physicians, gynecologists, gynecologic oncologists, radiation oncologists, and advanced practice practitioners provide diagnoses and care plans. Specialty care, ambulatory care, and oncology nurses should work with the team to coordinate care, support patient education, and provide feedback to the rest of the team. Pharmacists should evaluate vaccinations and medications, recognize drug-to-drug interactions, provide patient education, and monitor compliance. Together, the team can improve outcomes for patients with cervical cancer.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Brisson M, Drolet M. Global elimination of cervical cancer as a public health problem. The Lancet. Oncology. 2019 Mar:20(3):319-321. doi: 10.1016/S1470-2045(19)30072-5. Epub 2019 Feb 19 [PubMed PMID: 30795952]
Pimple SA, Mishra GA. Global strategies for cervical cancer prevention and screening. Minerva ginecologica. 2019 Aug:71(4):313-320. doi: 10.23736/S0026-4784.19.04397-1. Epub 2019 Feb 22 [PubMed PMID: 30808155]
. Cervical Cancer Screening Every 5 Years OK. Cancer discovery. 2018 Oct:8(10):1204. doi: 10.1158/2159-8290.CD-NB2018-118. Epub 2018 Sep 11 [PubMed PMID: 30206109]
Farghaly H, Bourgeois D, Houser PM, Padmanabhan V, Lage JM, Hoda RS. Routine vaginal Pap test is not useful in women status-post hysterectomy for benign disease. Diagnostic cytopathology. 2006 Sep:34(9):640-3 [PubMed PMID: 16900480]
Foran C, Brennan A. Prevention and early detection of cervical cancer in the UK. British journal of nursing (Mark Allen Publishing). 2015 May 28-Jun 10:24(10):S22-4, S26, S28-9. doi: 10.12968/bjon.2015.24.Sup10.S22. Epub [PubMed PMID: 26018178]
Pierre-Victor D, Stephens DP, Omondi A, Clarke R, Jean-Baptiste N, Madhivanan P. Barriers to HPV Vaccination Among Unvaccinated, Haitian American College Women. Health equity. 2018:2(1):90-97. doi: 10.1089/heq.2017.0028. Epub 2018 Jun 1 [PubMed PMID: 29904749]
Manini I, Montomoli E. Epidemiology and prevention of Human Papillomavirus. Annali di igiene : medicina preventiva e di comunita. 2018 Jul-Aug:30(4 Supple 1):28-32. doi: 10.7416/ai.2018.2231. Epub [PubMed PMID: 30062377]
Ghosh I, Mandal R, Kundu P, Biswas J. Association of Genital Infections Other Than Human Papillomavirus with Pre-Invasive and Invasive Cervical Neoplasia. Journal of clinical and diagnostic research : JCDR. 2016 Feb:10(2):XE01-XE06. doi: 10.7860/JCDR/2016/15305.7173. Epub 2016 Feb 1 [PubMed PMID: 27042571]
Habtemariam LW, Zewde ET, Simegn GL. Cervix Type and Cervical Cancer Classification System Using Deep Learning Techniques. Medical devices (Auckland, N.Z.). 2022:15():163-176. doi: 10.2147/MDER.S366303. Epub 2022 Jun 16 [PubMed PMID: 35734419]
Kuhn L, Denny L. The time is now to implement HPV testing for primary screening in low resource settings. Preventive medicine. 2017 May:98():42-44. doi: 10.1016/j.ypmed.2016.12.030. Epub 2017 Feb 6 [PubMed PMID: 28279263]
Rauh-Hain JA, Melamed A, Schaps D, Bregar AJ, Spencer R, Schorge JO, Rice LW, Del Carmen MG. Racial and ethnic disparities over time in the treatment and mortality of women with gynecological malignancies. Gynecologic oncology. 2018 Apr:149(1):4-11. doi: 10.1016/j.ygyno.2017.12.006. Epub [PubMed PMID: 29605048]
Level 2 (mid-level) evidenceWang X, Huang X, Zhang Y. Involvement of Human Papillomaviruses in Cervical Cancer. Frontiers in microbiology. 2018:9():2896. doi: 10.3389/fmicb.2018.02896. Epub 2018 Nov 28 [PubMed PMID: 30546351]
Romero-Masters JC, Lambert PF, Munger K. Molecular Mechanisms of MmuPV1 E6 and E7 and Implications for Human Disease. Viruses. 2022 Sep 28:14(10):. doi: 10.3390/v14102138. Epub 2022 Sep 28 [PubMed PMID: 36298698]
Choi PW, Liu TL, Wong CW, Liu SK, Lum YL, Ming WK. The Dysregulation of MicroRNAs in the Development of Cervical Pre-Cancer-An Update. International journal of molecular sciences. 2022 Jun 27:23(13):. doi: 10.3390/ijms23137126. Epub 2022 Jun 27 [PubMed PMID: 35806128]
Nowakowski A, Cybulski M, Buda I, Janosz I, Olszak-Wąsik K, Bodzek P, Śliwczyński A, Teter Z, Olejek A, Baranowski W. Cervical Cancer Histology, Staging and Survival before and after Implementation of Organised Cervical Screening Programme in Poland. PloS one. 2016:11(5):e0155849. doi: 10.1371/journal.pone.0155849. Epub 2016 May 19 [PubMed PMID: 27196050]
Lax S. Histopathology of cervical precursor lesions and cancer. Acta dermatovenerologica Alpina, Pannonica, et Adriatica. 2011 Sep:20(3):125-33 [PubMed PMID: 22131112]
Jenkins D. Histopathology and cytopathology of cervical cancer. Disease markers. 2007:23(4):199-212 [PubMed PMID: 17627056]
Zhang S, Xu H, Zhang L, Qiao Y. Cervical cancer: Epidemiology, risk factors and screening. Chinese journal of cancer research = Chung-kuo yen cheng yen chiu. 2020 Dec 31:32(6):720-728. doi: 10.21147/j.issn.1000-9604.2020.06.05. Epub [PubMed PMID: 33446995]
Level 2 (mid-level) evidenceHutchcraft ML, Miller RW. Bleeding from Gynecologic Malignancies. Obstetrics and gynecology clinics of North America. 2022 Sep:49(3):607-622. doi: 10.1016/j.ogc.2022.02.022. Epub [PubMed PMID: 36122988]
Zhdan VM, Holovanova IA, Vovk OY, Korosh MV. RELATIONSHIP BETWEEN CERVICAL CANCER AND THE LEVEL OF PREVENTIVE ONCOLOGICAL EXAMINATIONS. Wiadomosci lekarskie (Warsaw, Poland : 1960). 2021:74(6):1428-1432 [PubMed PMID: 34159932]
Burness JV, Schroeder JM, Warren JB. Cervical Colposcopy: Indications and Risk Assessment. American family physician. 2020 Jul 1:102(1):39-48 [PubMed PMID: 32603071]
Mirpour S, Mhlanga JC, Logeswaran P, Russo G, Mercier G, Subramaniam RM. The role of PET/CT in the management of cervical cancer. AJR. American journal of roentgenology. 2013 Aug:201(2):W192-205. doi: 10.2214/AJR.12.9830. Epub [PubMed PMID: 23883234]
Senol T, Polat M, Ozkaya E, Karateke A. Comparison of Two Step LEEP and Cold Conisation For Cervical Intraepithelial Lesions to Decrease Positive Surgical Margins. Asian Pacific journal of cancer prevention : APJCP. 2016:17(7):3317-20 [PubMed PMID: 27509969]
Shachner TR, Van Meter SE. Metastatic melanoma of the uterine cervix diagnosed on cervical Pap smear: Case report and literature review. Diagnostic cytopathology. 2018 Dec:46(12):1045-1049. doi: 10.1002/dc.24058. Epub 2018 Oct 24 [PubMed PMID: 30354020]
Level 3 (low-level) evidenceLarsson G, Gullberg B, Grundsell H. A comparison of complications of laser and cold knife conization. Obstetrics and gynecology. 1983 Aug:62(2):213-7 [PubMed PMID: 6408545]
Brun JL, Youbi A, Hocké C. [Complications, sequellae and outcome of cervical conizations: evaluation of three surgical technics]. Journal de gynecologie, obstetrique et biologie de la reproduction. 2002 Oct:31(6):558-64 [PubMed PMID: 12407327]
Smith ES, Moon AS, O'Hanlon R, Leitao MM Jr, Sonoda Y, Abu-Rustum NR, Mueller JJ. Radical Trachelectomy for the Treatment of Early-Stage Cervical Cancer: A Systematic Review. Obstetrics and gynecology. 2020 Sep:136(3):533-542. doi: 10.1097/AOG.0000000000003952. Epub [PubMed PMID: 32769648]
Level 1 (high-level) evidenceCibula D, Abu-Rustum NR, Benedetti-Panici P, Köhler C, Raspagliesi F, Querleu D, Morrow CP. New classification system of radical hysterectomy: emphasis on a three-dimensional anatomic template for parametrial resection. Gynecologic oncology. 2011 Aug:122(2):264-8. doi: 10.1016/j.ygyno.2011.04.029. Epub 2011 May 17 [PubMed PMID: 21592548]
Querleu D, Morrow CP. Classification of radical hysterectomy. The Lancet. Oncology. 2008 Mar:9(3):297-303. doi: 10.1016/S1470-2045(08)70074-3. Epub [PubMed PMID: 18308255]
Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, Buda A, Yan X, Shuzhong Y, Chetty N, Isla D, Tamura M, Zhu T, Robledo KP, Gebski V, Asher R, Behan V, Nicklin JL, Coleman RL, Obermair A. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. The New England journal of medicine. 2018 Nov 15:379(20):1895-1904. doi: 10.1056/NEJMoa1806395. Epub 2018 Oct 31 [PubMed PMID: 30380365]
Li RZ, Sun LF, Li R, Wang HJ. Survival after minimally invasive radical hysterectomy without using uterine manipulator for early-stage cervical cancer: A systematic review and meta-analysis. BJOG : an international journal of obstetrics and gynaecology. 2023 Jan:130(2):176-183. doi: 10.1111/1471-0528.17339. Epub 2022 Nov 13 [PubMed PMID: 36331008]
Level 2 (mid-level) evidenceBogani G, Di Donato V, Scambia G, Raspagliesi F, Chiantera V, Sozzi G, Golia D'Augè T, Muzii L, Benedetti Panici P, D'Oria O, Vizza E, Giannini A, On Behalf Of The Investigators Of The Italian Gynecological Cancer Study Group. Radical Hysterectomy for Early Stage Cervical Cancer. International journal of environmental research and public health. 2022 Sep 15:19(18):. doi: 10.3390/ijerph191811641. Epub 2022 Sep 15 [PubMed PMID: 36141917]
Touhami O, Plante M. Minimally Invasive Surgery for Cervical Cancer in Light of the LACC Trial: What Have We Learned? Current oncology (Toronto, Ont.). 2022 Feb 14:29(2):1093-1106. doi: 10.3390/curroncol29020093. Epub 2022 Feb 14 [PubMed PMID: 35200592]
Lécuru F, Mathevet P, Querleu D, Leblanc E, Morice P, Daraï E, Marret H, Magaud L, Gillaizeau F, Chatellier G, Dargent D. Bilateral negative sentinel nodes accurately predict absence of lymph node metastasis in early cervical cancer: results of the SENTICOL study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 May 1:29(13):1686-91. doi: 10.1200/JCO.2010.32.0432. Epub 2011 Mar 28 [PubMed PMID: 21444878]
Mathevet P, Lécuru F, Uzan C, Boutitie F, Magaud L, Guyon F, Querleu D, Fourchotte V, Baron M, Bats AS, Senticol 2 group. Sentinel lymph node biopsy and morbidity outcomes in early cervical cancer: Results of a multicentre randomised trial (SENTICOL-2). European journal of cancer (Oxford, England : 1990). 2021 May:148():307-315. doi: 10.1016/j.ejca.2021.02.009. Epub 2021 Mar 24 [PubMed PMID: 33773275]
Level 1 (high-level) evidenceBaboudjian M, Gondran-Tellier B, Michel F, Abdallah R, Rouy M, Gaillet S, Sichez PC, Boissier R, Bladou F, Lechevallier E, Karsenty G. Miami Pouch: A Simple Technique for Efficient Continent Cutaneous Urinary Diversion. Urology. 2021 Jun:152():178-183. doi: 10.1016/j.urology.2021.02.004. Epub 2021 Feb 10 [PubMed PMID: 33581233]
von Knobloch R, Seybold M, Fischer HP, Kibele M, Samad WA. Modification of the Indiana Pouch Ileo-Caecal Cutaneous Continent Urinary Diversion: Tubular Ileal Afferent Limb for Ureteral Anastomosis Has Low Stricture Rate and Allows Ileal Ureter Replacement. Urologia internationalis. 2022:106(2):180-185. doi: 10.1159/000518561. Epub 2021 Sep 23 [PubMed PMID: 34569528]
Matsuzaki S, Klar M, Chang EJ, Matsuzaki S, Maeda M, Zhang RH, Roman LD, Matsuo K. Minimally Invasive Surgery and Surgical Volume-Specific Survival and Perioperative Outcome: Unmet Need for Evidence in Gynecologic Malignancy. Journal of clinical medicine. 2021 Oct 19:10(20):. doi: 10.3390/jcm10204787. Epub 2021 Oct 19 [PubMed PMID: 34682910]
Bizzarri N, Chiantera V, Ercoli A, Fagotti A, Tortorella L, Conte C, Cappuccio S, Di Donna MC, Gallotta V, Scambia G, Vizzielli G. Minimally Invasive Pelvic Exenteration for Gynecologic Malignancies: A Multi-Institutional Case Series and Review of the Literature. Journal of minimally invasive gynecology. 2019 Nov-Dec:26(7):1316-1326. doi: 10.1016/j.jmig.2018.12.019. Epub 2019 Jan 4 [PubMed PMID: 30611973]
Level 2 (mid-level) evidenceWestin SN, Rallapalli V, Fellman B, Urbauer DL, Pal N, Frumovitz MM, Ramondetta LM, Bodurka DC, Ramirez PT, Soliman PT. Overall survival after pelvic exenteration for gynecologic malignancy. Gynecologic oncology. 2014 Sep:134(3):546-51. doi: 10.1016/j.ygyno.2014.06.034. Epub 2014 Jul 9 [PubMed PMID: 25014540]
Level 2 (mid-level) evidenceHöckel M, Dornhöfer N. Pelvic exenteration for gynaecological tumours: achievements and unanswered questions. The Lancet. Oncology. 2006 Oct:7(10):837-47 [PubMed PMID: 17012046]
Level 2 (mid-level) evidenceMagrina JF, Stanhope CR, Weaver AL. Pelvic exenterations: supralevator, infralevator, and with vulvectomy. Gynecologic oncology. 1997 Jan:64(1):130-5 [PubMed PMID: 8995561]
Level 2 (mid-level) evidenceBourke J, Wong K, Srinivasjois R, Pereira G, Shepherd CCJ, White SW, Stanley F, Leonard H. Predicting Long-Term Survival Without Major Disability for Infants Born Preterm. The Journal of pediatrics. 2019 Dec:215():90-97.e1. doi: 10.1016/j.jpeds.2019.07.056. Epub 2019 Sep 4 [PubMed PMID: 31493909]
Zanotti-Fregonara P, Laforest R, Wallis JW. Fetal Radiation Dose from 18F-FDG in Pregnant Patients Imaged with PET, PET/CT, and PET/MR. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2015 Aug:56(8):1218-22. doi: 10.2967/jnumed.115.157032. Epub 2015 Jun 18 [PubMed PMID: 26089550]
Alouini S, Rida K, Mathevet P. Cervical cancer complicating pregnancy: implications of laparoscopic lymphadenectomy. Gynecologic oncology. 2008 Mar:108(3):472-7. doi: 10.1016/j.ygyno.2007.12.006. Epub 2008 Jan 16 [PubMed PMID: 18201752]
Level 2 (mid-level) evidenceBernardini F, Ferrandina G, Ricci C, Fagotti A, Fanfani F, Cavaliere AF, Gui B, Scambia G, De Vincenzo R. Neoadjuvant Chemotherapy in Pregnant Patients with Cervical Cancer: A Monocentric Retrospective Study. Current oncology (Toronto, Ont.). 2022 Aug 14:29(8):5702-5714. doi: 10.3390/curroncol29080450. Epub 2022 Aug 14 [PubMed PMID: 36005188]
Level 2 (mid-level) evidenceAmant F, Berveiller P, Boere IA, Cardonick E, Fruscio R, Fumagalli M, Halaska MJ, Hasenburg A, Johansson ALV, Lambertini M, Lok CAR, Maggen C, Morice P, Peccatori F, Poortmans P, Van Calsteren K, Vandenbroucke T, van Gerwen M, van den Heuvel-Eibrink M, Zagouri F, Zapardiel I. Gynecologic cancers in pregnancy: guidelines based on a third international consensus meeting. Annals of oncology : official journal of the European Society for Medical Oncology. 2019 Oct 1:30(10):1601-1612. doi: 10.1093/annonc/mdz228. Epub [PubMed PMID: 31435648]
Level 3 (low-level) evidenceLandoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, Favini G, Ferri L, Mangioni C. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet (London, England). 1997 Aug 23:350(9077):535-40 [PubMed PMID: 9284774]
Level 1 (high-level) evidenceLandoni F, Colombo A, Milani R, Placa F, Zanagnolo V, Mangioni C. Randomized study between radical surgery and radiotherapy for the treatment of stage IB-IIA cervical cancer: 20-year update. Journal of gynecologic oncology. 2017 May:28(3):e34. doi: 10.3802/jgo.2017.28.e34. Epub 2017 Feb 24 [PubMed PMID: 28382797]
Level 1 (high-level) evidencevan Kol K, Ebisch R, Piek J, Beugeling M, Vergeldt T, Bekkers R. Adjuvant Hysterectomy for Cervical Cancer Patients Treated with Chemoradiation Therapy: A Systematic Review on the Pathology-Proven Residual Disease Rate. Cancers. 2021 Dec 8:13(24):. doi: 10.3390/cancers13246190. Epub 2021 Dec 8 [PubMed PMID: 34944810]
Level 1 (high-level) evidenceMorris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, Rotman M, Gershenson DM, Mutch DG. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. The New England journal of medicine. 1999 Apr 15:340(15):1137-43 [PubMed PMID: 10202164]
Level 1 (high-level) evidenceSedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: A Gynecologic Oncology Group Study. Gynecologic oncology. 1999 May:73(2):177-83 [PubMed PMID: 10329031]
Level 1 (high-level) evidenceLevinson K, Beavis AL, Purdy C, Rositch AF, Viswanathan A, Wolfson AH, Kelly MG, Tewari KS, McNally L, Guntupalli SR, Ragab O, Lee YC, Miller DS, Huh WK, Wilkinson KJ, Spirtos NM, Van Le L, Casablanca Y, Holman LL, Waggoner SE, Fader AN. Beyond Sedlis-A novel histology-specific nomogram for predicting cervical cancer recurrence risk: An NRG/GOG ancillary analysis. Gynecologic oncology. 2021 Sep:162(3):532-538. doi: 10.1016/j.ygyno.2021.06.017. Epub 2021 Jul 1 [PubMed PMID: 34217544]
Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, Souhami L, Grigsby P, Gordon W Jr, Alberts DS. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2000 Apr:18(8):1606-13 [PubMed PMID: 10764420]
Level 1 (high-level) evidenceHuang H, Feng YL, Wan T, Zhang YN, Cao XP, Huang YW, Xiong Y, Huang X, Zheng M, Li YF, Li JD, Chen GD, Li H, Chen YL, Ma LG, Yang HY, Li L, Yao SZ, Ye WJ, Tu H, Huang QD, Liang LZ, Liu FY, Liu Q, Liu JH. Effectiveness of Sequential Chemoradiation vs Concurrent Chemoradiation or Radiation Alone in Adjuvant Treatment After Hysterectomy for Cervical Cancer: The STARS Phase 3 Randomized Clinical Trial. JAMA oncology. 2021 Mar 1:7(3):361-369. doi: 10.1001/jamaoncol.2020.7168. Epub [PubMed PMID: 33443541]
Level 1 (high-level) evidenceMell LK, Sirák I, Wei L, Tarnawski R, Mahantshetty U, Yashar CM, McHale MT, Xu R, Honerkamp-Smith G, Carmona R, Wright M, Williamson CW, Kasaová L, Li N, Kry S, Michalski J, Bosch W, Straube W, Schwarz J, Lowenstein J, Jiang SB, Saenz CC, Plaxe S, Einck J, Khorprasert C, Koonings P, Harrison T, Shi M, Mundt AJ, INTERTECC Study Group. Bone Marrow-sparing Intensity Modulated Radiation Therapy With Concurrent Cisplatin For Stage IB-IVA Cervical Cancer: An International Multicenter Phase II Clinical Trial (INTERTECC-2). International journal of radiation oncology, biology, physics. 2017 Mar 1:97(3):536-545. doi: 10.1016/j.ijrobp.2016.11.027. Epub 2016 Nov 23 [PubMed PMID: 28126303]
Level 1 (high-level) evidenceYeung AR, Pugh SL, Klopp AH, Gil KM, Wenzel L, Westin SN, Gaffney DK, Small W Jr, Thompson S, Doncals DE, Cantuaria GHC, Yaremko BP, Chang A, Kundapur V, Mohan DS, Haas ML, Kim YB, Ferguson CL, Deshmukh S, Bruner DW, Kachnic LA. Improvement in Patient-Reported Outcomes With Intensity-Modulated Radiotherapy (RT) Compared With Standard RT: A Report From the NRG Oncology RTOG 1203 Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020 May 20:38(15):1685-1692. doi: 10.1200/JCO.19.02381. Epub 2020 Feb 19 [PubMed PMID: 32073955]
Gonzalez VJ, Hullett CR, Burt L, Rassiah-Szegedi P, Sarkar V, Tward JD, Hazard LJ, Huang YJ, Salter BJ, Gaffney DK. Impact of prone versus supine positioning on small bowel dose with pelvic intensity modulated radiation therapy. Advances in radiation oncology. 2017 Apr-Jun:2(2):235-243. doi: 10.1016/j.adro.2017.01.005. Epub 2017 Jan 24 [PubMed PMID: 28740937]
Level 3 (low-level) evidenceSmall W Jr, Mell LK, Anderson P, Creutzberg C, De Los Santos J, Gaffney D, Jhingran A, Portelance L, Schefter T, Iyer R, Varia M, Winter K, Mundt AJ. Consensus guidelines for delineation of clinical target volume for intensity-modulated pelvic radiotherapy in postoperative treatment of endometrial and cervical cancer. International journal of radiation oncology, biology, physics. 2008 Jun 1:71(2):428-34 [PubMed PMID: 18037584]
Level 3 (low-level) evidenceRíos I, Vásquez I, Cuervo E, Garzón Ó, Burbano J. Problems and solutions in IGRT for cervical cancer. Reports of practical oncology and radiotherapy : journal of Greatpoland Cancer Center in Poznan and Polish Society of Radiation Oncology. 2018 Nov-Dec:23(6):517-527. doi: 10.1016/j.rpor.2018.05.002. Epub 2018 May 26 [PubMed PMID: 30534015]
Kavanagh BD, Pan CC, Dawson LA, Das SK, Li XA, Ten Haken RK, Miften M. Radiation dose-volume effects in the stomach and small bowel. International journal of radiation oncology, biology, physics. 2010 Mar 1:76(3 Suppl):S101-7. doi: 10.1016/j.ijrobp.2009.05.071. Epub [PubMed PMID: 20171503]
Level 1 (high-level) evidenceViswanathan AN, Thomadsen B, American Brachytherapy Society Cervical Cancer Recommendations Committee, American Brachytherapy Society. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part I: general principles. Brachytherapy. 2012 Jan-Feb:11(1):33-46. doi: 10.1016/j.brachy.2011.07.003. Epub [PubMed PMID: 22265436]
Level 3 (low-level) evidenceViswanathan AN, Beriwal S, De Los Santos JF, Demanes DJ, Gaffney D, Hansen J, Jones E, Kirisits C, Thomadsen B, Erickson B, American Brachytherapy Society. American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part II: high-dose-rate brachytherapy. Brachytherapy. 2012 Jan-Feb:11(1):47-52. doi: 10.1016/j.brachy.2011.07.002. Epub [PubMed PMID: 22265437]
Level 3 (low-level) evidenceCharra-Brunaud C, Harter V, Delannes M, Haie-Meder C, Quetin P, Kerr C, Castelain B, Thomas L, Peiffert D. Impact of 3D image-based PDR brachytherapy on outcome of patients treated for cervix carcinoma in France: results of the French STIC prospective study. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2012 Jun:103(3):305-13. doi: 10.1016/j.radonc.2012.04.007. Epub 2012 May 25 [PubMed PMID: 22633469]
Level 1 (high-level) evidencePötter R, Tanderup K, Schmid MP, Jürgenliemk-Schulz I, Haie-Meder C, Fokdal LU, Sturdza AE, Hoskin P, Mahantshetty U, Segedin B, Bruheim K, Huang F, Rai B, Cooper R, van der Steen-Banasik E, Van Limbergen E, Pieters BR, Tan LT, Nout RA, De Leeuw AAC, Ristl R, Petric P, Nesvacil N, Kirchheiner K, Kirisits C, Lindegaard JC, EMBRACE Collaborative Group. MRI-guided adaptive brachytherapy in locally advanced cervical cancer (EMBRACE-I): a multicentre prospective cohort study. The Lancet. Oncology. 2021 Apr:22(4):538-547. doi: 10.1016/S1470-2045(20)30753-1. Epub [PubMed PMID: 33794207]
Dimopoulos JC, Petrow P, Tanderup K, Petric P, Berger D, Kirisits C, Pedersen EM, van Limbergen E, Haie-Meder C, Pötter R. Recommendations from Gynaecological (GYN) GEC-ESTRO Working Group (IV): Basic principles and parameters for MR imaging within the frame of image based adaptive cervix cancer brachytherapy. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2012 Apr:103(1):113-22. doi: 10.1016/j.radonc.2011.12.024. Epub 2012 Jan 30 [PubMed PMID: 22296748]
Level 2 (mid-level) evidenceBanerjee R, Kamrava M. Brachytherapy in the treatment of cervical cancer: a review. International journal of women's health. 2014:6():555-64. doi: 10.2147/IJWH.S46247. Epub 2014 May 28 [PubMed PMID: 24920937]
Liu R, Wang X, Tian JH, Yang K, Wang J, Jiang L, Hao XY. High dose rate versus low dose rate intracavity brachytherapy for locally advanced uterine cervix cancer. The Cochrane database of systematic reviews. 2014 Oct 9:2014(10):CD007563. doi: 10.1002/14651858.CD007563.pub3. Epub 2014 Oct 9 [PubMed PMID: 25300170]
Level 1 (high-level) evidence. Report 89. Journal of the ICRU. 2013 Apr:13(1-2):NP. doi: 10.1093/jicru/ndw042. Epub [PubMed PMID: 27335498]
Mazeron R, Fokdal LU, Kirchheiner K, Georg P, Jastaniyah N, Šegedin B, Mahantshetty U, Hoskin P, Jürgenliemk-Schulz I, Kirisits C, Lindegaard JC, Dörr W, Haie-Meder C, Tanderup K, Pötter R, EMBRACE collaborative group. Dose-volume effect relationships for late rectal morbidity in patients treated with chemoradiation and MRI-guided adaptive brachytherapy for locally advanced cervical cancer: Results from the prospective multicenter EMBRACE study. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2016 Sep:120(3):412-419. doi: 10.1016/j.radonc.2016.06.006. Epub 2016 Jul 7 [PubMed PMID: 27396811]
Lucidarme D, Marteau P, Foucault M, Vautrin B, Filoche B. Efficacy and tolerance of mesalazine suppositories vs. hydrocortisone foam in proctitis. Alimentary pharmacology & therapeutics. 1997 Apr:11(2):335-40 [PubMed PMID: 9146772]
Level 1 (high-level) evidenceRigaud J, Hetet JF, Bouchot O. [Management of radiation cystitis]. Progres en urologie : journal de l'Association francaise d'urologie et de la Societe francaise d'urologie. 2004 Sep:14(4):568-72 [PubMed PMID: 15776916]
Pereira D, Ferreira C, Catarino R, Correia T, Cardoso A, Reis F, Cerqueira M, Prisco R, Camacho O. Hyperbaric oxygen for radiation-induced cystitis: A long-term follow-up. Actas urologicas espanolas. 2020 Oct:44(8):561-567. doi: 10.1016/j.acuro.2020.03.010. Epub 2020 Jul 28 [PubMed PMID: 32736899]
Boice JD Jr, Day NE, Andersen A, Brinton LA, Brown R, Choi NW, Clarke EA, Coleman MP, Curtis RE, Flannery JT. Second cancers following radiation treatment for cervical cancer. An international collaboration among cancer registries. Journal of the National Cancer Institute. 1985 May:74(5):955-75 [PubMed PMID: 3858584]
Level 2 (mid-level) evidenceWright JD, St Clair CM, Deutsch I, Burke WM, Gorrochurn P, Sun X, Herzog TJ. Pelvic radiotherapy and the risk of secondary leukemia and multiple myeloma. Cancer. 2010 May 15:116(10):2486-92. doi: 10.1002/cncr.25067. Epub [PubMed PMID: 20209618]
Wallace WH, Thomson AB, Saran F, Kelsey TW. Predicting age of ovarian failure after radiation to a field that includes the ovaries. International journal of radiation oncology, biology, physics. 2005 Jul 1:62(3):738-44 [PubMed PMID: 15936554]
Small W Jr, Kim YS, Joyce C, Surucu M, Leshyk M, Harkenrider MM, Potkul RK, Liotta M, Winder A, Altoos B. Uterine perforation during brachytherapy for cervical cancer: Complications, outcomes, and best practices for forward treatment planning and management. Brachytherapy. 2021 May-Jun:20(3):557-564. doi: 10.1016/j.brachy.2021.02.001. Epub 2021 Mar 17 [PubMed PMID: 33741275]
van Dyk S, Schneider M, Kondalsamy-Chennakesavan S, Bernshaw D, Narayan K. Ultrasound use in gynecologic brachytherapy: Time to focus the beam. Brachytherapy. 2015 May-Jun:14(3):390-400. doi: 10.1016/j.brachy.2014.12.001. Epub 2015 Jan 22 [PubMed PMID: 25620161]
Rowlands S, Oloto E, Horwell DH. Intrauterine devices and risk of uterine perforation: current perspectives. Open access journal of contraception. 2016:7():19-32. doi: 10.2147/OAJC.S85546. Epub 2016 Mar 16 [PubMed PMID: 29386934]
Level 3 (low-level) evidenceRose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, Clarke-Pearson DL, Insalaco S. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. The New England journal of medicine. 1999 Apr 15:340(15):1144-53 [PubMed PMID: 10202165]
Level 1 (high-level) evidenceDueñas-González A, Zarbá JJ, Patel F, Alcedo JC, Beslija S, Casanova L, Pattaranutaporn P, Hameed S, Blair JM, Barraclough H, Orlando M. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 May 1:29(13):1678-85. doi: 10.1200/JCO.2009.25.9663. Epub 2011 Mar 28 [PubMed PMID: 21444871]
Level 1 (high-level) evidenceMoore DH, Blessing JA, McQuellon RP, Thaler HT, Cella D, Benda J, Miller DS, Olt G, King S, Boggess JF, Rocereto TF. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a gynecologic oncology group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004 Aug 1:22(15):3113-9 [PubMed PMID: 15284262]
Level 1 (high-level) evidenceMonk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, Benda J, Cella D. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Oct 1:27(28):4649-55. doi: 10.1200/JCO.2009.21.8909. Epub 2009 Aug 31 [PubMed PMID: 19720909]
Level 1 (high-level) evidenceTewari KS, Sill MW, Penson RT, Huang H, Ramondetta LM, Landrum LM, Oaknin A, Reid TJ, Leitao MM, Michael HE, DiSaia PJ, Copeland LJ, Creasman WT, Stehman FB, Brady MF, Burger RA, Thigpen JT, Birrer MJ, Waggoner SE, Moore DH, Look KY, Koh WJ, Monk BJ. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet (London, England). 2017 Oct 7:390(10103):1654-1663. doi: 10.1016/S0140-6736(17)31607-0. Epub 2017 Jul 27 [PubMed PMID: 28756902]
Level 1 (high-level) evidenceMarabelle A, Le DT, Ascierto PA, Di Giacomo AM, De Jesus-Acosta A, Delord JP, Geva R, Gottfried M, Penel N, Hansen AR, Piha-Paul SA, Doi T, Gao B, Chung HC, Lopez-Martin J, Bang YJ, Frommer RS, Shah M, Ghori R, Joe AK, Pruitt SK, Diaz LA Jr. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2020 Jan 1:38(1):1-10. doi: 10.1200/JCO.19.02105. Epub 2019 Nov 4 [PubMed PMID: 31682550]
Colombo N, Dubot C, Lorusso D, Caceres MV, Hasegawa K, Shapira-Frommer R, Tewari KS, Salman P, Hoyos Usta E, Yañez E, Gümüş M, Olivera Hurtado de Mendoza M, Samouëlian V, Castonguay V, Arkhipov A, Toker S, Li K, Keefe SM, Monk BJ, KEYNOTE-826 Investigators. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. The New England journal of medicine. 2021 Nov 11:385(20):1856-1867. doi: 10.1056/NEJMoa2112435. Epub 2021 Sep 18 [PubMed PMID: 34534429]
Nishio S, Yonemori K, Usami T, Minobe S, Yunokawa M, Iwata T, Okamoto A, Aoki Y, Itamochi H, Takekuma M, Harano K, Yamamoto K, Maruko T, Ugai H, Tekin C, Colombo N, Fujiwara K, Hasegawa K, Ushijima K. Pembrolizumab plus chemotherapy in Japanese patients with persistent, recurrent or metastatic cervical cancer: Results from KEYNOTE-826. Cancer science. 2022 Nov:113(11):3877-3887. doi: 10.1111/cas.15479. Epub 2022 Sep 15 [PubMed PMID: 35792064]
Kitagawa R, Katsumata N, Shibata T, Kamura T, Kasamatsu T, Nakanishi T, Nishimura S, Ushijima K, Takano M, Satoh T, Yoshikawa H. Paclitaxel Plus Carboplatin Versus Paclitaxel Plus Cisplatin in Metastatic or Recurrent Cervical Cancer: The Open-Label Randomized Phase III Trial JCOG0505. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015 Jul 1:33(19):2129-35. doi: 10.1200/JCO.2014.58.4391. Epub 2015 Mar 2 [PubMed PMID: 25732161]
Level 1 (high-level) evidenceBhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri: 2021 update. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2021 Oct:155 Suppl 1(Suppl 1):28-44. doi: 10.1002/ijgo.13865. Epub [PubMed PMID: 34669203]
Saleh M, Virarkar M, Javadi S, Elsherif SB, de Castro Faria S, Bhosale P. Cervical Cancer: 2018 Revised International Federation of Gynecology and Obstetrics Staging System and the Role of Imaging. AJR. American journal of roentgenology. 2020 May:214(5):1182-1195. doi: 10.2214/AJR.19.21819. Epub 2020 Mar 17 [PubMed PMID: 32182097]
Salib MY, Russell JHB, Stewart VR, Sudderuddin SA, Barwick TD, Rockall AG, Bharwani N. 2018 FIGO Staging Classification for Cervical Cancer: Added Benefits of Imaging. Radiographics : a review publication of the Radiological Society of North America, Inc. 2020 Oct:40(6):1807-1822. doi: 10.1148/rg.2020200013. Epub 2020 Sep 18 [PubMed PMID: 32946322]
Castellano T, Ding K, Moore KN, Landrum LM. Simple Hysterectomy for Cervical Cancer: Risk Factors for Failed Screening and Deviation From Screening Guidelines. Journal of lower genital tract disease. 2019 Apr:23(2):124-128. doi: 10.1097/LGT.0000000000000463. Epub [PubMed PMID: 30817687]
Chen HH, Meng WY, Li RZ, Wang QY, Wang YW, Pan HD, Yan PY, Wu QB, Liu L, Yao XJ, Kang M, Leung EL. Potential prognostic factors in progression-free survival for patients with cervical cancer. BMC cancer. 2021 May 10:21(1):531. doi: 10.1186/s12885-021-08243-3. Epub 2021 May 10 [PubMed PMID: 33971846]
Rauh-Hain JA, Clemmer JT, Bradford LS, Clark RM, Growdon WB, Goodman A, Boruta DM 2nd, Schorge JO, del Carmen MG. Racial disparities in cervical cancer survival over time. Cancer. 2013 Oct 15:119(20):3644-52. doi: 10.1002/cncr.28261. Epub 2013 Jul 31 [PubMed PMID: 23913530]
Quinn BA, Deng X, Colton A, Bandyopadhyay D, Carter JS, Fields EC. Increasing age predicts poor cervical cancer prognosis with subsequent effect on treatment and overall survival. Brachytherapy. 2019 Jan-Feb:18(1):29-37. doi: 10.1016/j.brachy.2018.08.016. Epub 2018 Oct 22 [PubMed PMID: 30361045]
Pergialiotis V, Bellos I, Thomakos N, Haidopoulos D, Perrea DN, Kontzoglou K, Daskalakis G, Rodolakis A. Survival outcomes of patients with cervical cancer and accompanying hydronephrosis: A systematic review of the literature. Oncology reviews. 2019 Jan 14:13(1):387. doi: 10.4081/oncol.2019.387. Epub 2019 Jan 15 [PubMed PMID: 30746036]
Level 1 (high-level) evidenceMendu S, Boukhechba M, Gordon JR, Datta D, Molina E, Arroyo G, Proctor SK, Wells KJ, Barnes LE. Design of a Culturally-Informed Virtual Human for Educating Hispanic Women about Cervical Cancer. International Conference on Pervasive Computing Technologies for Healthcare : [proceedings]. International Conference on Pervasive Computing Technologies for Healthcare. 2018 May:2018():360-366. doi: 10.1145/3240925.3240968. Epub [PubMed PMID: 30555731]
Nasser S, Berek J, Ullrich A, Giordano L, Sehouli J. A report on the Marrakech International Women's Cancer Days: dialogs and implications. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2019 Feb:29(2):417-421. doi: 10.1136/ijgc-2018-000059. Epub 2018 Dec 21 [PubMed PMID: 30718317]
Level 2 (mid-level) evidenceCunningham-Erves J, Forbes L, Ivankova N, Mayo-Gamble T, Kelly-Taylor K, Deakings J. Black mother's intention to vaccinate daughters against HPV: A mixed methods approach to identify opportunities for targeted communication. Gynecologic oncology. 2018 Jun:149(3):506-512. doi: 10.1016/j.ygyno.2018.03.047. Epub 2018 Mar 24 [PubMed PMID: 29588103]
Mabelele MM, Materu J, Ng'ida FD, Mahande MJ. Knowledge towards cervical cancer prevention and screening practices among women who attended reproductive and child health clinic at Magu district hospital, Lake Zone Tanzania: a cross-sectional study. BMC cancer. 2018 May 16:18(1):565. doi: 10.1186/s12885-018-4490-7. Epub 2018 May 16 [PubMed PMID: 29769124]
Level 2 (mid-level) evidenceLai D, Bodson J, Davis FA, Lee D, Tavake-Pasi F, Napia E, Villalta J, Mukundente V, Mooney R, Coulter H, Stark LA, Sanchez-Birkhead AC, Kepka D. Diverse Families' Experiences with HPV Vaccine Information Sources: A Community-Based Participatory Approach. Journal of community health. 2017 Apr:42(2):400-412. doi: 10.1007/s10900-016-0269-4. Epub [PubMed PMID: 27734247]
Spinner C, Ding L, Bernstein DI, Brown DR, Franco EL, Covert C, Kahn JA. Human Papillomavirus Vaccine Effectiveness and Herd Protection in Young Women. Pediatrics. 2019 Feb:143(2):. doi: 10.1542/peds.2018-1902. Epub 2019 Jan 22 [PubMed PMID: 30670582]
Bayu H, Berhe Y, Mulat A, Alemu A. Cervical Cancer Screening Service Uptake and Associated Factors among Age Eligible Women in Mekelle Zone, Northern Ethiopia, 2015: A Community Based Study Using Health Belief Model. PloS one. 2016:11(3):e0149908. doi: 10.1371/journal.pone.0149908. Epub 2016 Mar 10 [PubMed PMID: 26963098]
Kung TH, Gordon JR, Abdullahi A, Barve A, Chaudhari V, Kosambiya JK, Kumar A, Gamit S, Wells KJ. "My husband says this: If you are alive, you can be someone…": Facilitators and barriers to cervical cancer screening among women living with HIV in India. Cancer causes & control : CCC. 2019 Apr:30(4):365-374. doi: 10.1007/s10552-019-01145-7. Epub 2019 Feb 26 [PubMed PMID: 30809741]
McPherson GS, Fairbairn-Dunlop P, Payne D. Overcoming Barriers to Cervical Screening Among Pacific Women: A Narrative Review. Health equity. 2019:3(1):22-29. doi: 10.1089/heq.2018.0076. Epub 2019 Feb 14 [PubMed PMID: 30783634]
Level 3 (low-level) evidence