Introduction
Cervical cancer incidence and mortality have decreased due primarily to screening programs using the pap smear. As more outcome data has become available, screening, and treatment guidelines for cervical intraepithelial neoplasia (CIN) have evolved. Detection of the disease in a precancerous state, close monitoring, and treatment are paramount in the prevention of cervical cancer. The screening process for cervical cancer involves pap smear cytology of the cervix, along with with human papillomavirus (HPV) testing in certain circumstances.[1]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Human papillomavirus (HPV) infection of the cervix is a sexually transmitted disease and a significant risk factor for the development of cervical intraepithelial neoplasia. However, only a relatively small percentage of women with the infection will develop severe CIN or invasive cervical cancer. Several factors determine whether the infection will progress to CIN or carcinoma, the greatest of which is the HPV genotype causing the infection. Although there are approximately 100 subtypes of HPV, a small subgroup has a known association with cervical dysplasia and carcinoma. HPV subtypes are considered either oncogenic or non-oncogenic. Persistence of the virus in tissues is another critical factor in the development of CIN and ultimately, carcinoma.[1][2][3][4]
HPV 16 is the most carcinogenic and accounts for 55 to 60% of cervical cancers worldwide. HPV 18 is the second most carcinogenic and accounts for 10 to 15% of cervical cancer. Risk factors such as smoking, immunocompromised state, or HIV infection likely lead to persistence of HPV infection and an increased risk for the development of CIN.[5][6][7]
Although the terminology is changing, cytologic abnormalities on Pap smear are typically described as "squamous intraepithelial lesions" and further classified as "low-grade" or "high-grade."
Epidemiology
HPV infection occurs in sexually active women of any age but is more common in adolescent women and women under the age of 30. The highest incidence is in women ages 20 to 24. These young women are the most likely to clear the infection to undetectable levels in an average of 8 months; this is the rationale for increasing the initiation of Pap smear screening to age 21.
Women over age 30 with HPV detected are more likely to have a persistent infection and warrant more aggressive follow-up to rule out cervical intraepithelial neoplasia.[1]
Pathophysiology
Cervical intraepithelial neoplasia results from HPV infection within cervical cells. These changes, especially in young women, commonly revert to normal cells due to an intact immune response and rapid turnover of cells on the cervix. About 60% of CIN-1 will regress to normal after 1 year. Women with CIN-2 and CIN-3 are at high risk for developing invasive cancer, although the average time for progression is still several years. Therefore, women with CIN-2/3 should receive treatment. Exceptions to this recommendation are women in the 20 to 24 year age group and pregnant women. Since a significant percentage of low-grade squamous intraepithelial lesions (LGSIL) on Pap smear will have CIN-2 or 3, it makes sense that these pap smears still require colposcopy and biopsy in most cases. The same holds for older women with atypical squamous cells of undetermined significance (ASCUS) pap smears who also have a high-risk HPV. Cytology is the screening test, but histologic characteristics of a tissue biopsy make the diagnosis.[1][8]
Histopathology
The classic microscopic description of HPV infection of cervical epithelial cells is "koilocytosis." This term refers to the appearance of a perinuclear "halo" within the cell, along with enlarged and irregular nuclei that show evidence of mitosis. The proportion of cervical epithelium exhibiting dysplastic cells determines the grade of the dysplasia. CIN-1 (low-grade) involves the lower 1/3 or less of the epithelium, whereas the more significant CIN-2 and CIN-3 (high-grade) progress to include the entire thickness of the epithelium. Dysplasia becomes cancer when it invades the basement membrane.
History and Physical
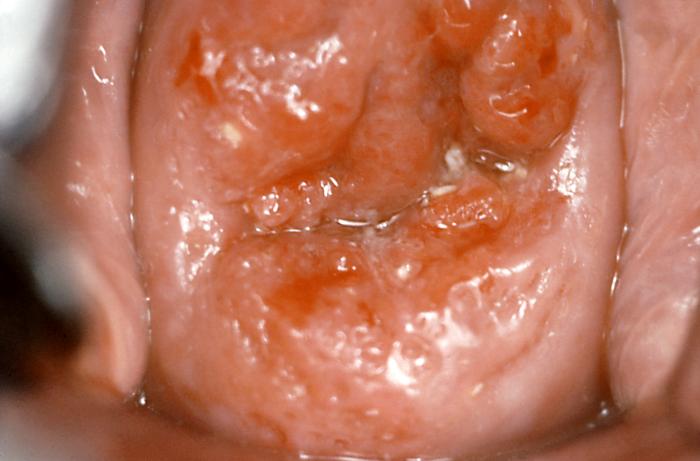
In most instances, dysplastic lesions of the cervix are not visible to the naked eye, and it is the pap smear which detects abnormalities requiring further evaluation. Some lesions appear as exophytic or plaque-like growths on the cervix. HPV can cause anogenital warts and thus prompt further investigation into other abnormalities caused by HPV.
Evaluation
Colposcopy with directed biopsy is the preferred method for evaluation of an abnormal pap smear result. "Co-testing" combines cytology and HPV testing for high-risk types, but is still considered screening. The diagnosis ultimately requires tissue sampling.
There are exceptions to this rule:
For women aged 21 to 24 with LGSIL cytology because of the high rate of disease resolution, repeat cytology at 12-month intervals is the recommendation. This same age-group of women with Pap smear results showing atypical squamous cells-cannot exclude high grade (ASC-H), atypical glandular cells, or HGSIL results on repeat cytology; colposcopy is the recommendation. For follow-up Pap smears showing ASCUS, LGSIL or negative, the recommendation is to repeat in another 12 months. For those patients with repeated ASCUS or LGSIL at 24 months, colposcopy is the next step.[1]
Patients older than 24 years of age with ASCUS with positive high-risk HPV and LGSIL or higher should undergo colposcopy. Regardless of age, women with HGSIL or ASCUS-H should have a colposcopy. With a diagnosis of CIN II or greater, excisional treatment is the recommendation. With the subgroup of younger women, close observation with colposcopy may be appropriate if they are compliant with care.[1]
Treatment / Management
CIN-1 can undergo observation and co-testing repeated in 1 year. If CIN-1 is persistent after 2 years or progresses within that time, treatment is the recommendation.[1](B3)
As stated earlier, CIN -2 or higher requires treatment. Treatment is also recommended when there is more than one degree of difference between pap results and biopsy results. For example, if the pap smear is high-grade intraepithelial lesion (HGSIL), but the biopsy is negative, the potential reasons are a misread of the specimen, or there was a missed a lesion at the time of colposcopy. In this case, a diagnostic excisional procedure is the preferred mode of treatment because it is both therapeutic and diagnostic. The margins of the cervical specimen may then undergo evaluation for complete removal of any abnormal cells.[1](B3)
The usual treatment is via ablation or excision of abnormal cells. Ablation of abnormal cells includes cryosurgery or laser ablation (CO2 laser). Ablation is only acceptable when the endocervical sampling is negative, there are no glandular abnormalities, the entire borders of the lesion are visible, and the patient has not failed other treatments. These techniques were more common before the development of LEEP (loop electrosurgical excision procedure). Ablative procedures have a higher recurrence rate in the setting of severe dysplasia when compared to LEEP.[1](B3)
Excisional procedures for the treatment of CIN include LEEP, cold knife conization, and laser conization. Whether any of these procedures increase a patient’s risk for preterm labor is controversial since the risks for preterm delivery and dysplasia overlap considerably. That said, in women younger than 25 with CIN-2 or 3, there may be a role for close observation with colposcopy in 6 months rather than excision. However, that is not the currently preferred treatment option. During pregnancy, treatment is postponed until after delivery unless colposcopic surveillance during pregnancy reveals progression to invasive cervical cancer.[1](B3)
Women treated for CIN-2, or greater should have a Pap smear and HPV testing 12 and 24 months after the procedure. Even with positive endocervical margins on an excised specimen, the procedure is deemed 70 to 80% effective. When margins are positive, repeat cytology testing in 4-6 months accompanied by an endocervical curettage is the course of action. A repeat excisional procedure is one option for treatment of persistent or recurrent CIN-2 or 3. In some circumstances, patients will opt for a hysterectomy, which is also appropriate for recurrent CIN.[1](B3)
Differential Diagnosis
The differential diagnosis should include normal squamous cells, cervical warty lesions, inflammation, infection, and carcinoma.
Prognosis
The prognosis for cervical intraepithelial neoplasia differs depending on the severity. With adherence to ASCCP guidelines, the risk for progression to carcinoma is low. The risk of overt cervical cancer is significantly higher when a woman has missed screening for more than 10 years.
Complications
Complications can arise from cervical biopsy include excessive bleeding or infection, but are rare. Surgical treatments such as a cold knife cone or LEEP carry increased risks, including risks of anesthesia. In those individuals treated with an excisional procedure, there have been concerns regarding the risk of pregnancy complications such as preterm delivery or cervical incompetence. However, the risk factors for these complications overlap those for cervical dysplasia, so it is difficult to discern the actual impact of the excisional procedure on preterm delivery.[9][10]
Pearls and Other Issues
The American Society for Colposcopy and Cervical Pathology (ASCCP) has published a smartphone application entitled “ASSCP Mobile” that is updated regularly. It has a user-friendly algorithm for screening guidelines and management recommendations. It is available for Android and IOS platforms for a minimal fee.
Enhancing Healthcare Team Outcomes
Healthcare providers, including the nurse practitioner, should discuss cervical cancer screening protocols and implement a reliable system for follow up, especially for abnormal results. This system should include a combination of verbal and written notifications about the process of evaluation and the importance of appropriate follow-up.
Consistent condom use is among the most effective methods for women to protect themselves from HPV transmission. HPV vaccines also protect against HPV-related diseases.[11] They are FDA approved and show nearly 100% efficacy in preventing cervical neoplasia from HPV subtypes included in the vaccine. These vaccines provide immunity for several subtypes of HPV. All women should receive cervical cancer screening per ASCCP guidelines, regardless of their HPV immunization status.
Cervical intraepithelial neoplasia requires an interprofessional team approach, including physicians, specialists, specialty-trained nurses, and pharmacists, all collaborating across disciplines to achieve optimal patient results. [Level V]
Media
(Click Image to Enlarge)
References
Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, Solomon D, Wentzensen N, Lawson HW, 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Journal of lower genital tract disease. 2013 Apr:17(5 Suppl 1):S1-S27. doi: 10.1097/LGT.0b013e318287d329. Epub [PubMed PMID: 23519301]
Level 3 (low-level) evidencede Sanjose S, Quint WG, Alemany L, Geraets DT, Klaustermeier JE, Lloveras B, Tous S, Felix A, Bravo LE, Shin HR, Vallejos CS, de Ruiz PA, Lima MA, Guimera N, Clavero O, Alejo M, Llombart-Bosch A, Cheng-Yang C, Tatti SA, Kasamatsu E, Iljazovic E, Odida M, Prado R, Seoud M, Grce M, Usubutun A, Jain A, Suarez GA, Lombardi LE, Banjo A, Menéndez C, Domingo EJ, Velasco J, Nessa A, Chichareon SC, Qiao YL, Lerma E, Garland SM, Sasagawa T, Ferrera A, Hammouda D, Mariani L, Pelayo A, Steiner I, Oliva E, Meijer CJ, Al-Jassar WF, Cruz E, Wright TC, Puras A, Llave CL, Tzardi M, Agorastos T, Garcia-Barriola V, Clavel C, Ordi J, Andújar M, Castellsagué X, Sánchez GI, Nowakowski AM, Bornstein J, Muñoz N, Bosch FX, Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. The Lancet. Oncology. 2010 Nov:11(11):1048-56. doi: 10.1016/S1470-2045(10)70230-8. Epub 2010 Oct 15 [PubMed PMID: 20952254]
Level 2 (mid-level) evidenceWheeler CM, Hunt WC, Joste NE, Key CR, Quint WG, Castle PE. Human papillomavirus genotype distributions: implications for vaccination and cancer screening in the United States. Journal of the National Cancer Institute. 2009 Apr 1:101(7):475-87. doi: 10.1093/jnci/djn510. Epub 2009 Mar 24 [PubMed PMID: 19318628]
Level 2 (mid-level) evidenceBosch FX,de Sanjosé S, Chapter 1: Human papillomavirus and cervical cancer--burden and assessment of causality. Journal of the National Cancer Institute. Monographs. 2003 [PubMed PMID: 12807939]
Xi LF, Koutsky LA, Castle PE, Edelstein ZR, Meyers C, Ho J, Schiffman M. Relationship between cigarette smoking and human papilloma virus types 16 and 18 DNA load. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009 Dec:18(12):3490-6. doi: 10.1158/1055-9965.EPI-09-0763. Epub [PubMed PMID: 19959700]
Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: Updating the natural history of HPV and anogenital cancer. Vaccine. 2006 Aug 31:24 Suppl 3():S3/42-51 [PubMed PMID: 16950017]
Hildesheim A, Schiffman MH, Gravitt PE, Glass AG, Greer CE, Zhang T, Scott DR, Rush BB, Lawler P, Sherman ME. Persistence of type-specific human papillomavirus infection among cytologically normal women. The Journal of infectious diseases. 1994 Feb:169(2):235-40 [PubMed PMID: 8106758]
Wright TC Jr,Massad LS,Dunton CJ,Spitzer M,Wilkinson EJ,Solomon D, 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. American journal of obstetrics and gynecology. 2007 Oct [PubMed PMID: 17904957]
Level 3 (low-level) evidenceParaskevaidis E, Koliopoulos G, Kalantaridou S, Pappa L, Navrozoglou I, Zikopoulos K, Lolis DE. Management and evolution of cervical intraepithelial neoplasia during pregnancy and postpartum. European journal of obstetrics, gynecology, and reproductive biology. 2002 Aug 5:104(1):67-9 [PubMed PMID: 12128266]
Boardman LA, Goldman DL, Cooper AS, Heber WW, Weitzen S. CIN in pregnancy: antepartum and postpartum cytology and histology. The Journal of reproductive medicine. 2005 Jan:50(1):13-8 [PubMed PMID: 15730167]
Level 2 (mid-level) evidenceMoss JL, Reiter PL, Brewer NT. Correlates of human papillomavirus vaccine coverage: a state-level analysis. Sexually transmitted diseases. 2015 Feb:42(2):71-5. doi: 10.1097/OLQ.0000000000000225. Epub [PubMed PMID: 25585064]