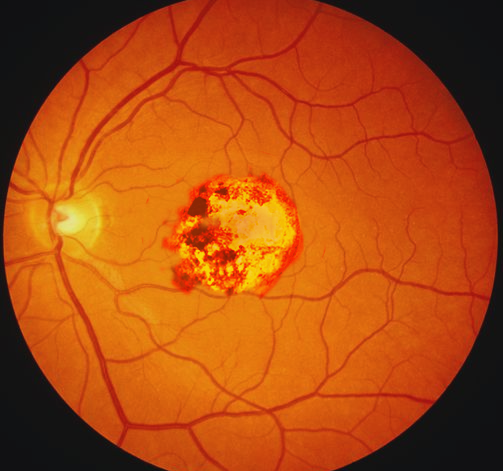
Introduction
Chorioretinitis is a type of uveitis involving the posterior segment of the eye, which includes inflammation of the choroid and the retina of the eye. Though the term uveitis means inflammation of the uveal tract (iris, ciliary body, and choroid), it can involve the adjacent structures like the retina, retinal vessels, vitreous, optic nerve head, and sclera. The Standardization of Uveitis Nomenclature (SUN)[1] consensus conference workshop classified uveitis into anterior (iritis, iridocyclitis, anterior cyclitis), intermediate (pars planitis, posterior cyclitis, hyalitis), posterior uveitis (choroiditis, chorioretinitis, retinochoroiditis, retinitis, and neuroretinitis) and panuveitis based on the anatomical location. Uveitis also classifies as granulomatous or nongranulomatous, acute, chronic or recurrent, insidious, or sudden, based on criteria like appearance, duration, or type of onset. Chorioretinitis is a type of posterior uveitis. The choroid is the vascular layer of the eye, which lies between the retina and the sclera. Since the choroid is responsible for the vascular support of the outer layers of the retina, inflammation of these layers can lead to vision-threatening complications.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Uveitis correlates with a wide range of underlying causes. Based on the etiology, posterior uveitis can classify as infectious or noninfectious.[2] Infection can be congenital or acquired. It can be idiopathic or associated with trauma.
Infectious causes include[3][4][5][6]:
- Toxoplasmosis
- Viruses like cytomegalovirus (CMV), herpes simplex, rubella, lymphocytic choriomeningitis virus, West Nile virus,
- HIV related eye diseases
- Tuberculosis
- Toxocara
- Syphilis
- Bartonella
- Fungal infections caused by Candida spp.
- Coccidioidomycosis[6] histoplasmosis
Noninfectious etiology with systemic association:
- Sarcoidosis
- Behcets disease
Noninfectious etiology without any systemic association:
- Multifocal choroiditis and panuveitis
- Punctate inner choroidopathy
- Multiple evanescent white dots syndrome
- Unilateral acute idiopathic maculopathy
- Birdshot choroidopathy
- Serpiginous choroidopathy
Other conditions are malignancies like lymphoma masquerading as uveitis, cancer-associated retinopathy.[7] Sympathetic ophthalmia and Vogt Koyanagi Harada syndrome have choroidal inflammation as a part of panuveitis. Posterior scleritis may also simulate choroiditis, but posterior scleritis is associated with severe pain and on ultrasound shows subtenon fluid (T sign).
Epidemiology
The epidemiological data from various studies showed that uveitis occurs between 52 and 341 per 100000 person-years in the US.[8][9] It is responsible for 2.8 to 10% of blindness cases in the United States.[10] Toxoplasma chorioretinitis is the most common cause of posterior uveitis worldwide. It has a higher prevalence in tropical countries with hot and humid climates. It accounts for 25% of cases of posterior uveitis in the US.[11] In the United States, antibodies to Toxoplasma are present in nearly 20% of people. It is a major opportunistic pathogen in AIDS.[12] Increased age was associated with a higher incidence of uveitis.[13] It is responsible for an estimated 30000 new cases of legal blindness annually in the USA and accounts for about 10 to 15% of all cases of total blindness in the country.[14] Ocular inflammatory disorders affect women more than men, and the majority of affected women are of childbearing age.[15][16]
Pathophysiology
Chorioretinitis is usually due to an infectious etiology. The extent of ocular involvement varies depending on the organism. Toxoplasma gondii is the most common cause of infectious posterior uveitis worldwide. Infection usually occurs by either consumption of tissue cysts present in raw or undercooked meat or by ingesting oocysts in the feces of cats. It can be transmitted transplacentally.[17] It can cause severe infection in immunosuppressed individuals and pregnant women.
Congenital Toxoplasmosis: In congenital toxoplasmosis, the mother is often asymptomatic or has mild constitutional manifestations. The severity of fetal involvement is related to the gestational period at which infection occurred, with more severe in early trimesters, when fetal death may result. In the first trimester rate of transmission to the fetus is around 10 to 25%, in the third trimester the transplacental transmission is approximately 60 to 80%. However, fetuses infected in early pregnancy are more likely to show clinical signs of infection.[18] The presence of chorioretinitis, intracranial calcifications, and hydrocephalus are considered the classic triad of congenital toxoplasmosis.[19] Congenital toxoplasmosis is associated with retinochoroidal lesions in 80% of infected newborns. It can be bilateral. Typical features are excavated macular lesions with associated pigmentation.
Acquired Toxoplasmosis: Most infections are thought to occur congenitally and can remain asymptomatic for several years and can become clinically evident commonly in the second through fourth decades. In the inactive stage, it can present as quiescent atrophic chorioretinal scars, which appear as pigmented lesions in clusters. In 70 to 80% of cases, it can occur as a unilateral focal chorioretinal lesion.[20] In the active phase, it occurs as necrotizing chorioretinitis with overlying vitritis. On fundoscopy, it can appear as a yellow-white lesion with indistinct margins, often described as a “headlight in a fog” appearance. The 'fog' is due to the vitritis, which causes vitreous haze, and the lesion of chorioretinitis appears as a focal area of yellow/whitish-yellow lesion ('headlight'). Lesions can be solitary, multiple, or satellite to an old chorioretinal scar. Numerous or bilateral active lesions are present in immunocompromised patients. In immunocompromised patients, it can mimic severe acute retinal necrosis (ARN).
In immunocompetent patients, the parasite can occur as a tissue cyst and can cause chronic infection. In patients with HIV, toxoplasmosis reactivation occurs when cell-mediated immunity fails. In HIV patients, with CD4 count less than 100, cotrimoxazole or dapsone and pyrimethamine can be given for six months.[21]
Congenital CMV: Cytomegalovirus (CMV) is the most common congenital viral infection in the United States. Congenital CMV infection presents with ocular manifestations like chorioretinitis, cataract, and optic atrophy along with jaundice, petechiae, hepatosplenomegaly, hearing loss, periventricular calcifications, ventricular calcifications, and microcephaly.[22][23] The majority of children with congenital CMV have asymptomatic infection. Chorioretinitis and/or optic atrophy is present in 10% of symptomatic infants with CMV.
CMV retinitis occurs in severely immunocompromised AIDS patients, with a CD4 lymphocyte count less than 50cells/micro L. CMV can cause necrotizing retinitis, retinal vasculitis, and optic neuropathy, with minimal vitritis. The characteristic fundus appearance of white retinitis lesions with hemorrhages is called "pizza-pie" appearance. It can cause anterior uveitis in healthy adults.
Ocular toxocariasis: It results from the nematodes Toxocara canis and Toxocara cati. It usually affects young patients. It is unilateral in 90% of cases. The child can have leukocoria, eye pain, strabismus, and profound monocular loss of vision. It commonly causes chronic endophthalmitis, granulomas in the posterior pole, or peripheral retina. Tractional retinal detachment can occur in these patients. Atypically it can cause diffuse chorioretinitis. It can be diagnosed based on the history of contact with pets and the characteristic ocular features. Retinoblastoma is an important differential diagnosis to be considered, which has a similar presentation. Serological tests like ELISA for the detection of anti-Toxocara excretory-secretory antigen IgE levels can confirm the diagnosis.[24][25] The use of anti-helminthic drugs is limited. Systemic steroids are given to treat severe inflammation.
Ocular syphilis: It has earned the name the great masquerader as the clinical presentation is diverse, with various inflammatory presentations such as iritis, chorioretinitis, vitritis, scleritis, neurosyphilis with Argyll Robertson pupil, and panuveitis. The most common presentation is iritis or iridocyclitis. In syphilis patients with HIV, placoid lesions have been described called acute syphilitic posterior placoid chorioretinitis.[26][27] It can occur in secondary or tertiary syphilis. Treatment is the same as neurosyphilis. The diagnosis should be suspected in patients with high-risk sexual behavior or with a history of other sexually transmitted diseases.
Ocular Tuberculosis (TB) is extrapulmonary tuberculosis commonly seen in endemic countries like India. The presentation can be variable, with lesions involving any layer of the eye. It can present as granulomatous anterior uveitis. Posterior segment manifestations including retinal vasculitis, multifocal serpiginous choroiditis, multifocal choroiditis, choroidal tubercles, tuberculoma, subretinal hypopyon, and subretinal abscesses are present.[28][29][30][29][28] Serpiginous like choroiditis may be associated with tuberculosis. Choroidal tubercles, tuberculoma, and choroidal abscess are generally considered as direct infection by tuberculosis bacillus. However, whether other manifestations are caused by infection (which needs antitubercular therapy/ATT) or by a hypersensitivity reaction (which is treated by steroids and/or immunosuppressive therapy) is controversial.[31] Mantoux test, interferon-gamma release assay/IGRA, polymerase chain reaction (PCR) from the intraocular specimen can suggest the diagnosis, though confirmation of direct causation by the bacteria is difficult. In most of the cases, the diagnosis is presumptive.[32] In low and middle-income countries, the World Health Organization's policy statement concluded that 'Neither IGRAs nor the TST should be used for the diagnosis of active TB disease.'[33] Ascertaining which cases need ATT, is difficult; this is important as ATT might have serious adverse effects also. Ocular tuberculosis is usually treated with ATT of at least nine months and oral steroids if needed. Healed ocular tuberculosis may be associated with a choroidal neovascular membrane, which might respond well with anti-vascular endothelial growth factor agents.[34][35]
Ocular histoplasmosis syndrome occurs in regions endemic for Histoplasma capsulatum. It can present with asymptomatic chorioretinitis, discrete oval–round whitish lesions less than 400 micrometers in diameter that may develop into classic punched out histo spots or choroidal neovascular membrane, which can cause central vision loss.[36]
Chorioretinitis has also been described in congenital infections with the Zika virus, rubella, and varicella-zoster virus.[37][38][39][40][41][40][38][42]
Zika virus is a neurotropic virus that can cross the placenta and infect the fetus. Classical findings include microcephaly, ventriculomegaly, cerebellar hypoplasia, lissencephaly with hydrocephalus, and fetal akinesia deformation sequence called arthrogryposis. Infants with the Zika virus have vision-threatening fundus abnormalities like macular chorioretinal atrophy, focal pigmentary changes, and optic nerve abnormalities. Diagnostic confirmation is by Zika RNA with PCR.
Congenital Varicella-Zoster virus infection can cause microphthalmia, chorioretinitis, and cataract in 44 to 52% of cases.[43]
EBV (Epstein Barr virus) uveitis: EBV uveitis can present as iritis, whereas posterior segment involvement may include multifocal choroiditis with punched-out areas of pigment epithelial changes and vitritis.[44] Reports exist of chorioretinitis with West Nile virus infection.[45] The chorioretinitis associated with the West Nile virus is typically linear, which might be related to the organization of the retinal nerve fiber layer.[46]
Non-infective posterior uveitis with systemic illness:
Sarcoidosis: It is a multi-system disease characterized by noncaseating granulomas in tissues, with a high incidence reported in African American women. It can cause granulomatous anterior uveitis, periphlebitis, with candle wax drippings and yellow waxy spots, vitritis, snowball opacities, and snowbanking. Chorioretinal granulomas, placoid lesions, and optic nerve head granuloma are presenting features.
Bechet’s disease presents with recurrent anterior and posterior uveitis, recurrent oral or genital ulcers, and skin lesions. Ocular manifestation can be seen in 70% of cases of Behcets disease and is one of the main diagnostic criteria of the disease. It can cause nongranulomatous anterior uveitis. Posterior segment involvement characteristically shows by vitritis/media haze, retinal vasculitis, hyalitis, necrotizing retinitis, and chorioretinitis.[47][48]
Non-infective posterior uveitis without systemic illness (white dot syndromes)
Common non-infective posterior uveitic entities include the white dot syndromes (WDS), named according to their clinical appearance and behavior. Laboratory investigations are not routinely needed for their diagnosis. Fundus fluorescein angiogram (FFA), indocyanine green angiogram (ICGA), optical coherence tomography (OCT), and multimodal imaging are used to evaluate WDS.
Birdshot chorioretinopathy (BSCR)/vitiliginous choroiditis: More than 90% of the patients with BCR are HLA-A29 positive. The term “birdshot” is because the lesions scattered around the optic disc and radiate to the equator resembling the shotgun scatter of birdshot.[49]
Acute Posterior Multifocal Placoid Pigment Epitheliopathy (APMPEE): It is a bilateral inflammatory retinal/choroidal disease characterized by a sudden loss of vision caused by the sudden appearance of multiple yellow-white, flat inflammatory lesions lying deep within the sensory retina, Retinal pigment epithelium (RPE) and the choriocapillaris. The lesions of APMPPE are unique but may be confused with viral retinitis, syphilis, serpiginous choroiditis, and toxoplasma retinochoroiditis. In APMPPE, lesions are flat, and there is no significant vitritis. Fundus Fluorescein Angiography (FFA) shows an early hypofluorescence of the white placoid lesions with late staining.
Multiple Evanescent White Dot Syndrome (MEWDS): MEWDS is an inflammatory chorioretinopathy characterized by the presence of white/gray lesions deep in the retina. It is a rare disorder seen in healthy young women. It can also demonstrate optic disc edema, mild vitritis, panuveitis, diffuse choroidal thickening. A characteristic granular appearance of the fovea is present. Contrary to other white dot syndromes, it is usually unilateral. FFA shows early and late hyperfluorescence at the lesions.
Serpiginous Choroiditis (SC): It is a rare progressive recurrent bilateral disease that affects the outer retina, retinal pigment epithelium, choriocapillaris, and inner choroid. The lesions are gray or gray-yellow areas that begin either in the peripapillary region or macula. FFA demonstrates early hypofluorescence and late hyperfluorescence of active lesions.
Multi-Focal Choroiditis/Punctate Inner Choroidopathy (MFC and PIC): They occur predominantly in myopic women, usually involving both eyes. The presence of cystoid macular edema /choroidal neovascular membrane is associated with poor visual prognosis.
Masquerade syndromes: Malignancies like intraocular lymphoma and leukemia can present with uveitis. Choroidal melanoma and cancer-associated retinopathy must be ruled out in non-resolving and atypical presentations of uveitis.[50]
Histopathology
Histopathologically, diffuse or focal inflammatory infiltrates appear in the retina and choroid. With active ocular toxoplasmosis, a focal retinochoroiditis with necrotizing granulomatous inflammation of the retina is present. Parasites may appear as free tachyzoites, or tissue cysts with mononuclear inflammatory infiltrates surrounding retinal blood vessels.
History and Physical
Patients usually present with symptoms such as the sudden onset of floaters, blurred vision, loss of vision, scotoma, or distorted vision. Pain and redness may be present if the anterior segment has also become inflamed as a part of panuveitis. Patients may be asymptomatic when the lesions are present in the periphery of the retina, away from the macula. Some patients may have associated systemic symptoms. Congenital toxoplasmosis is associated with prematurity, intrauterine growth restriction, jaundice, hepatosplenomegaly, rash, chorioretinal lesions, hydrocephalus, intracranial calcification, microcephaly, and seizures. Young children may present with reduced visual acuity, nystagmus, strabismus, or leukocoria. A history of contact with pets is in the patient history with parasitic infections like toxocariasis and toxoplasmosis.
Most infections have characteristic fundus features. Choroiditis and retinitis a distinguishable on fundus examination. Retinitis appears as a whitish patch with ill-defined borders. It is superficial, with severe overlying vitritis. In contrast, choroiditis lesions appear as yellow patches, with regular borders, deeper to the retinal vessels, causing mild vitritis. Active lesions have fuzzy borders, whereas healed lesions have a sharply demarcated margin with pigmentation. However, in most cases, both retina and choroid are inflamed, resulting in chorioretinitis.
Evaluation
The approach to the uveitis patient must be comprehensive, as it may be associated with multiple systemic conditions. The diagnosis can be made mostly clinically by direct and indirect ophthalmoscopy, based on the characteristic fundus findings. The method of developing and narrowing the differentials has been called naming and meshing based on the clinical presentation.[51] The condition requires a targeted approach where investigations get tailored to each patient depending on the history and clinical features and the differentials generated. Ancillary investigations like fundus fluorescein angiography (FFA), Indocyanine green angiography (ICG), B scan ultrasonography, Optical coherence tomography (OCT), Fundus autofluorescence can help in the diagnosis.
Fundus Fluorescein Angiography (FFA): It provides fundus photographs for diagnosis, documentation as well as monitoring the progress of the disease. It can detect whether a lesion is active or inactive, active lesions will show late hyperfluorescence on FFA. It can also detect neovascularization, vasculitis, areas of capillary nonperfusion, and cystoid macular edema.
Indocyanine Green Angiography (ICG) is more helpful in the case of white dot syndromes and other choroidal pathologies, which FFA cannot detect. It can provide additional information in diagnosis along with FFA. The infrared light in indocyanine green angiography (ICGA) penetrates the pigments in the retinal pigment epithelium. So it is better than FFA in delineating choriocapillaris.
Optical coherence tomography (OCT) is a noninvasive imaging technique using near-infrared light interferometry used to evaluate pathological macular changes like cystoid macular edema, subretinal fluid, choroidal thickening, and epiretinal membrane.
B scan ultrasonography for ultrasound imaging of the posterior segment, when the view becomes obscured by small pupillary size or poor view of the fundus due to the opacity of the media.
The basic workup for uveitis depends on the history, clinical features, and anatomical location of the lesion. Always exclude syphilis and sarcoidosis. Lab investigations include intraocular fluid evaluation for polymerase chain reaction or the parasite DNA and serum antibody titers. It aids in differentiating infectious from noninfectious etiology. Serum antibodies can detect whether the infection is recent or chronic. Anti-Toxoplasma Ig M antibodies appear in the first week of infection and then decline in the next few months. Toxoplasma IgG appears within 1 to 2 weeks of infection, peaks within 1 to 2 months, and will remain detectable for the lifetime of the patient. The positive predictive value of the IgG Toxoplasma antibody is low. A rise in titer in Toxoplasma IgG over three weeks has been useful as an indicator of a recent infection. For detection of infection in the newborn, Ig A or IgM antibodies are used. Maternal IgG antibodies present in the newborn reflect past or recent infection in the mother. In babies, maternally transmitted IgG will disappear within 6 to 12 months.[52]
Treatment / Management
The goal of the treatment is to preserve visual function. Treatment is directed at the elimination of infection if present and suppression of host inflammatory response. In infective conditions, the specific antimicrobial therapy gets coupled with anti-inflammatory agents, which are started 48 to 72 hours after the initiation of anti-infective agents. Treatment of noninfectious posterior uveitis is mainly corticosteroids and immunosuppressive agents. Therapy administration can be via multiple routes, including topical, periocular, and intraocular injections or systemic. It is essential to treat the underlying systemic disease, as well.
Ocular Toxoplasmosis: Treatment is a combination of antiparasitic drugs with systemic corticosteroids. Pyrimethamine, which is a folic acid antagonist, gets administered as a loading dose of 75 to 100 mg for 1 to 2 days, followed by 25 to 50 mg daily for four weeks. It is combined with oral folinic acid 5 mg three times a week to prevent folate deficiency. Sulfadiazine 1 g four times daily for 3 to 4 weeks is usually in combination with pyrimethamine. Systemic steroids are typically started 72 hours after the initiation of antimicrobial therapy.
In the case of allergy to sulfonamides, clindamycin 300 mg four times daily or azithromycin 250 to 500 mg/day are therapeutic options. Sulfamethoxazole-trimethoprim 800 mg/160 mg is another combination given. Atovaquone 750 mg four times daily can be dosed for 4 to 6 weeks. Spiramycin 2 g/day is the safest antiparasitic in pregnancy.
Systemic steroids serve as the mainstay of therapy in non-infective posterior uveitis. Oral Prednisolone is the most commonly given steroid at a dose of 1 to 1.5 mg/kg body weight. Vision-threatening lesions, such as those with optic nerve head or macular/foveal involvement, will require administration of intravenous methylprednisolone 1 g daily for three consecutive days should be administered, followed by oral prednisolone in tapering doses.
Steroid sparing immunomodulatory agents are useful in steroid-resistant cases or cases of complications from long term use of steroids. Systemic cyclosporine, azathioprine, mycophenolate mofetil, methotrexate may be administered. Immunomodulators like tumor necrosis factor-alpha inhibitor like adalimumab or infliximab are also options when first-line agents fail.[53]
Treatment of complications like choroidal neovascular membrane includes anti-VEGF agents such as bevacizumab, ranibizumab, local and systemic corticosteroids, photodynamic therapy. Surgical treatment like pars plana vitrectomy, cryotherapy, laser photocoagulation is for treatment of complications.[54](B3)
Differential Diagnosis
The clinical presentation of the various clinical entities causing posterior uveitis can overlap. Sometimes lesions of age-related macular degeneration, multiple leaking central serous retinopathy, posterior scleritis, intraocular malignancies can be confused with lesions of posterior uveitis. The infective chorioretinitis lesions can be distinguished from white dot syndromes by their fundus appearance, and ICGA and FFA give conclusive evidence in most cases. In newborns with congenital infection, exclude TORCHeS. Other less common congenital infections simulating toxoplasmosis include West Nile virus fever, acute lymphocytic choriomeningitis, and, more recently, Zika virus, the latter particularly in the setting of microcephaly. Tumors such as retinoblastoma and congenital anomalies of the retina or choroid such as retinochoroidal coloboma and persistent hyperplastic vitreous are also considerations in the differential diagnosis in children.
Prognosis
Visual prognosis depends on the location of the chorioretinal lesion, recurrences, and other posterior segment complications.[55][56] Lesions adjacent to the macula and optic nerve causes severe visual impairment (20/200 or worse). Toxoplasma has a high recurrence after an active episode. The prognosis is good in immunocompetent individuals if the central macula is not involved.
Complications
Uveitis can cause visual loss and blindness due to a variety of causes, including secondary complications like cataract, secondary glaucoma, choroidal neovascularization cystoid macular edema, optic neuropathy, retinal detachment, retinal vascular occlusion, hemorrhage, and phthisis.[57][58] Cystoid macular edema (CME) is the most common complication in noninfectious posterior uveitis.
CME is common in birdshot chorioretinitis, sarcoidosis, and other uveitis, which cause extensive vitreous inflammation. Choroidal neovascularization (CNV) is seen more with choroidal involvement and disruption of the Bruch's membrane. Causes of CNV include presumed ocular histoplasmosis syndrome, multifocal choroiditis and panuveitis, serpiginous choroiditis, and punctate inner choroidopathy.
Deterrence and Patient Education
Primary prophylactic measures in pregnant women and HIV infected individuals to prevent Toxoplasmosis include avoiding ingestion of raw/ uncooked meat. Eating raw oysters, clams, or mussels should be avoided, as they may be contaminated.[12] Toxoplasma cysts will die at 60 degrees C for 15 minutes or by freezing at -20 degrees C for 24 hours, overnight. Drink only well filtered or boiled water. Avoiding contact with cats and their feces in soil or litter boxes, using gloves, peeling, or cleaning of fruits and vegetables thoroughly before eating, as well as washing hands after contact with soil and cat litter boxes, is recommended.
Women who acquire toxoplasmosis infection during pregnancy require counseling about the risk of congenital infection and its clinical sequelae.
Enhancing Healthcare Team Outcomes
The approach to the uveitis patient must be comprehensive. The importance of a careful, complete history, including family, social, travel, and medication histories and review of systems cannot be overemphasized. It is best managed by an interprofessional team that includes an ophthalmologist, neurologist, neonatologist, obstetrician, infectious disease expert, internist, infectious disease pharmacist, and specialty-trained ophthalmology nurse. Immunosuppressed patients usually need long term treatment and follow up. The primary care provider and nurse practitioner should educate the patient on preventive measures. Early detection and treatment are necessary to prevent vision-threatening complications.
The clinician or specialist will initiate treatment, and agent selection should receive a consult with a board-certified infectious disease pharmacist with the latest antibiogram data; this will lead to targeted therapy and help prevent antibiotic resistance. The pharmacist can also advise on other medications used in management, and perform medication reconciliation. Nursing will work with the patient on counseling, followup, and monitor treatment effectiveness and check for potential adverse drug reactions, informing the clinicians of any concerns. This type of interprofessional approach is crucial to the successful treatment and management of chorioretinitis/uveitis. [Level V]
Media
(Click Image to Enlarge)
Fundoscopic Image of Late Neuroocular Syphilis. This is a fundoscopic image of the effect of late neuroocular syphilis on the optic disk and retina, including pathology, severe optic-nerve atrophy, chorioretinitis, and inflammation of the choroidal and neural layers of the retina.
Contributed by S Lindsley
References
Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. American journal of ophthalmology. 2005 Sep:140(3):509-16 [PubMed PMID: 16196117]
Vadot E. [Retinitis and chorioretinitis]. La Revue du praticien. 1989 May 11:39(14):1213-6 [PubMed PMID: 2734581]
Lazzarotto T, Guerra B, Gabrielli L, Lanari M, Landini MP. Update on the prevention, diagnosis and management of cytomegalovirus infection during pregnancy. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2011 Sep:17(9):1285-93. doi: 10.1111/j.1469-0691.2011.03564.x. Epub 2011 Jun 1 [PubMed PMID: 21631642]
Grutzmacher RD, Henderson D, McDonald PJ, Coster DJ. Herpes simplex chorioretinitis in a healthy adult. American journal of ophthalmology. 1983 Dec:96(6):788-96 [PubMed PMID: 6660268]
Level 3 (low-level) evidenceChen KJ, Chou HD, Teh WM. Early-Stage Multifocal Candida chorioretinitis in an Immunocompromised Woman. Ophthalmology. Retina. 2019 Oct:3(10):887. doi: 10.1016/j.oret.2019.05.023. Epub [PubMed PMID: 31585711]
Nordstrom B, Sherpa N, Marshall M, Chawla A, Heidari A, Johnson R. Coccidioidomycosis Chorioretinitis. Journal of investigative medicine high impact case reports. 2019 Jan-Dec:7():2324709619881561. doi: 10.1177/2324709619881561. Epub [PubMed PMID: 31597500]
Level 3 (low-level) evidenceCarrera W, Tsamis KA, Shah R. A case of cancer-associated retinopathy with chorioretinitis and optic neuritis associated with occult small cell lung cancer. BMC ophthalmology. 2019 May 2:19(1):101. doi: 10.1186/s12886-019-1103-4. Epub 2019 May 2 [PubMed PMID: 31046716]
Level 3 (low-level) evidenceReeves SW, Sloan FA, Lee PP, Jaffe GJ. Uveitis in the elderly: epidemiological data from the National Long-term Care Survey Medicare Cohort. Ophthalmology. 2006 Feb:113(2):307.e1 [PubMed PMID: 16406541]
Level 2 (mid-level) evidenceGritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004 Mar:111(3):491-500; discussion 500 [PubMed PMID: 15019324]
Level 2 (mid-level) evidenceAcharya NR, Tham VM, Esterberg E, Borkar DS, Parker JV, Vinoya AC, Uchida A. Incidence and prevalence of uveitis: results from the Pacific Ocular Inflammation Study. JAMA ophthalmology. 2013 Nov:131(11):1405-12. doi: 10.1001/jamaophthalmol.2013.4237. Epub [PubMed PMID: 24008391]
Level 2 (mid-level) evidenceKijlstra A, Petersen E. Epidemiology, pathophysiology, and the future of ocular toxoplasmosis. Ocular immunology and inflammation. 2014 Apr:22(2):138-47. doi: 10.3109/09273948.2013.823214. Epub 2013 Oct 16 [PubMed PMID: 24131274]
Level 3 (low-level) evidenceJones JL, Dargelas V, Roberts J, Press C, Remington JS, Montoya JG. Risk factors for Toxoplasma gondii infection in the United States. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009 Sep 15:49(6):878-84. doi: 10.1086/605433. Epub [PubMed PMID: 19663709]
Level 3 (low-level) evidenceGonzález MM, Solano MM, Porco TC, Oldenburg CE, Acharya NR, Lin SC, Chan MF. Epidemiology of uveitis in a US population-based study. Journal of ophthalmic inflammation and infection. 2018 Apr 17:8(1):6. doi: 10.1186/s12348-018-0148-5. Epub 2018 Apr 17 [PubMed PMID: 29666980]
Thorne JE, Suhler E, Skup M, Tari S, Macaulay D, Chao J, Ganguli A. Prevalence of Noninfectious Uveitis in the United States: A Claims-Based Analysis. JAMA ophthalmology. 2016 Nov 1:134(11):1237-1245. doi: 10.1001/jamaophthalmol.2016.3229. Epub [PubMed PMID: 27608193]
Suttorp-Schulten MS, Rothova A. The possible impact of uveitis in blindness: a literature survey. The British journal of ophthalmology. 1996 Sep:80(9):844-8 [PubMed PMID: 8962842]
Level 3 (low-level) evidenceYeh S, Forooghian F, Suhler EB. Implications of the Pacific Ocular Inflammation uveitis epidemiology study. JAMA. 2014 May 14:311(18):1912-3. doi: 10.1001/jama.2014.2294. Epub [PubMed PMID: 24825646]
Kato K. How does Toxoplama gondii invade host cells? The Journal of veterinary medical science. 2018 Nov 23:80(11):1702-1706. doi: 10.1292/jvms.18-0344. Epub 2018 Oct 4 [PubMed PMID: 30282883]
Dunn D, Wallon M, Peyron F, Petersen E, Peckham C, Gilbert R. Mother-to-child transmission of toxoplasmosis: risk estimates for clinical counselling. Lancet (London, England). 1999 May 29:353(9167):1829-33 [PubMed PMID: 10359407]
Tripathy K, Sharma YR, Chawla R, Basu K, Vohra R, Venkatesh P. Triads in Ophthalmology: A Comprehensive Review. Seminars in ophthalmology. 2017:32(2):237-250. doi: 10.3109/08820538.2015.1045150. Epub 2015 Jul 6 [PubMed PMID: 26148300]
Bonfioli AA, Orefice F. Toxoplasmosis. Seminars in ophthalmology. 2005 Jul-Sep:20(3):129-41 [PubMed PMID: 16282146]
Level 3 (low-level) evidenceBasavaraju A. Toxoplasmosis in HIV infection: An overview. Tropical parasitology. 2016 Jul-Dec:6(2):129-135 [PubMed PMID: 27722101]
Level 3 (low-level) evidenceBoppana SB, Pass RF, Britt WJ, Stagno S, Alford CA. Symptomatic congenital cytomegalovirus infection: neonatal morbidity and mortality. The Pediatric infectious disease journal. 1992 Feb:11(2):93-9 [PubMed PMID: 1311066]
Picone O, Vauloup-Fellous C, Cordier AG, Guitton S, Senat MV, Fuchs F, Ayoubi JM, Grangeot Keros L, Benachi A. A series of 238 cytomegalovirus primary infections during pregnancy: description and outcome. Prenatal diagnosis. 2013 Aug:33(8):751-8. doi: 10.1002/pd.4118. Epub 2013 May 1 [PubMed PMID: 23553686]
Level 2 (mid-level) evidenceAhn SJ, Ryoo NK, Woo SJ. Ocular toxocariasis: clinical features, diagnosis, treatment, and prevention. Asia Pacific allergy. 2014 Jul:4(3):134-41. doi: 10.5415/apallergy.2014.4.3.134. Epub 2014 Jul 29 [PubMed PMID: 25097848]
Tian JX, O'Hagan S. Toxocara polymerase chain reaction on ocular fluids in bilateral granulomatous chorioretinitis. International medical case reports journal. 2015:8():107-10. doi: 10.2147/IMCRJ.S84185. Epub 2015 May 18 [PubMed PMID: 26056495]
Level 3 (low-level) evidenceFranco M, Nogueira V. Severe acute syphilitic posterior placoid chorioretinitis with complete spontaneous resolution: The natural course. GMS ophthalmology cases. 2016:6():Doc02. doi: 10.3205/oc000039. Epub 2016 Feb 16 [PubMed PMID: 27625961]
Level 3 (low-level) evidenceGass JD, Braunstein RA, Chenoweth RG. Acute syphilitic posterior placoid chorioretinitis. Ophthalmology. 1990 Oct:97(10):1288-97 [PubMed PMID: 2243679]
Level 3 (low-level) evidenceChawla R, Venkatesh P, Tripathy K, Chaudhary S, Sharma SK. Successful Management of Proliferative Diabetic Retinopathy and Multiple Choroidal Tubercles in a Patient with Miliary Tuberculosis. Journal of ophthalmic & vision research. 2018 Apr-Jun:13(2):210-211. doi: 10.4103/jovr.jovr_203_16. Epub [PubMed PMID: 29719654]
Tripathy K, Chawla R. Choroidal tuberculoma. The National medical journal of India. 2016 Mar-Apr:29(2):106 [PubMed PMID: 27586221]
Chawla R, Tripathy K, Meena S, Behera AK. Subretinal Hypopyon in Presumed Tubercular Uveitis: A Report of Two Cases. Middle East African journal of ophthalmology. 2018 Jul-Dec:25(3-4):163-166. doi: 10.4103/meajo.MEAJO_187_17. Epub [PubMed PMID: 30765956]
Level 3 (low-level) evidenceShakarchi FI. Ocular tuberculosis: current perspectives. Clinical ophthalmology (Auckland, N.Z.). 2015:9():2223-7. doi: 10.2147/OPTH.S65254. Epub 2015 Nov 26 [PubMed PMID: 26648690]
Level 3 (low-level) evidenceTripathy K. Comment on Trad et al.'s "Update on Immunological Test (Quantiferon-TB Gold) Contribution in the Management of Tuberculosis-Related Ocular Inflammation". Ocular immunology and inflammation. 2019:27(1):138-139. doi: 10.1080/09273948.2017.1371766. Epub 2017 Oct 11 [PubMed PMID: 29020492]
Level 3 (low-level) evidence. Use of Tuberculosis Interferon-Gamma Release Assays (IGRAs) in Low- and Middle- Income Countries: Policy Statement. 2011:(): [PubMed PMID: 26269875]
Tripathy K. Choroidal neovascular membrane in intraocular tuberculosis. GMS ophthalmology cases. 2017:7():Doc24. doi: 10.3205/oc000075. Epub 2017 Sep 1 [PubMed PMID: 28944155]
Level 3 (low-level) evidenceTripathy K, Chawla R, Sharma YR. Intravitreal Bevacizumab for Choroidal Neovascular Membrane at the Edge of a Healed Choroidal Tuberculoma. Ocular immunology and inflammation. 2018:26(2):239-241. doi: 10.1080/09273948.2016.1206205. Epub 2016 Aug 19 [PubMed PMID: 27541084]
Diaz RI, Sigler EJ, Rafieetary MR, Calzada JI. Ocular histoplasmosis syndrome. Survey of ophthalmology. 2015 Jul-Aug:60(4):279-95. doi: 10.1016/j.survophthal.2015.02.005. Epub 2015 Mar 5 [PubMed PMID: 25841248]
Level 3 (low-level) evidenceMets MB. Eye manifestations of intrauterine infections. Ophthalmology clinics of North America. 2001 Sep:14(3):521-31 [PubMed PMID: 11705152]
O'Neill JF. The ocular manifestations of congenital infection: a study of the early effect and long-term outcome of maternally transmitted rubella and toxoplasmosis. Transactions of the American Ophthalmological Society. 1998:96():813-79 [PubMed PMID: 10360309]
Level 3 (low-level) evidenceAlvarado MG, Schwartz DA. Zika Virus Infection in Pregnancy, Microcephaly, and Maternal and Fetal Health: What We Think, What We Know, and What We Think We Know. Archives of pathology & laboratory medicine. 2017 Jan:141(1):26-32. doi: 10.5858/arpa.2016-0382-RA. Epub 2016 Sep 16 [PubMed PMID: 27636525]
Henry CR, Al-Attar L, Cruz-Chacón AM, Davis JL. Chorioretinal Lesions Presumed Secondary to Zika Virus Infection in an Immunocompromised Adult. JAMA ophthalmology. 2017 Apr 1:135(4):386-389. doi: 10.1001/jamaophthalmol.2017.0098. Epub [PubMed PMID: 28278327]
Clark WG, Register JC 3rd, Nejidat A, Eichholtz DA, Sanders PR, Fraley RT, Beachy RN. Tissue-specific expression of the TMV coat protein in transgenic tobacco plants affects the level of coat protein-mediated virus protection. Virology. 1990 Dec:179(2):640-7 [PubMed PMID: 2238465]
Lambert SR, Taylor D, Kriss A, Holzel H, Heard S. Ocular manifestations of the congenital varicella syndrome. Archives of ophthalmology (Chicago, Ill. : 1960). 1989 Jan:107(1):52-6 [PubMed PMID: 2910286]
Level 3 (low-level) evidenceAndreou A, Basiakos H, Hatzikoumi I, Lazarides A. Fetal varicella syndrome with manifestations limited to the eye. American journal of perinatology. 1995 Sep:12(5):347-8 [PubMed PMID: 8540940]
Level 3 (low-level) evidencePeponis VG, Chatziralli IP, Parikakis EA, Chaira N, Katzakis MC, Mitropoulos PG. Bilateral Multifocal Chorioretinitis and Optic Neuritis due to Epstein-Barr Virus: A Case Report. Case reports in ophthalmology. 2012 Sep:3(3):327-32. doi: 10.1159/000343704. Epub 2012 Oct 6 [PubMed PMID: 23139677]
Level 3 (low-level) evidenceShaikh S, Trese MT. West Nile virus chorioretinitis. The British journal of ophthalmology. 2004 Dec:88(12):1599-60 [PubMed PMID: 15548822]
Level 3 (low-level) evidenceKhairallah M, Ben Yahia S, Attia S, Zaouali S, Ladjimi A, Messaoud R. Linear pattern of West Nile virus-associated chorioretinitis is related to retinal nerve fibres organization. Eye (London, England). 2007 Jul:21(7):952-5 [PubMed PMID: 16628235]
Level 2 (mid-level) evidenceAuluck I, Karimi A, Taylor S. Lesson of the month 2: A case of Behçet's disease: 70% have ophthalmic involvement. Clinical medicine (London, England). 2019 Oct 22:():. pii: clinmed.2019-0149. doi: 10.7861/clinmed.2019-0149. Epub 2019 Oct 22 [PubMed PMID: 31641065]
Level 3 (low-level) evidenceDesbois AC, Terrada C, Cacoub P, Bodaghi B, Saadoun D. [Ocular manifestations in Behçet's disease]. La Revue de medecine interne. 2018 Sep:39(9):738-745. doi: 10.1016/j.revmed.2018.02.022. Epub 2018 Apr 4 [PubMed PMID: 29625716]
Minos E, Barry RJ, Southworth S, Folkard A, Murray PI, Duker JS, Keane PA, Denniston AK. Birdshot chorioretinopathy: current knowledge and new concepts in pathophysiology, diagnosis, monitoring and treatment. Orphanet journal of rare diseases. 2016 May 12:11(1):61. doi: 10.1186/s13023-016-0429-8. Epub 2016 May 12 [PubMed PMID: 27175923]
Rothova A, Ooijman F, Kerkhoff F, Van Der Lelij A, Lokhorst HM. Uveitis masquerade syndromes. Ophthalmology. 2001 Feb:108(2):386-99 [PubMed PMID: 11158819]
Level 3 (low-level) evidenceRathinam SR, Babu M. Algorithmic approach in the diagnosis of uveitis. Indian journal of ophthalmology. 2013 Jun:61(6):255-62. doi: 10.4103/0301-4738.114092. Epub [PubMed PMID: 23803476]
Garweg JG, de Groot-Mijnes JD, Montoya JG. Diagnostic approach to ocular toxoplasmosis. Ocular immunology and inflammation. 2011 Aug:19(4):255-61. doi: 10.3109/09273948.2011.595872. Epub [PubMed PMID: 21770803]
Mérida S, Palacios E, Navea A, Bosch-Morell F. New Immunosuppressive Therapies in Uveitis Treatment. International journal of molecular sciences. 2015 Aug 11:16(8):18778-95. doi: 10.3390/ijms160818778. Epub 2015 Aug 11 [PubMed PMID: 26270662]
Korol AR, Zborovska O, Kustryn T, Dorokhova O, Pasyechnikova N. Intravitreal aflibercept for choroidal neovascularization associated with chorioretinitis: a pilot study. Clinical ophthalmology (Auckland, N.Z.). 2017:11():1315-1320. doi: 10.2147/OPTH.S132923. Epub 2017 Jul 20 [PubMed PMID: 28769551]
Level 3 (low-level) evidenceAzevedo MH, Moura GL, Camilo EN, Muccioli C, Arantes TE. Visual function and macular architecture in patients with inactive zone 2 and 3 toxoplasmic retinochoroiditis. Arquivos brasileiros de oftalmologia. 2015 Sep-Oct:78(5):273-7. doi: 10.5935/0004-2749.20150073. Epub [PubMed PMID: 26466223]
Aleixo AL, Curi AL, Benchimol EI, Amendoeira MR. Toxoplasmic Retinochoroiditis: Clinical Characteristics and Visual Outcome in a Prospective Study. PLoS neglected tropical diseases. 2016 May:10(5):e0004685. doi: 10.1371/journal.pntd.0004685. Epub 2016 May 2 [PubMed PMID: 27136081]
Mukkamala L, Yiu G. Choriovitreal Neovascularization After Resolution of Infectious Chorioretinitis. Retina (Philadelphia, Pa.). 2019 Jun:39(6):e21-e22. doi: 10.1097/IAE.0000000000002558. Epub [PubMed PMID: 31090687]
Tripathy K, Chawla R, Temkar S, Sagar P, Kashyap S, Pushker N, Sharma YR. Phthisis Bulbi-a Clinicopathological Perspective. Seminars in ophthalmology. 2018:33(6):788-803. doi: 10.1080/08820538.2018.1477966. Epub 2018 Jun 14 [PubMed PMID: 29902388]
Level 3 (low-level) evidence