Introduction
Chronic myeloid leukemia (CML), BCR-ABL1-positive, is classified as a myeloproliferative neoplasm predominantly composed of proliferating granulocytes and determined to have the Philadelphia chromosome/translocation t(9;22)(q34;q11.2). CML affects both the peripheral blood and the bone marrow.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
There is an increased incidence of CML among atomic bomb survivors; however, the predisposing risk factors are unknown.[1]
Epidemiology
CML has a worldwide annual incidence rate of 0.87 people per 100,000, increasing with age up to 1.52 in patients older than 70 years. There is a slight male predominance. The median age of diagnosis is 56 years old.[2]
In the United States, the annual incidence rate between 2009 and 2013 was 1.4 and 2.2 per 100,000 for females and males, respectively.[3] Estimates for 2018 were 8490 new cases of CML and 1090 estimated deaths.[4]
Pathophysiology
The fusion oncoprotein BCR-ABL1 defines CML. 90% to 95% of patients with CML have a shortened chromosome 22 because of a reciprocal translocation t(9;22) (q34;q11.2) called the Philadelphia chromosome. The ABL1 gene encodes a non-receptor tyrosine kinase on chromosome 9, and BCR is a breakpoint cluster region on chromosome 22. The translated oncoprotein, in most cases, is 210-kd and called p210 BCR-ABL1. Alternative splicing results in p190 and p230 BCR-ABL1, which may show different presentations. This oncoprotein acts as a constitutively expressed defective tyrosine kinase. The downstream pathways affected include JAK/STAT, PI3K/AKT, and RAS/MEK; they involve cell growth, cell survival, inhibition of apoptosis, and activation of transcription factors.[5]
The remainder of patients has variant or complex translocations involving additional chromosomes detected by routine cytogenetics or a cryptic BCR-ABL1 translocation detected with fluorescent in situ hybridization (FISH) or reverse transcriptase-polymerase chain reaction (PCR).[6]
Histopathology
Chronic Phase
The peripheral blood smear will show a leukocytosis due to granulocytes in various stages of maturation. There will be a bimodal distribution with higher proportions of mature segmented neutrophils and myelocytes. Blast cells will account for less than 2% of the white blood cells. Increased basophils and eosinophils are common. Significant dysplasia affecting greater than 10% of the granulocyte population is absent. Monocytosis may be present; however, it is usually less than 3% of the white blood cells. Platelets usually range from the normal range to a significant increase. Thrombocytopenia is an uncommon finding.[7]
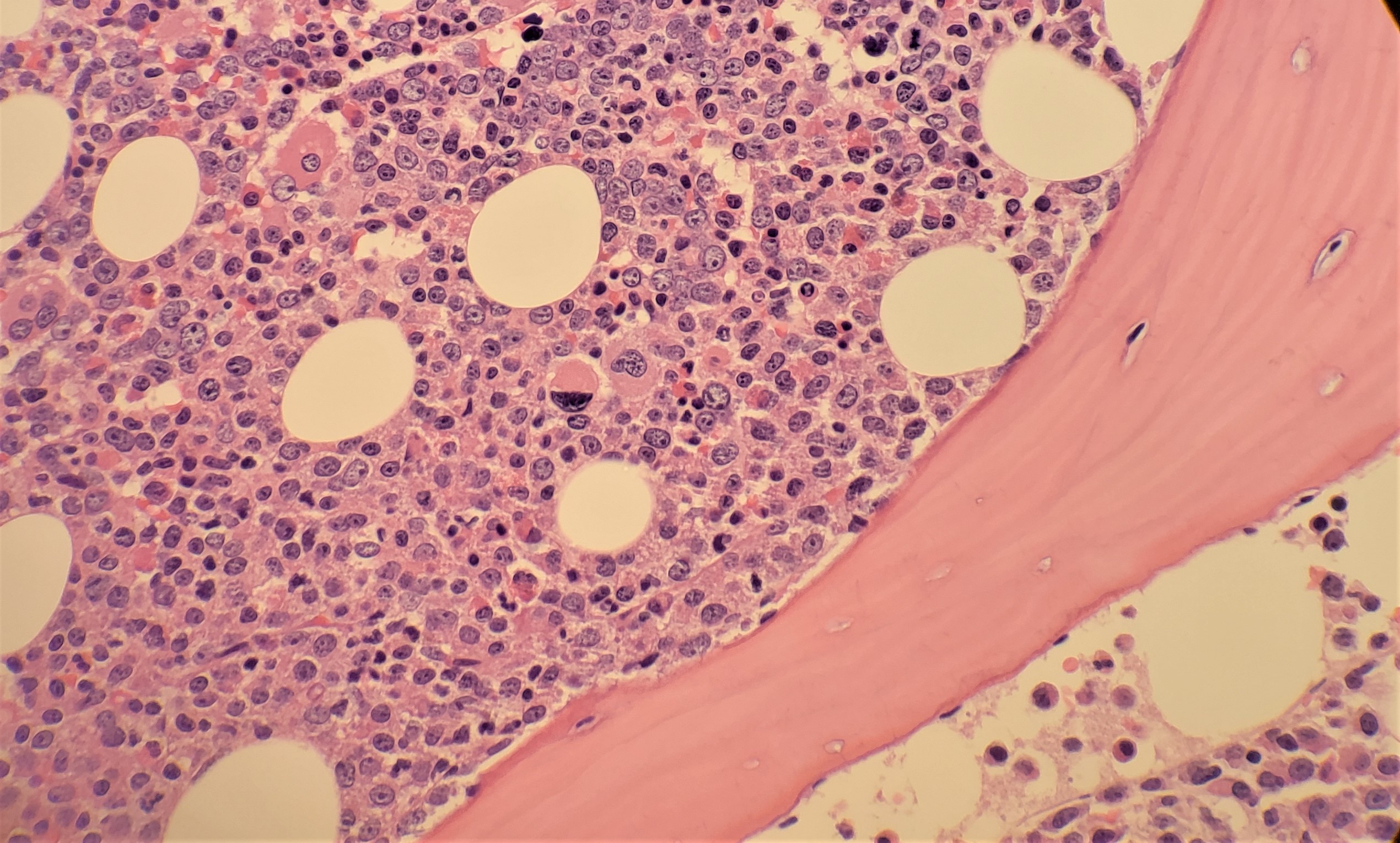
Bone marrow aspirate and biopsy will show hypercellularity with marked granulocytic proliferation and significantly increased myelocytes, although significant dysplasia should be absent (see Image. Chronic Myeloid Leukemia). Blasts are usually less than 5%. Erythroid precursors are decreased considerably, and there is an increased myeloid to erythroid ratio. Megakaryocytes may be reduced, normal, or increased. About half of the cases show a megakaryocytic proliferation. The megakaryocytes in CML show a small, hypo-lobate “dwarf” morphology. The biopsy will show immature granulocytes in a thickened band of 5 to 10 cells along bone trabeculae. Adjacent to bone trabeculae is the normal distribution site of immature granulocytes; however, it is usually 2 to 3 cells thick. The bone marrow may also show increased reticulin fibrosis.[8]
Accelerated Phase
The peripheral smear may or may not show increased blasts (10% to 19%). The bone marrow aspirate and biopsy will show similar changes to chronic phase CML with increased blasts (10% to 19%), possibly dysplastic changes in granulocytes, and increased reticulin and collagen fibrosis.[9]
Blast Phase
The peripheral smear and/or bone marrow aspirate will show greater than 20% blasts, or there will be an extramedullary proliferation of blasts. Most cases will show blasts with myeloid differentiation; however, other lineages or combinations may be present, including lymphoblasts. Extramedullary proliferation is most commonly seen in the skin, lymph nodes, bone, and the central nervous system (CNS).[10]
History and Physical
Approximately half of the patients with CML are asymptomatic and are diagnosed on routine complete blood count. Most patients are in the chronic phase of CML. CML, in the chronic phase, most often presents with symptoms related to anemia and splenomegaly. Symptomatic anemia includes symptoms such as fatigue and malaise. Splenomegaly may cause a mass effect resulting in early satiety, left upper quadrant fullness, or pain. CML may also cause thrombocytopenia or platelet dysfunction resulting in bleeding, thrombocytosis resulting in thrombosis or priapism, basophilia resulting in histamine release, and upper gastrointestinal ulcers. As CML progresses into the accelerated phase or blast phase, symptoms such as headaches, bone pain, fever, joint pain, bleeding, infections, and lymphadenopathy become more common.[6]
The physical exam should include assessing the spleen size by palpation, measured in centimeters below the costal margin.[11][12] Splenomegaly is the most common physical exam finding in patients suffering from CML. In more than half of the patients, the spleen size extends beyond 5 cm below the left costal margin at the time of diagnosis. A very large spleen is usually a herald sign of the transformation into an acute blast crisis from the disease. Hepatomegaly may also be found as it is a part of the extramedullary hematopoiesis occurring in the spleen. Physical exam findings may also include the signs of hyperviscosity. On fundoscopy, the retina may reveal papilledema, venous obstruction, and hemorrhages.
Evaluation
Initially, if CML is suspected, cytogenetic testing, fluorescent in situ hybridization (FISH), and/or reverse transcriptase-polymerase chain reaction (PCR) to determine the Philadelphia chromosome, or BCR-ABL1 oncoprotein presence can be performed on peripheral blood.
At the time of diagnosis, laboratory blood testing should include a complete blood count with differential, chemistry panel, hepatitis panel, and a quantitative PCR for BCR-ABL1. A baseline bone marrow aspirate and biopsy should be performed with cytogenetics. Quantitative PCR should be repeated every three months after initiation of therapy. After BCR-ABL1 is less than or equal to 1% by international scale, quantitative PCR should continue for two years and then every 3 to 6 months after two years.
If chronic phase CML is established, additional evaluation includes determining the risk score using Sokal et al. or Hasford et al. risk calculations before determining first-line therapy.[6]
- Sokal risk calculation uses age, spleen size, platelet count, and percentage of myeloblasts in peripheral blood to determine the risk group.[11]
- Hasford risk calculation uses age, spleen size, platelet count, and percentage of blasts, eosinophils, and basophils in the peripheral blood to determine the risk group.[12]
If accelerated or blast phase CML is diagnosed or progresses from chronic phase CML, additional testing should include flow cytometry to determine lineage, mutational analysis, and HLA testing if allogeneic hematopoietic stem cell transplant (HCT) is being considered. Additional bone marrow cytogenetics and mutational analysis should be considered when there is a failure to reach response milestones or any sign of hematologic or cytogenetic relapse.[6]
Treatment / Management
There are 4 FDA-approved, first-line treatments for chronic phase CML that are commercially available tyrosine kinase inhibitors, including first-generation imatinib and second-generation dasatinib, nilotinib, and bosutinib.
Dosing
- Imatinib: 400 mg daily[13][14]
- Bosutinib: 500 mg daily[15]
- Dasatinib: 100 mg daily[16]
- Nilotinib: 300 mg twice a day[17] (A1)
For chronic phase, CML with intermediate- or high-risk score, second-generation tyrosine kinase inhibitors (bosutinib, dasatinib, nilotinib) as first-line therapy may have an additional benefit over imatinib.[15][16][17](A1)
Ponatinib, a third-generation tyrosine kinase inhibitor, dosed at 45 mg daily, is a third-line treatment option in chronic phase CML for patients who have failed to respond to multiple tyrosine kinase inhibitors and for individuals who have the T315I mutation.[18](B3)
Advanced CML (accelerated or blast phase) has additional therapeutic considerations. Second- or third-generation tyrosine kinase inhibitor therapy should be initiated to reduce the CML burden and be considered for early allogeneic hematopoietic stem cell transplant (HSCT).[6] Omacetaxine is a chemotherapeutic agent that is an additional treatment option in cases refractory to tyrosine kinase inhibitor therapy that advanced from chronic phase CML.[19]
Allogenic HSCT should be considered in patients resistant to tyrosine kinase inhibitor therapy in chronic phase CML and cases of advanced CML.[6][20]
Clinical trial participation should be considered for all patients.
Differential Diagnosis
Differentiating CML from other causes of granulocytic leukocytoses, such as infections or drugs, is necessary. Most causes of reactive leukocytosis have a white blood cell count less than 50 x 10/L and show toxic changes such as toxic granulation and Dohle bodies. There will also be an absence of basophilia.
Other myeloproliferative disorders such as chronic neutrophilic leukemia (CNL) and polycythemia vera (PV) may present with leukocytosis and thrombocytosis. Both CNL and PV will lack the BCR- ABL1 fusion gene. CNL is rare and presents with mature segmented neutrophilia and lacks a bimodal leukocytosis with myelocytes.[6]
Pertinent Studies and Ongoing Trials
A search of ClinicalTrials.gov shows there are 221 active, interventional CML trials with 125 recruiting. These trials are widely variable, with study topics covering novel drugs, treatment optimization of current treatment modalities, potential drug treatment combination regimens, the efficacy of treatment discontinuation, and immunotherapy.
Ongoing trials of note include the randomized international phase 3 study Evaluating Nilotinib Efficacy and Safety in Clinical Trials-Newly Diagnosed Patients (ENESTnd)[17], and the phase 3 study Results of Efficacy and Safety of Radotinib Compared with Imatinib in Newly Diagnosed Chronic Phase Chronic Myeloid Leukemia (RERISE).[21]
Other pertinent studies include trials that supported the first-line treatment with imatinib and dosing include the CML Study IV,[13] the Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) study,[14] and the International Randomized Study of Interferon and STI571 trial (IRIS).[22] Important trials concerning the efficacy and dosing of the second generation FDA-approved tyrosine kinase inhibitors include Bosutinib Efficacy and Safety in Newly Diagnosed CML trial (BELA),[15] Bosutinib Versus Imatinib in Adult Patients With Newly Diagnosed Chronic Phase Chronic Myelogenous Leukemia trial (BFORE),[23] Dasatinib Versus Imatinib Study in Treatment-Naive Chronic Myeloid Leukemia Patients trial (DASISION),[16] and the ENESTnd study.[17]
Pertinent studies and ongoing trials assessing tyrosine kinase inhibitor therapy discontinuation efficacy and candidate recommendations include the imatinib discontinuation study (STIM1),[24] Evaluating Nilotinib Efficacy and Safety in Clinical Trials: Treatment-Free Remission study (ENESTfreedom),[25] Imatinib Suspension and Validation study (ISAV),[26] and Discontinuation of Tyrosine Kinase Inhibitor Therapy in Chronic Myeloid Leukaemia trial (EURO-SKI).[27]
Treatment Planning
Any of the four FDA-approved tyrosine kinase inhibitors are highly effective in treating chronic phase CML. In selecting front-line therapy, a patient’s age, comorbidities, and tyrosine kinase toxicities must be taken into account.
For patients with chronic phase CML, the use of the Sokal or Hasford risk stratification scores helps predict outcomes and choose the first-line tyrosine-kinase inhibitor.[11][12][11] For chronic phase, CML with intermediate- or high-risk score, second-generation tyrosine kinase inhibitors (bosutinib, dasatinib, nilotinib) may have the additional benefit over imatinib in achieving response milestones.[15][16][17][28]
At baseline, all patients should undergo bone marrow examination and cytogenetic analysis to monitor treatment response. The patient should be monitored at 3, 6, and 12 months after starting therapy using cytogenetic and molecular studies such as PCR performed on peripheral blood.[29] The major goal in chronic phase CML treatment is achieving complete cytogenetic response by 12 months after the initiation of tyrosine kinase inhibitors. Response milestones include complete hematologic response, cytogenetic response, and major molecular response.[6] A complete hematologic response is defined as complete normalization of peripheral blood counts with a leukocyte count below 10 x 10^9/L and platelet count below 450 x 10^9/L, the absence of myelocytes, promyelocytes, and blasts, and absence of signs and symptoms of the disease, including the disappearance of a palpable spleen.[30] A complete cytogenetic response shows no Philadelphia-positive metaphases, and a major molecular response is defined as BCR-ABL1 transcripts of less than or equal to 0.1% on the international scale (IS).[31]
The European Leukemia Net (ELN) 2013 criteria can be a guide to monitor failure or suboptimal response to first-line therapy or second-line therapy. These criteria evaluate patients with chronic phase CML at 3 months, 6 months, and 12 months and then any subsequent follow-up; the optimal response is defined by PCR as BCR-ABL1 transcripts (IS) of less than or equal to 10%, less than 1%, and less than or equal to 0.1%, respectively. Alternatively, if quantitative PCR is not available, cytogenetics can determine levels of the Philadelphia chromosome but is not useful in cryptic BCR-ABL1. If the optimal response is not achieved, there is a warning response group and a failure response group. In the warning group, consideration of dose escalation of imatinib or changing tyrosine kinase inhibitors is warranted.[32] However, if an optimal response is not achieved at three months, studies show that most patients will achieve an optimal response at six months without changes in therapy with a similar prognosis.[33] Another consideration is patient compliance; poor adherence has been shown to be a significant contributor to treatment failure and loss of complete response.[34] In high-risk cases, at the time of diagnosis or in case of treatment failure despite good patient compliance, the tyrosine kinase inhibitor therapy should be changed. The patient should be evaluated for the possibility of allogeneic HSCT.[6]
In the event of tyrosine kinase inhibitor resistance, analysis of the BCR-ABL1 mutational profile may be useful in choosing second- or third-line therapy. The T315I mutation has been shown to confer resistance to imatinib and all second-generation tyrosine kinase inhibitors. In these patients, ponatinib should be considered.[18] In cases of Y253H, E255K/V, or F359C/V mutations, dasatinib or bosutinib may have better responses, and in cases of V299L and F317L mutations, nilotinib may have promising outcomes.[6]
For accelerated or blast phase CML, treatment considerations include evaluation for allogeneic HSCT, whether the advanced phase presented while the patient was on or off tyrosine kinase inhibitor therapy, comorbidities, age, prior therapy, and analysis of BCR-ABL1 mutation profile. Chemotherapy is often required in advanced CML to reduce disease burden and prepare for allogeneic HSCT.[19][20][32]
In patients who achieve a long-term deep molecular response to therapy, discontinuation of tyrosine kinase inhibitors may be considered. Treatment-free remission can be achieved in approximately 40% to 60% of the low risk to intermediate-risk patients. Characteristics that predict treatment-free remission include cases with low Sokal risk, no history of tyrosine kinase inhibitor resistance, and duration of tyrosine kinase inhibitor use greater than five years. Most relapses will occur within the first six months of discontinuation, and molecular monitoring should occur monthly for the first year post-discontinuation and every 6 to 12 weeks indefinitely. If molecular relapse occurs, the tyrosine kinase inhibitor therapy should be re-initiated.[35]
Toxicity and Adverse Effect Management
Most patients will develop mild to moderate adverse events early in tyrosine kinase inhibitor therapy, and most will resolve spontaneously or be well-controlled.
Adverse events and side effects divide into four grades based on severity.
- Grade 1 would require no change in tyrosine kinase inhibitor therapy; however, it may require specific treatment.
- Grade 2 will involve withholding therapy until severity decreases or continuing therapy if symptoms decrease in severity with monitoring. If a Grade 2 side effect is recurrent, dose reduction should be considered.
- Grade 3 should involve withholding therapy until severity decreases and then restarting at a lower dose or withholding till symptoms reach a grade 1 level or less and resume prior dosage. If there is no resolution or Grade 3 side effects are recurrent, the tyrosine kinase inhibitor should be changed.
- Grade 4 events should involve switching tyrosine kinase inhibitor therapy when possible.[36]
Vascular System: Ischemic heart disease, ischemic cerebrovascular events, and peripheral arterial occlusive disease have correlations with nilotinib and ponatinib. Higher dosing has been related to higher risks of these events. Cardiovascular risk should undergo evaluation before therapy begins with these agents and should be closely monitored during treatment. Risk assessment and monitoring should include hemoglobin A1C, lipid profile, and serum creatinine. These drugs should be used with caution in patients with a high risk for vascular disease.
Cardiac Function: Ponatinib has an associated incidence of heart failure. The monitoring of cardiac function should be mandatory for patients on ponatinib.
Cardiac Rhythm Alterations: Tyrosine kinase inhibitors have the potential to alter the QT interval. Nilotinib has the highest incidence of QT interval prolongation. Ponatinib is associated with QT interval shortening. Electrocardiogram at baseline and periodically in select patients who are on any tyrosine kinase inhibitor and have QT interval prolongation, and for those who are on ponatinib. Nilotinib should be avoided in patients with a high risk for arrhythmias or when other drugs that may alter the QT interval are being administered. Potassium and magnesium levels should be replete before initiation of tyrosine kinase inhibitor therapy in all cases.
Pulmonary System: Pleural effusions can occur with all tyrosine kinase inhibitors but most commonly occurs with dasatinib. Patients with pre-existing lung injuries, chronic lung disease, congestive heart failure, and hypertension are at the highest risk of developing pleural effusions. Caution is necessary if patients develop a cough, shortness of breath, or chest pain during therapy, and a chest X-ray must be obtained in such cases. Reports also exist of pulmonary hypertension with dasatinib use. If the development of pulmonary hypertension is suspected, dasatinib should be stopped immediately.
Hepatobiliary System: Hepatotoxicity is common in all tyrosine kinase inhibitors. Ponatinib has the highest risk for developing high-grade increases in transaminases. Substances that alter liver metabolism by cytochrome P450 and acetaminophen should be used with caution or avoided entirely—prompt treatment with glucocorticoids in severe cases of hepatotoxicity assists in hepatic recovery.
Hyperglycemia: Nilotinib has been shown to induce hyperglycemia in non-diabetic and diabetic patients. Fasting glucose and hemoglobin A1C require monitoring.
Hypercholesterolemia: Nilotinib shows correlations with increases in LDL-cholesterol. Hypertriglyceridemia has been reported with ponatinib. If persistent high-risk hypercholesterolemia develops, appropriate statin initiation may be warranted.
Other Endocrinopathies: Hypophosphatemia and hypocalcemia have been reported with imatinib, nilotinib, and dasatinib. Phosphate, calcium, and Vitamin D levels should be tested at baseline and monitored after that. Hypothyroidism may also develop and is treated effectively with hormone replacement.
Myelosuppression: Development is common with all tyrosine kinase inhibitors but is usually limited to the first few weeks to months of therapy. Leukocytes, erythrocytes, and platelets can all be affected; this necessitates close monitoring of blood counts and response to avoid infection and bleeding. Tyrosine kinase interruption, dose reduction, and supportive management with blood products are considerations based on levels of cytopenias, the specific tyrosine kinase inhibitor, and the phase of the disease. Any nutritional deficiencies should be corrected before the initiation of therapy.
Gastrointestinal System: Nausea, diarrhea, abdominal pain, and vomiting are frequent side effects related to tyrosine kinase inhibitors. Diarrhea is especially significant with bosutinib and often requires dose interruption and/or dose reduction. Patients can take their medication with meals to avoid nausea and vomiting. In severe cases, antiemetic and/or antidiarrheal medication can be administered, and close monitoring of hydration status should occur. Gastrointestinal bleeding has been reported with dasatinib. There are reports of increased lipase levels and pancreatitis with nilotinib and ponatinib.
Dermatologic System: Rash development is common with all tyrosine kinase inhibitors. Most cases are dose-related and self-limited. Management can comprise topical therapies, antihistamines, and/or short courses of systemic steroids. In severe cases, prednisone merit consideration with the gradual reintroduction of the offending tyrosine kinase inhibitor.
Kidney Injury: Monitoring for tumor lysis syndrome should be done from the outset of treatment with all tyrosine kinase inhibitors. Otherwise, the development of acute kidney injury may result from imatinib therapy. Dasatinib and bosutinib have also been associated with acute kidney injury but at lower incidence rates. Serum creatinine requires monitoring in these patients, and caution is necessary for patients with renal insufficiency.
Other commonly reported side effects include myalgia, bone pain, ophthalmological changes, and headache.[6][36]
Staging
CML staging is determined by the phase of the disease, which includes a chronic phase, accelerated phase, and blast phase.
The chronic phase has less than 10% blasts, asymptomatic to mild symptoms, and responds to tyrosine kinase inhibitor therapy.
The World Health Organization (WHO) defines accelerated phase CML as having one or more of the following criteria:
- Persistent or increasing high white blood cell count (greater than 10 x 10^9/L) unresponsive to therapy
- Persistent or increasing splenomegaly unresponsive to therapy
- Persistent thrombocytosis (greater than 1000 x 10^9/L) unresponsive to therapy
- Persistent thrombocytopenia (less than 100 x 10^9/L)
- Greater than or equal to 20% basophils in peripheral blood
- 10-19% blasts in peripheral blood or bone marrow (without extramedullary blast proliferation)
- Additional chromosomal abnormalities in Philadelphia chromosome-positive cells at diagnosis
- Any new clonal chromosomal abnormality in Philadelphia chromosome-positive cells during therapy
The WHO also considers resistance to tyrosine kinase inhibitors as provisional criteria for determining accelerated phase CML.
Blast phase CML is defined by greater than or equal to 20% blasts in the peripheral blood and/or bone marrow or extramedullary blast proliferation.[9]
Prognosis
Before introducing imatinib, most CML cases progressed to the blast phase, and death occurred in under five years. Since tyrosine kinase inhibitors have been the first-line therapy for CML, the 5-year survival has risen from 33% to over 90%. The ten-year survival has risen from 11% to 84%, and complete cytogenetic response occurs in 70% to 90% of patients. Individuals diagnosed with chronic phase CML are expected to reach normal or near-normal life expectancy.[28]
Cytogenetic analysis for additional abnormalities, in addition to the classic translocation, may be important for prognostic information. Previously, any additional chromosomal abnormalities were considered a risk for disease progression, tyrosine kinase inhibitor resistance, and worse prognosis. However, studies have shown that single additional chromosomal abnormalities, including trisomy 8, loss of Y, and an extra copy of the Philadelphia chromosome, do not impact survival. The presence of two or more additional chromosomal abnormalities or a single additional chromosomal abnormality involving i(17)(q10), the loss of 7 or deletion of 7q, and 3q26.2 rearrangements are poor prognostic indicators.[37]
The BCR-ABL1 mutational analysis may also impact prognosis. The T315I mutation was the first mutation discovered in association with the development of tyrosine kinase inhibitor resistance. Over 100 point mutations in the BCR-ABL1 oncogene have been isolated in imatinib-resistant patients. These mutations may have an influence over treatment planning in first-line tyrosine kinase resistant patients.[38]
The future prognostic evaluation may include sequencing for mutations involving known cancer genes, such as IKZF1, RUNX1, ASXL1, BCORL1, and IDH1. These mutations were found in patients who underwent sequencing during blast-phase CML.[39]
Complications
Complications of CML can include:
- Hepatomegaly and/or splenomegaly
- Worsening anemia
- Changes in platelet count changes resulting in clotting or bleeding complications
- Recurrent infections
- Bone pain
- Fever
Consultations
The interprofessional team should involve hematology/oncology, hematopathology, and other specialties already involved with patient comorbidities. If complications or adverse events develop, transfusion medicine, cardiology, endocrinology, gastroenterology, and infectious disease, consultations should be considered in select circumstances.
Deterrence and Patient Education
Patients need to understand the testing needed following CML diagnosis to check for metastatic disease and determine what phase the disease has achieved. Chronic myelogenous leukemia has 3 phases chronic, acute and blastic. The phase will dictate the treatment. They must also understand that CML relapse is possible following treatment.
Lastly, they need to be counseled on what to expect and their prognosis. Prognosis is affected by several factors, including:
- Patient age
- The disease phase
- The number of blasts in the blood or bone marrow.
- Spleen size
- Overall patient health
They must also understand the ramifications and side effects of their therapy.
Pearls and Other Issues
- Most CML patients are asymptomatic in the chronic phase and are found by routine complete blood count showing leukocytosis with bimodal increases in myelocytes and mature segmented neutrophils.
- The diagnosis of CML requires the BCR-ABL1 translocation. A traditional karyotype can detect most cases; however, a small subset of cases are cryptic and require FISH.
- CML is divided into 3 phases: chronic, accelerated, and blast phase.
- The BCR-ABL1 oncoprotein acts as a constitutively active tyrosine kinase. The mainstay of treatment in CML is tyrosine kinase inhibitors. Risk stratification scores are useful in determining first-line therapy choices.
- The mutational analysis may be important in cases with resistance to tyrosine kinase inhibitor therapy. Ponatinib is a tyrosine kinase inhibitor especially useful for those with a T315I BCR-ABL1 mutation.
- Allogenic HSCT is an important consideration for resistant chronic phase and advanced phases of CML.
Enhancing Healthcare Team Outcomes
CML is a common leukemia found in adults, and the advancement of tyrosine kinase inhibitor treatment has a good prognosis. However, identifying patients with CML can be challenging in instances of cryptic translocations and presentations in advanced phases. The World Health Organization and the European LeukemiaNet provide evidence-based guidance on evaluation, diagnosis, treatment, and milestones for CML. To avoid misdiagnosis and delayed treatment, a bone marrow aspirate and collaboration with pathology is necessary in suspected cases to ensure adequate sampling to identify the BCR-ABL1 translocation.[32] (Level I)
Interprofessional teamwork and decision making with the patient are vitally important, and side effects that may hinder treatment adherence should be discussed directly with the patient. Tyrosine kinase inhibitors are effective in most cases; however, the patient's non-compliance is a prominent cause of failure. The pharmacist must verify all chemotherapeutic agent dosing and compatibility, check for drug interactions, and educate the team on adverse effects. The health care team needs to identify side effects early, make patient concerns a priority, and combat any challenges as soon as possible.[34] [Level 2] A coordinated interprofessional team approach involving nurses, primary care providers, pharmacists, and specialists will result in the most successful care of patients with CML. [Level 5]
Media
(Click Image to Enlarge)
References
Corso A, Lazzarino M, Morra E, Merante S, Astori C, Bernasconi P, Boni M, Bernasconi C. Chronic myelogenous leukemia and exposure to ionizing radiation--a retrospective study of 443 patients. Annals of hematology. 1995 Feb:70(2):79-82 [PubMed PMID: 7880928]
Level 2 (mid-level) evidenceHoffmann VS, Baccarani M, Hasford J, Lindoerfer D, Burgstaller S, Sertic D, Costeas P, Mayer J, Indrak K, Everaus H, Koskenvesa P, Guilhot J, Schubert-Fritschle G, Castagnetti F, Di Raimondo F, Lejniece S, Griskevicius L, Thielen N, Sacha T, Hellmann A, Turkina AG, Zaritskey A, Bogdanovic A, Sninska Z, Zupan I, Steegmann JL, Simonsson B, Clark RE, Covelli A, Guidi G, Hehlmann R. The EUTOS population-based registry: incidence and clinical characteristics of 2904 CML patients in 20 European Countries. Leukemia. 2015 Jun:29(6):1336-43. doi: 10.1038/leu.2015.73. Epub 2015 Mar 18 [PubMed PMID: 25783795]
Level 2 (mid-level) evidenceSiegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA: a cancer journal for clinicians. 2017 Jan:67(1):7-30. doi: 10.3322/caac.21387. Epub 2017 Jan 5 [PubMed PMID: 28055103]
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians. 2018 Jan:68(1):7-30. doi: 10.3322/caac.21442. Epub 2018 Jan 4 [PubMed PMID: 29313949]
Faderl S, Talpaz M, Estrov Z, O'Brien S, Kurzrock R, Kantarjian HM. The biology of chronic myeloid leukemia. The New England journal of medicine. 1999 Jul 15:341(3):164-72 [PubMed PMID: 10403855]
Jabbour E, Kantarjian H. Chronic myeloid leukemia: 2018 update on diagnosis, therapy and monitoring. American journal of hematology. 2018 Mar:93(3):442-459. doi: 10.1002/ajh.25011. Epub [PubMed PMID: 29411417]
Spiers AS, Bain BJ, Turner JE. The peripheral blood in chronic granulocytic leukaemia. Study of 50 untreated Philadelphia-positive cases. Scandinavian journal of haematology. 1977 Jan:18(1):25-38 [PubMed PMID: 265093]
Level 3 (low-level) evidenceThiele J,Kvasnicka HM,Fischer R, Bone marrow histopathology in chronic myelogenous leukemia (CML)--evaluation of distinctive features with clinical impact. Histology and histopathology. 1999 Oct [PubMed PMID: 10506940]
Level 3 (low-level) evidenceArber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016 May 19:127(20):2391-405. doi: 10.1182/blood-2016-03-643544. Epub 2016 Apr 11 [PubMed PMID: 27069254]
Cortes JE, Talpaz M, O'Brien S, Faderl S, Garcia-Manero G, Ferrajoli A, Verstovsek S, Rios MB, Shan J, Kantarjian HM. Staging of chronic myeloid leukemia in the imatinib era: an evaluation of the World Health Organization proposal. Cancer. 2006 Mar 15:106(6):1306-15 [PubMed PMID: 16463391]
Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, Tso CY, Braun TJ, Clarkson BD, Cervantes F. Prognostic discrimination in "good-risk" chronic granulocytic leukemia. Blood. 1984 Apr:63(4):789-99 [PubMed PMID: 6584184]
Hasford J,Pfirrmann M,Hehlmann R,Allan NC,Baccarani M,Kluin-Nelemans JC,Alimena G,Steegmann JL,Ansari H, A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. Journal of the National Cancer Institute. 1998 Jun 3 [PubMed PMID: 9625174]
Level 1 (high-level) evidenceHehlmann R, Lauseker M, Saußele S, Pfirrmann M, Krause S, Kolb HJ, Neubauer A, Hossfeld DK, Nerl C, Gratwohl A, Baerlocher GM, Heim D, Brümmendorf TH, Fabarius A, Haferlach C, Schlegelberger B, Müller MC, Jeromin S, Proetel U, Kohlbrenner K, Voskanyan A, Rinaldetti S, Seifarth W, Spieß B, Balleisen L, Goebeler MC, Hänel M, Ho A, Dengler J, Falge C, Kanz L, Kremers S, Burchert A, Kneba M, Stegelmann F, Köhne CA, Lindemann HW, Waller CF, Pfreundschuh M, Spiekermann K, Berdel WE, Müller L, Edinger M, Mayer J, Beelen DW, Bentz M, Link H, Hertenstein B, Fuchs R, Wernli M, Schlegel F, Schlag R, de Wit M, Trümper L, Hebart H, Hahn M, Thomalla J, Scheid C, Schafhausen P, Verbeek W, Eckart MJ, Gassmann W, Pezzutto A, Schenk M, Brossart P, Geer T, Bildat S, Schäfer E, Hochhaus A, Hasford J. Assessment of imatinib as first-line treatment of chronic myeloid leukemia: 10-year survival results of the randomized CML study IV and impact of non-CML determinants. Leukemia. 2017 Nov:31(11):2398-2406. doi: 10.1038/leu.2017.253. Epub 2017 Aug 14 [PubMed PMID: 28804124]
Level 1 (high-level) evidenceBaccarani M, Druker BJ, Branford S, Kim DW, Pane F, Mongay L, Mone M, Ortmann CE, Kantarjian HM, Radich JP, Hughes TP, Cortes JE, Guilhot F. Long-term response to imatinib is not affected by the initial dose in patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: final update from the Tyrosine Kinase Inhibitor Optimization and Selectivity (TOPS) study. International journal of hematology. 2014:99(5):616-24 [PubMed PMID: 24658916]
Brümmendorf TH, Cortes JE, de Souza CA, Guilhot F, Duvillié L, Pavlov D, Gogat K, Countouriotis AM, Gambacorti-Passerini C. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukaemia: results from the 24-month follow-up of the BELA trial. British journal of haematology. 2015 Jan:168(1):69-81. doi: 10.1111/bjh.13108. Epub 2014 Sep 8 [PubMed PMID: 25196702]
Level 1 (high-level) evidenceCortes JE, Saglio G, Kantarjian HM, Baccarani M, Mayer J, Boqué C, Shah NP, Chuah C, Casanova L, Bradley-Garelik B, Manos G, Hochhaus A. Final 5-Year Study Results of DASISION: The Dasatinib Versus Imatinib Study in Treatment-Naïve Chronic Myeloid Leukemia Patients Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2016 Jul 10:34(20):2333-40. doi: 10.1200/JCO.2015.64.8899. Epub 2016 May 23 [PubMed PMID: 27217448]
Nakamae H, Fukuda T, Nakaseko C, Kanda Y, Ohmine K, Ono T, Matsumura I, Matsuda A, Aoki M, Ito K, Shibayama H. Nilotinib vs. imatinib in Japanese patients with newly diagnosed chronic myeloid leukemia in chronic phase: long-term follow-up of the Japanese subgroup of the randomized ENESTnd trial. International journal of hematology. 2018 Mar:107(3):327-336. doi: 10.1007/s12185-017-2353-7. Epub 2017 Oct 26 [PubMed PMID: 29076005]
Level 1 (high-level) evidenceMüller MC, Cervantes F, Hjorth-Hansen H, Janssen JJWM, Milojkovic D, Rea D, Rosti G. Ponatinib in chronic myeloid leukemia (CML): Consensus on patient treatment and management from a European expert panel. Critical reviews in oncology/hematology. 2017 Dec:120():52-59. doi: 10.1016/j.critrevonc.2017.10.002. Epub 2017 Oct 8 [PubMed PMID: 29198338]
Level 3 (low-level) evidenceKhoury HJ, Cortes J, Baccarani M, Wetzler M, Masszi T, Digumarti R, Craig A, Benichou AC, Akard L. Omacetaxine mepesuccinate in patients with advanced chronic myeloid leukemia with resistance or intolerance to tyrosine kinase inhibitors. Leukemia & lymphoma. 2015 Jan:56(1):120-7. doi: 10.3109/10428194.2014.889826. Epub 2014 Apr 28 [PubMed PMID: 24650054]
Nair AP, Barnett MJ, Broady RC, Hogge DE, Song KW, Toze CL, Nantel SH, Power MM, Sutherland HJ, Nevill TJ, Abou Mourad Y, Narayanan S, Gerrie AS, Forrest DL. Allogeneic Hematopoietic Stem Cell Transplantation Is an Effective Salvage Therapy for Patients with Chronic Myeloid Leukemia Presenting with Advanced Disease or Failing Treatment with Tyrosine Kinase Inhibitors. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2015 Aug:21(8):1437-44. doi: 10.1016/j.bbmt.2015.04.005. Epub 2015 Apr 10 [PubMed PMID: 25865648]
Kwak JY, Kim SH, Oh SJ, Zang DY, Kim H, Kim JA, Do YR, Kim HJ, Park JS, Choi CW, Lee WS, Mun YC, Kong JH, Chung JS, Shin HJ, Kim DY, Park J, Jung CW, Bunworasate U, Comia NS, Jootar S, Reksodiputro AH, Caguioa PB, Lee SE, Kim DW. Phase III Clinical Trial (RERISE study) Results of Efficacy and Safety of Radotinib Compared with Imatinib in Newly Diagnosed Chronic Phase Chronic Myeloid Leukemia. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017 Dec 1:23(23):7180-7188. doi: 10.1158/1078-0432.CCR-17-0957. Epub 2017 Sep 22 [PubMed PMID: 28939746]
Level 1 (high-level) evidenceGuilhot F, Druker B, Larson RA, Gathmann I, So C, Waltzman R, O'Brien SG. High rates of durable response are achieved with imatinib after treatment with interferon alpha plus cytarabine: results from the International Randomized Study of Interferon and STI571 (IRIS) trial. Haematologica. 2009 Dec:94(12):1669-75. doi: 10.3324/haematol.2009.010629. Epub 2009 Jul 31 [PubMed PMID: 19648168]
Level 1 (high-level) evidenceCortes JE, Gambacorti-Passerini C, Deininger MW, Mauro MJ, Chuah C, Kim DW, Dyagil I, Glushko N, Milojkovic D, le Coutre P, Garcia-Gutierrez V, Reilly L, Jeynes-Ellis A, Leip E, Bardy-Bouxin N, Hochhaus A, Brümmendorf TH. Bosutinib Versus Imatinib for Newly Diagnosed Chronic Myeloid Leukemia: Results From the Randomized BFORE Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018 Jan 20:36(3):231-237. doi: 10.1200/JCO.2017.74.7162. Epub 2017 Nov 1 [PubMed PMID: 29091516]
Level 1 (high-level) evidenceEtienne G, Guilhot J, Rea D, Rigal-Huguet F, Nicolini F, Charbonnier A, Guerci-Bresler A, Legros L, Varet B, Gardembas M, Dubruille V, Tulliez M, Noel MP, Ianotto JC, Villemagne B, Carré M, Guilhot F, Rousselot P, Mahon FX. Long-Term Follow-Up of the French Stop Imatinib (STIM1) Study in Patients With Chronic Myeloid Leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017 Jan 20:35(3):298-305. doi: 10.1200/JCO.2016.68.2914. Epub 2016 Oct 31 [PubMed PMID: 28095277]
Ross DM, Masszi T, Gómez Casares MT, Hellmann A, Stentoft J, Conneally E, Garcia-Gutierrez V, Gattermann N, le Coutre PD, Martino B, Saussele S, Giles FJ, Radich JP, Saglio G, Deng W, Krunic N, Bédoucha V, Gopalakrishna P, Hochhaus A. Durable treatment-free remission in patients with chronic myeloid leukemia in chronic phase following frontline nilotinib: 96-week update of the ENESTfreedom study. Journal of cancer research and clinical oncology. 2018 May:144(5):945-954. doi: 10.1007/s00432-018-2604-x. Epub 2018 Feb 22 [PubMed PMID: 29468438]
Mori S, Vagge E, le Coutre P, Abruzzese E, Martino B, Pungolino E, Elena C, Pierri I, Assouline S, D'Emilio A, Gozzini A, Giraldo P, Stagno F, Iurlo A, Luciani M, De Riso G, Redaelli S, Kim DW, Pirola A, Mezzatesta C, Petroccione A, Lodolo D'Oria A, Crivori P, Piazza R, Gambacorti-Passerini C. Age and dPCR can predict relapse in CML patients who discontinued imatinib: the ISAV study. American journal of hematology. 2015 Oct:90(10):910-4. doi: 10.1002/ajh.24120. Epub 2015 Sep 10 [PubMed PMID: 26178642]
Saussele S, Richter J, Guilhot J, Gruber FX, Hjorth-Hansen H, Almeida A, Janssen JJWM, Mayer J, Koskenvesa P, Panayiotidis P, Olsson-Strömberg U, Martinez-Lopez J, Rousselot P, Vestergaard H, Ehrencrona H, Kairisto V, Machová Poláková K, Müller MC, Mustjoki S, Berger MG, Fabarius A, Hofmann WK, Hochhaus A, Pfirrmann M, Mahon FX, EURO-SKI investigators. Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. The Lancet. Oncology. 2018 Jun:19(6):747-757. doi: 10.1016/S1470-2045(18)30192-X. Epub 2018 May 4 [PubMed PMID: 29735299]
Level 2 (mid-level) evidenceHehlmann R. CML--Where do we stand in 2015? Annals of hematology. 2015 Apr:94 Suppl 2():S103-5. doi: 10.1007/s00277-015-2331-1. Epub 2015 Mar 27 [PubMed PMID: 25814076]
Lima L, Bernal-Mizrachi L, Saxe D, Mann KP, Tighiouart M, Arellano M, Heffner L, McLemore M, Langston A, Winton E, Khoury HJ. Peripheral blood monitoring of chronic myeloid leukemia during treatment with imatinib, second-line agents, and beyond. Cancer. 2011 Mar 15:117(6):1245-52. doi: 10.1002/cncr.25678. Epub 2010 Nov 2 [PubMed PMID: 21381013]
Level 2 (mid-level) evidenceFaderl S, Talpaz M, Estrov Z, Kantarjian HM. Chronic myelogenous leukemia: biology and therapy. Annals of internal medicine. 1999 Aug 3:131(3):207-19 [PubMed PMID: 10428738]
Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J, Baccarani M, Cortes J, Cross NC, Druker BJ, Gabert J, Grimwade D, Hehlmann R, Kamel-Reid S, Lipton JH, Longtine J, Martinelli G, Saglio G, Soverini S, Stock W, Goldman JM. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006 Jul 1:108(1):28-37 [PubMed PMID: 16522812]
Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, Cervantes F, Clark RE, Cortes JE, Guilhot F, Hjorth-Hansen H, Hughes TP, Kantarjian HM, Kim DW, Larson RA, Lipton JH, Mahon FX, Martinelli G, Mayer J, Müller MC, Niederwieser D, Pane F, Radich JP, Rousselot P, Saglio G, Saußele S, Schiffer C, Silver R, Simonsson B, Steegmann JL, Goldman JM, Hehlmann R. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013 Aug 8:122(6):872-84. doi: 10.1182/blood-2013-05-501569. Epub 2013 Jun 26 [PubMed PMID: 23803709]
Nazha A, Kantarjian H, Jain P, Romo C, Jabbour E, Quintas-Cardama A, Luthra R, Abruzzo L, Borthakur G, Ravandi F, Pierce S, O'Brien S, Cortes J. Assessment at 6 months may be warranted for patients with chronic myeloid leukemia with no major cytogenetic response at 3 months. Haematologica. 2013 Nov:98(11):1686-8. doi: 10.3324/haematol.2013.090282. Epub 2013 Jun 28 [PubMed PMID: 23812943]
Ibrahim AR, Eliasson L, Apperley JF, Milojkovic D, Bua M, Szydlo R, Mahon FX, Kozlowski K, Paliompeis C, Foroni L, Khorashad JS, Bazeos A, Molimard M, Reid A, Rezvani K, Gerrard G, Goldman J, Marin D. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood. 2011 Apr 7:117(14):3733-6. doi: 10.1182/blood-2010-10-309807. Epub 2011 Feb 23 [PubMed PMID: 21346253]
Caldemeyer L, Akard LP. Rationale and motivating factors for treatment-free remission in chronic myeloid leukemia. Leukemia & lymphoma. 2016 Dec:57(12):2739-2751 [PubMed PMID: 27562641]
Steegmann JL, Baccarani M, Breccia M, Casado LF, García-Gutiérrez V, Hochhaus A, Kim DW, Kim TD, Khoury HJ, Le Coutre P, Mayer J, Milojkovic D, Porkka K, Rea D, Rosti G, Saussele S, Hehlmann R, Clark RE. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016 Aug:30(8):1648-71. doi: 10.1038/leu.2016.104. Epub 2016 Apr 28 [PubMed PMID: 27121688]
Wang W, Cortes JE, Tang G, Khoury JD, Wang S, Bueso-Ramos CE, DiGiuseppe JA, Chen Z, Kantarjian HM, Medeiros LJ, Hu S. Risk stratification of chromosomal abnormalities in chronic myelogenous leukemia in the era of tyrosine kinase inhibitor therapy. Blood. 2016 Jun 2:127(22):2742-50. doi: 10.1182/blood-2016-01-690230. Epub 2016 Mar 22 [PubMed PMID: 27006386]
Ernst T, La Rosée P, Müller MC, Hochhaus A. BCR-ABL mutations in chronic myeloid leukemia. Hematology/oncology clinics of North America. 2011 Oct:25(5):997-1008, v-vi. doi: 10.1016/j.hoc.2011.09.005. Epub [PubMed PMID: 22054731]
Branford S, Wang P, Yeung DT, Thomson D, Purins A, Wadham C, Shahrin NH, Marum JE, Nataren N, Parker WT, Geoghegan J, Feng J, Shanmuganathan N, Mueller MC, Dietz C, Stangl D, Donaldson Z, Altamura H, Georgievski J, Braley J, Brown A, Hahn C, Walker I, Kim SH, Choi SY, Park SH, Kim DW, White DL, Yong ASM, Ross DM, Scott HS, Schreiber AW, Hughes TP. Integrative genomic analysis reveals cancer-associated mutations at diagnosis of CML in patients with high-risk disease. Blood. 2018 Aug 30:132(9):948-961. doi: 10.1182/blood-2018-02-832253. Epub 2018 Jul 2 [PubMed PMID: 29967129]