Introduction
Minimally invasive techniques continue to evolve in many surgical specialties, and vascular surgery is no exception. Angiography is one of the commonest procedures performed today in a wide variety of specialties including diagnostic and interventional cardiology, vascular surgery and interventional radiology. Traditionally, hemostasis at the access site for these procedures has been obtained via manual compression. While manual compression is the gold standard in this regard, a wide array of vascular access closure devices have been developed to aid the proceduralist in achieving that goal. These devices can be useful in the setting of a large body habitus, anticoagulation and antiplatelet therapy, and in situations where extended bedrest may not be desirable (such as a patient with extensive pressure ulcers). In the case of the former two scenarios, manual compression needs to be applied for an extended period or with considerable force. Complications that can result from inadequate manual compression include hematoma and pseudoaneurysm formation. Studies have shown that use of these devices is safe even when patients have received thrombolytic therapy. We usually employ vascular access closure devices whenever a sheath that is a 6F catheter or larger is used for an arterial procedure.[1][2][3][4]
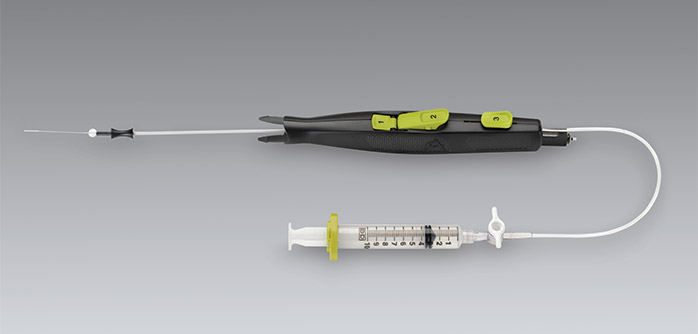
Vascular access closure devices can be divided into the following categories: suture-mediated closure devices, mechanical non-suture closure devices, intra-vascular sealant devices, extra-vascular sealant devices and manual compression assistance devices. Perclose (Abbott Vascular) is an example of a suture-mediated closure device that provides a pre-tied knot for the closure of the arteriotomy site while allowing maintenance of wire access. StarClose (Abbott Vascular) is an example of a mechanical non-suture closure device. The mechanism of this device is based on a nitinol clip which approximates the adventitia of the artery. Mynx device (CardinalHealth/Cordis) provides an extravascular sealant (polyethylene glycol) while Angioseal (St. Jude Medical) provides an intravascular sealant (bioabsorbable anchor with a collagen plug). The Catalyst II and III devices (Cardiva Medical) provide adjunctive hemostasis with manual compression. Many other devices are also available.[5][6][7]
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
These closure devices are designed for use in the closure of common femoral artery access site. The common femoral artery becomes the external iliac artery once it traverses the inguinal ligament and is a source of blood to the lower extremity. The common femoral artery divides into the profunda femoris and superficial femoral artery. The superficial femoral artery continues as the popliteal artery as it traverses the adductor canal and then it trifurcates into the tibial vessels below the knee.
Indications
These devices are approved for use in the common femoral artery.
Contraindications
Relative contraindications to the use of vascular access closure devices include dense arterial calcifications in access vessel, site of entry into the vessel above the inguinal ligament, and small size of the access artery.
Equipment
The following equipment is required:
- Sterile drapes
- Local anesthetic
- Scalpel
- Hemostat
- Micropuncture kit for percutaneous access to the common femoral artery
- Ultrasound machine
- A .035 wire such as a stiff angled guidewire or Bentson wire, contrast
- A 5F or 6F catheter short sheath
- A fluoroscopic imaging system (C-arm)
- Lead shielding, heparinized saline
- Heparin
- Vascular access closure device
Personnel
The following personnel is required for the procedure: proceduralist (such as a vascular surgeon), first assistant (resident, fellow, cath lab technician, physician assistant), radiology technician and nursing personnel. The anesthesiologist may or may not be present depending on the exact nature of the procedure.
Preparation
Both groins should be prepped and draped for the procedure. We usually recommend a complete pulse exam of the lower extremity before starting the procedure to have a baseline examination for comparison to ensure that it does not change after application of the vascular access closure device. Using a Doppler device, the location of the pedal signals is marked with a pen.
Technique or Treatment
For all vascular access closure devices, some general guidelines should be kept in mind. The common femoral artery should be accessed under ultrasonographic guidance in addition to evaluation of anatomic landmarks and fluoroscopic guidance. A micropuncture kit should be used for initial access. The puncture should be below the inguinal ligament, below the most superior aspect of the femoral head, should avoid accessing a densely calcified part of the artery and should avoid accessing the superficial and deep femoral arteries. Once the micropuncture needle and wire are in place, we usually use a scalpel to make a small skin nick at the access site. Using a hemostat, tissues are spread down to the level of the artery. Performance of this step helps in placement of the closure mechanism later on by reducing excess tissue between the skin and the artery. Once the 4F micropuncture catheter is in place, the micropuncture wire is removed. A femoral angiogram is obtained with the appropriate obliquity of the image intensifier to ensure an appropriate position of access, suitability of vessel for closure device (especially size and arterial plaque), and to rule out any complications from the access such as perforation or dissection.
Complications
Complications associated with vascular access closure devices can be categorized into the following three types:
Ischemic
Lower extremity ischemia from vascular access closure devices can be related to the stenosis/occlusion of the access artery or distal embolization. Device selection according to the characteristics of the access artery is important in minimizing this complication. These complications are usually managed with open surgical exploration. Endovascular approaches have also been employed in select cases.
Hemorrhagic
The risk of hemorrhagic complications is higher if the device is deployed in a vessel with unfavorable characteristics such as dense calcifications or at an inappropriate anatomic location (above inguinal ligament). Other factors can increase the risk of bleeding including increased age, interventional procedure, and end-stage renal disease. On-going hemorrhage from device failure can lead to blood loss anemia and hemodynamic instability requiring conversion to open surgical femoral exploration particularly if wire access is no longer in place. Alternatively, if the sheath size used was small and patient is hemodynamically stable, manual compression may be applied while either allowing heparin to wear off or administering protamine to reverse the heparin that was given during the procedure. Incomplete closure can also lead to the development of pseudoaneurysm from the access site. Depending on the clinical situation and imaging characteristics, this pseudoaneurysm can be managed either with ultrasound-guided thrombin injection or open surgical exploration.
Infectious
Vascular access closure devices introduce foreign material in the body (such as braided suture, nitinol, polyethylene glycol) and this can rarely serve as a nidus for infection. Particular attention should be paid to sterile technique throughout the procedure to minimize infectious complications from closure devices.
Clinical Significance
A wide variety of vascular access closure devices are available to the proceduralist today to aid in the hemostasis of the vascular access site. The proceduralist should familiarize himself with the indications and contraindications of some of these devices. These devices certainly have a utility in allowing earlier patient ambulation and discharge when compared to the traditional manual compression approach. Traditionally, we observe patients on bedrest for 4 to 6 hours after manual compression post-procedure whereas this time is reduced to 1 to 2 hours if a vascular closure device has been utilized. However, it should be noted that no one device has proven to be definitively superior. Also, closure device use has not resulted in a reduction in vascular complications when compared to manual compression. Although designed and approved for use in the common femoral artery, these devices have been utilized at other sites such as the brachial/subclavian arteries.[8][9]
Enhancing Healthcare Team Outcomes
After percutaneous procedures, today there are a number of vascular closure devices available. These devices are an alternative to manual compression to prevent bleeding. Healthcare workers including invasive cardiologists need to be familiar with these devices as there are frequently used. Most of these devices function in a similar fashion but the patient still needs to be observed for 30-120 minutes to ensure that there is no bleeding. Small case series reveal that there is no real difference between these devices and the success rate of vessel closure ranges from 80-100%. (Level V)
Media
(Click Image to Enlarge)
References
Malekzadeh S,Rolf T,Doenz F,Chouiter A,Jouannic AM,Qanadli SD, Safety of elective percutaneous peripheral revascularization in outpatients: A 10-year single-center experience. Diagnostic and interventional imaging. 2018 Dec 17; [PubMed PMID: 30573349]
Vora AN,Rao SV, Percutaneous or surgical access for transfemoral transcatheter aortic valve implantation. Journal of thoracic disease. 2018 Nov; [PubMed PMID: 30505540]
Kaki A,Blank N,Alraies MC,Kajy M,Grines CL,Hasan R,Htun WW,Glazier J,Mohamad T,Elder M,Schreiber T, Access and closure management of large bore femoral arterial access. Journal of interventional cardiology. 2018 Dec; [PubMed PMID: 30456854]
Shatila W,Krajcer Z, Plug vs. suture: Who wins in large bore access closure? Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography [PubMed PMID: 30450709]
Baldino G,Persi F,Mortola P,Gori A, An Alternative Technique to Achieve Haemostasis During PEVAR Using Perclose ProGlide. EJVES short reports. 2018; [PubMed PMID: 30426095]
Gewalt SM,Helde SM,Ibrahim T,Mayer K,Schmidt R,Bott-Flügel L,Hoppe K,Ott I,Hieber J,Morath T,Byrne RA,Kufner S,Cassese S,Hoppmann P,Fusaro M,Schunkert H,Laugwitz KL,Kastrati A,Schüpke S, Comparison of Vascular Closure Devices Versus Manual Compression After Femoral Artery Puncture in Women. Circulation. Cardiovascular interventions. 2018 Aug; [PubMed PMID: 30354782]
Han Y,Kwon JH,Park S, Korean single-center experience with femoral access closure using the ExoSeal device. World journal of radiology. 2018 Sep 28; [PubMed PMID: 30310545]
Tuna Katırcıbaşı M,Güneş H,Çağrı Aykan A,Aksu E,Özgül S, Comparison of Ultrasound Guidance and Conventional Method for Common Femoral Artery Cannulation: A Prospective Study of 939 Patients. Acta Cardiologica Sinica. 2018 Sep; [PubMed PMID: 30271089]
Wareham J,Luppe S,Youssef A,Crossley R,Mortimer A, Safety profile of an 8F femoral arteriotomy closure using the Angio-Seal device in thrombolysed acute stroke patients undergoing thrombectomy. Interventional neuroradiology : journal of peritherapeutic neuroradiology, surgical procedures and related neurosciences. 2018 Oct; [PubMed PMID: 29871562]