Introduction
The cingulate cortex is a fascinating area of the human brain that has been the subject of neuroanatomical and therapeutic investigation owing to its role in various functions and pathologies. It is a paired structure that resides within the medial surface of the cerebral hemisphere and is perhaps most well-known as part of the limbic system (see Image. Principal Systems of Association Fibers in the Cerebrum). It is involved in many vital neural circuits, which include other structures such as reward centers, amygdala, lateral prefrontal cortex, parietal cortex, motor areas, spinal cord, hippocampus, and limbic regions.[1] Although previously viewed as a mysterious territory of the brain, evidence from cognitive and fMRI studies has established a better understanding of the cingulate cortex in the last decade. Its functional role continues to be heavily studied to this day. The following sections shed more light on the cingulate cortical structure, function, and clinical and surgical significance.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
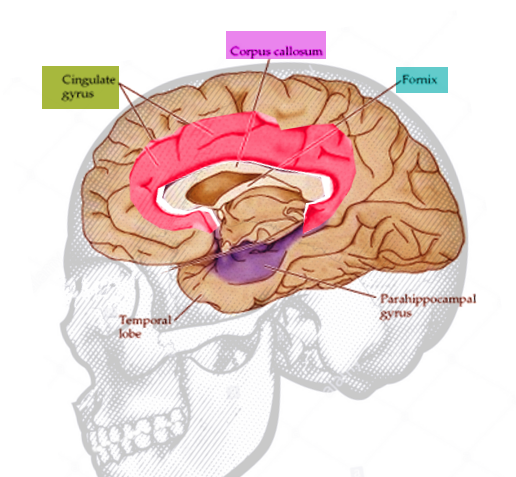
The cingulate cortex is made up of the cingulate gyrus and the cortical gray matter lining the superior and inferior borders of the cingulate sulcus (see Image. Cingulate Gyrus, Anatomy). As the Latin translation of "cingulate" would suggest, the cingulate gyrus wraps around the corpus callosum like a "belt." It begins beneath the rostrum of the corpus callosum, curves around the genu, and projects above the superior surface of the body of the corpus callosum, reaching its posterior end where it terminates at the isthmus of the cingulate gyrus. The isthmus is continuous inferiorly to the parahippocampal gyrus in the temporal lobe. The cingulate gyrus is separated from the corpus callosum inferiorly by the callosal sulcus and from the superior frontal gyrus superiorly by the cingulate sulcus.[2] See Image. Cingulate Gyrus. The cingulate cortex, therefore, reaches the cortical surface at no point in its trajectory, and in the intact brain, it is enclosed by the exterior frontal, temporal, parietal, and occipital cerebral lobes.
The cingulate cortex divides into four functionally distinct regions: the anterior cingulate cortex (ACC), midcingulate cortex, posterior cingulate cortex (PCC), and retrosplenial cortex.[3] The ACC comprises Brodmann's areas (BA) 24, 25, 32, and 33, and can be further broken down into three subregions. The perigenual anterior cingulate cortex (pACC) is mainly responsible for processing emotions and regulating the endocrine and autonomic responses to emotions. The dorsal anterior cingulate cortex (dACC), often known as the midcingulate cortex, is thought to carry out cognitive processing, specifically reward-based decision-making.[4] The cingulate motor areas (CMA) are higher-order motor areas in the cortex, located within the cingulate sulcus next to the primary and supplementary motor cortices. The CMAs process information from our internal and external states (e.g., emotional state signals from the limbic system) and translate them into motor commands executed by the primary and supplementary motor cortices and spinal cord.[5]
The PCC houses BA 23, 29, 30, and 31. The thick granular layer IV of the PCC marks a clear histologic distinction from the thin agranular layer IV of the ACC. The PCC consists of the posterior cingulate cortex proper (ventral and dorsal PCC), which is responsible for visuospatial orientation. Although contained within the PCC, the retrosplenial cortex is considered a distinct region of the cingulate cortex. It is implicated in imagination and the formation and consolidation of episodic memory.[6][7]
The cingulum, or cingulum bundle, is an important white matter tract that connects the orbitofrontal cortex to the temporal pole, nearly forming a near-complete ring that lies beneath the cingulate gyrus[8]. It connects functionally diverse parts of the brain, including both cortical and subcortical structures, and thus facilitates an astounding variety of functions, including memory processes, visuospatial processing, emotional and behavioral regulation, and executive function[9]. Furthermore, it forms a crucial part of the so-called 'Papez circuit,' a fundamental connective network governing emotional function, linking the hippocampal formation, fornix, anterior thalamic nucleus, cingulum, and entorhinal cortex. This was originally proposed and theorized by the neuroanatomist James Papez as he theorized the need for a circuit that connects the hypothalamus, limbic lobe, and brainstem as a method for emotional processing. This was later reclassified in the context of the limbic system by MacLean.[10] See Image. Mammillary Bodies.
As seen from the extensive neural pathways it shares with other brain regions, the cingulate cortex can be considered, in a sense, a connecting hub of emotions, sensations, and action. Some of these pathways are those involved in motivational processing, which is apparent through the connections with the orbitofrontal cortex, basal ganglia, and insula, which together make up the reward centers of the brain. Also, the cingulate cortex projects pathways to the lateral prefrontal cortex, which is involved in executive control, working memory, and learning. Pathways between the cingulate cortex and motor areas, like the primary and supplementary motor cortices, spinal cord, and frontal eye fields, suggest an important role in motor control. Moreover, the cingulate cortex and frontal and parietal lobes comprise a neural network for orienting attention, and expectedly, injury to any of these areas is known to cause hemineglect. The neural circuits cingulate cortex shares with the hippocampus and amygdala suggest a role in consolidating long-term memories and processing emotionally relevant stimuli, respectively.[11]
Embryology
During embryonic development, the prosencephalon (forebrain) gives rise to the telencephalon and diencephalon at around week 6. The telencephalon differentiates into the two cerebral hemispheres, which include the cingulate gyrus on their medial surface.
During early life and adolescent development, the functional connectivity of the cingulate cortex undergoes significant adaptive change. Interestingly, it appears that different functions ascribed to this anatomical area mature at different rates, with motor control and simple cognitive controls (e.g., inhibition and attention) maturing in early childhood, in contrast to more complex emotional behaviors such as social cognition, which may mature as late as adolescence.[12]
Blood Supply and Lymphatics
The anterior cerebral artery supplies the majority of the medial surface of the cerebral hemisphere, including the cingulate gyrus. As a direct continuation of the anterior cerebral artery, the pericallosal artery travels within the callosal sulcus and gives off many small cortical branches that supply the rostrum of the cingulate gyrus. The callomarginal artery, the largest branch of the anterior cerebral artery, runs within the cingulate sulcus and supplies the portion of the cingulate gyrus underlying the paracentral lobule. Posteriorly, the precuneus and posterior cingulate gyrus receive vascular supply from the precuneal artery, a branch of the pericallosal artery.[13]
Physiologic Variants
Some of the normal anatomic variations of the cingulate gyrus include a single cingulate sulcus or double parallel cingulate sulci. MRI-volumetric studies on normal brains showed no significant volume asymmetries between the left and right hemispheres, except in the cingulate gyrus, which was larger on the left. Pujol et al. studied the brains of 100 healthy subjects and found larger right ACC to be more frequent in females than males.[3] Interestingly, subjects with a larger right ACC had more worrisome personalities, greater fear in the face of uncertainty, and shyness around strangers.[14] In light of physiological variations in the size and morphology of the cingulate cortex between individuals, it has also been hypothesized that such anatomical variability may be part of the underlying reason for variations in response to surgical intervention in this area.[15]
Surgical Considerations
Bilateral anterior cingulotomy is a neurosurgical procedure that can be performed for chronic refractory depression, pain, or obsessive-compulsive disorder. It involves stereotactic ablation of the bilateral anterior cingulate gyri, thus severing the supracallosal fibers of the cingulum.[16] It is commonly used to treat chronic pain refractory to medications, as in patients with metastatic cancer. This procedure is thought to reduce the unpleasant perception of pain rather than eliminate the pain itself.
In the context of the treatment of refractory depression, a better clinical response was observed in patients who had relatively anteriorly placed lesioning of their ACC, in line with neuroimaging studies demonstrating that patients with major depression have anatomical abnormalities compared to the healthy population in the rostral part of the cingulate cortex.[15] Counterintuitively, it was found that a better response was experienced in patients with small lesions in this area, challenging the assumption that larger lesions would confer larger clinical benefits.
In the context of the treatment of obsessive-compulsive disorder, the target is the dorsal part of the ACC, and better clinical results have been found following lesioning of the superior part of the cingulate cortex here, as measured by the Yale-Brown Obsessive-Compulsive Scale (YBOCS) score.[17] A large meta-analysis of studies reporting outcomes of surgical treatment for obsessive-compulsive disorder found a mean reduction of 37% in the YBOCS score following cingulotomy[18]. Furthermore, neuroimaging studies of patients who responded well to cingulotomy found that they tended to have undergone lesions in the posterior part of Brodmann area 32, and in those who did not respond initially, a second lesion surgery can be beneficial to clinical outcomes.[17][19] In general, a better reduction (55%) in the YBOCS score was observed in patients undergoing capsulotomy, a procedure in which the anterior limb of the internal capsule is lesioned instead of the cingulate cortex.[20] However, the serious adverse effects associated with cingulotomy were much lower compared to those associated with capsulotomy (5.25 and 21.4%, respectively.)[18]
Clinical Significance
Subfalcine Herniation
Owing to the anatomical organization of the brain within a fixed compartment (the bony cranium) and subdivided by dural reflections (the falx cerebri and tentorium cerebelli), it is vulnerable to damage from an increase in intracranial pressure (ICP).[21] This may be caused by an increased amount of intracranial cerebrospinal fluid (i.e., hydrocephalus), hematoma in the extradural, subdural, subarachnoid, or intraparenchymal spaces, presence of a space-occupying lesion, or edema from a disease process.[22] Following an increase in ICP, the brain can herniate between the spaces bound by the dural reflections, causing corresponding neurological damage to the structures involved.
The most common brain herniation syndrome is subfalcine herniation, in which raised ICP forces a cerebral hemisphere to the opposite side of the cranium, forcing the cingulate gyrus beneath the free edge of the falx cerebri.[23] This happens anteriorly because the falx cerebri is rigid posteriorly, so the herniation will occur preferentially anteriorly, where there is less resistance from the falx cerbri (see Image. Compression of the Pericallosal Artery).[24] Although it does not usually produce severe symptomatology, if subfalcine herniation progresses, it can be further complicated by compression of the anterior cerebral artery (ACA) against the falx, resulting in an ipsilateral ACA stroke which most commonly manifests as contralateral leg weakness.[25]
Schizophrenia
Given the complexity of higher cognitive functions and the extensive connections of the cingulate cortex with other brain regions, it is not surprising that it plays a role in the pathogenesis of schizophrenia, a finding which has been confirmed in post-mortem anatomical studies.[26] In particular, the ACC is one of the brain areas which shows abnormalities in schizophrenic patients when compared to healthy subjects. These anomalies include reduced cortical grey matter, decreased gyrus volume, and disrupted neuronal arrangement, particularly in the anterior segment of the cingulate gyrus. Concerning cellular level characteristics, reduced neuronal size and glial density are observed in this region.[27][28] The relevance of these morphological findings to clinical practice and future therapeutic interventions relevant to schizophrenia remains to be elucidated and will require further detailed neuroimaging studies and clinical trials.
Media
(Click Image to Enlarge)
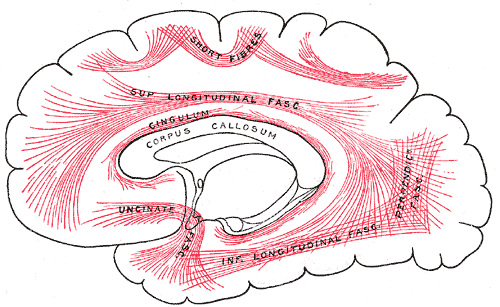
Principal Systems of Association Fibers in the Cerebrum. This diagram shows the principal systems of association fibers in the cerebrum, short fibers, superior longitudinal fascia, cingulum, corpus callosum, uncinate fascia, inferior longitudinal fascia, and perpendicular fascia.
Henry Vandyke Carter, Public Domain, via Wikimedia Commons
(Click Image to Enlarge)
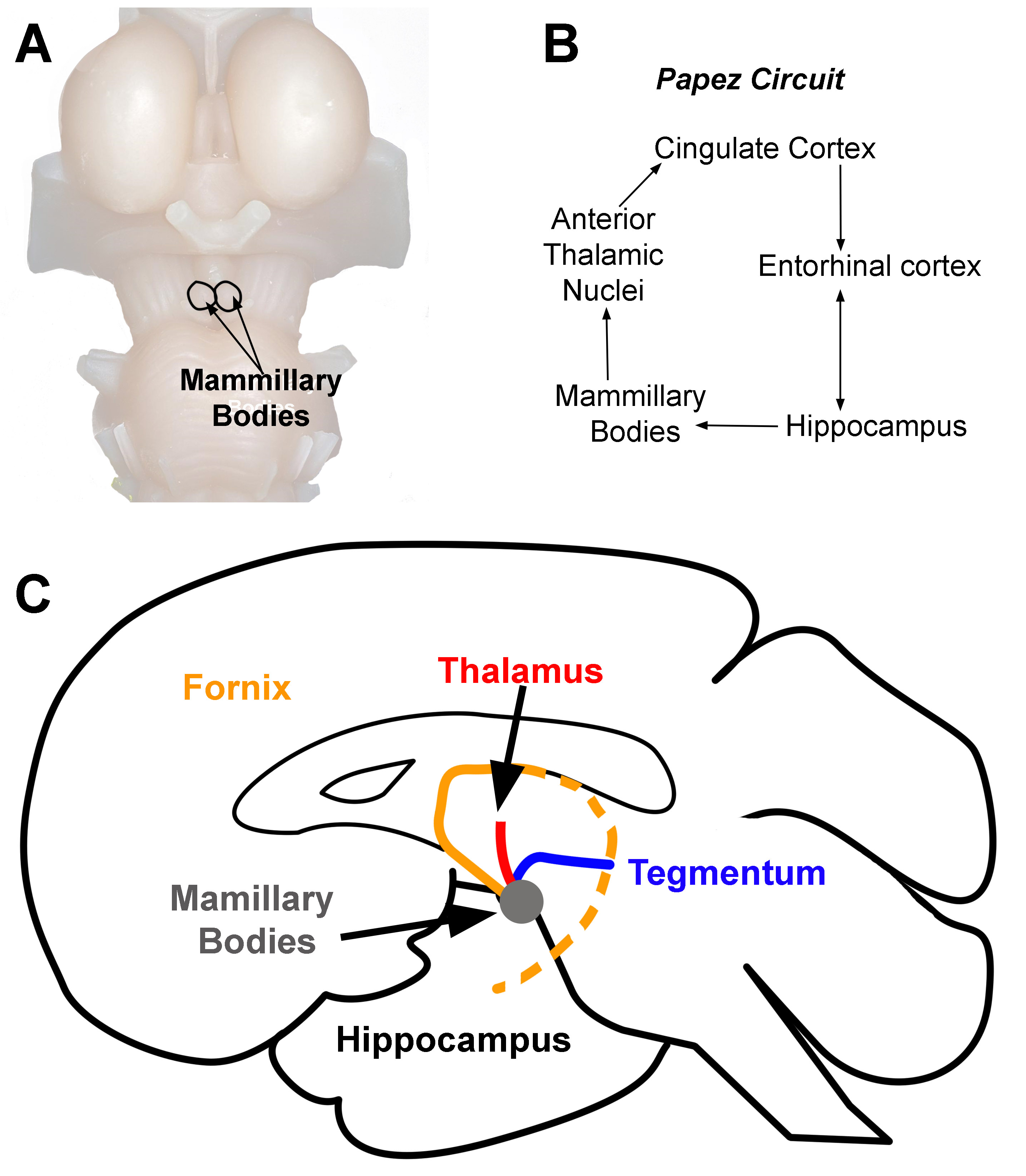
Mammillary Bodies. Location of mammillary bodies on the brainstem (A). Papez circuit (B). Arrows indicate the direction of information flow (C). Connections of the mammillary bodies (grey area). The three primary connections of the mammillary bodies are from (1) the hippocampus to the mammillary bodies (orange line). This pathway is the fornix. (2) The mammillary bodies to the thalamus (red line), and (3) the mammillary bodies to and from the tegmentum (blue line).
Contributed by D Peterson, PhD
(Click Image to Enlarge)
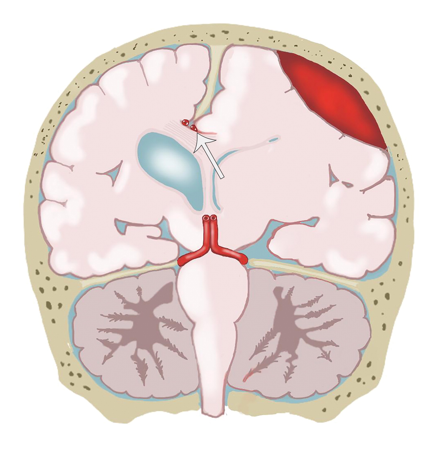
Compression of the Pericallosal Artery. The illustration shows compression of the pericallosal artery (arrow) against the falx cerebri due to a subfalcine hernia.
Gilardi BR, López JIM, Hernández Villegas AC, et al. Types of cerebral herniation and their imaging features. RadioGraphics. 2019;39:6:1598-1610. Doi: 10.1148/rg.2019190018.
References
Rolls ET. The cingulate cortex and limbic systems for action, emotion, and memory. Handbook of clinical neurology. 2019:166():23-37. doi: 10.1016/B978-0-444-64196-0.00002-9. Epub [PubMed PMID: 31731913]
Stevens FL, Hurley RA, Taber KH. Anterior cingulate cortex: unique role in cognition and emotion. The Journal of neuropsychiatry and clinical neurosciences. 2011 Spring:23(2):121-5 [PubMed PMID: 21677237]
Vogt BA, Nimchinsky EA, Vogt LJ, Hof PR. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. The Journal of comparative neurology. 1995 Aug 28:359(3):490-506 [PubMed PMID: 7499543]
Level 2 (mid-level) evidenceVogt BA, Midcingulate cortex: Structure, connections, homologies, functions and diseases. Journal of chemical neuroanatomy. 2016 Jul [PubMed PMID: 26993424]
Strick PL, Dum RP, Picard N. Motor areas on the medial wall of the hemisphere. Novartis Foundation symposium. 1998:218():64-75; discussion 75-80, 104-8 [PubMed PMID: 9949816]
Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Progress in brain research. 2005:150():205-17 [PubMed PMID: 16186025]
Level 3 (low-level) evidenceLeech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain : a journal of neurology. 2014 Jan:137(Pt 1):12-32. doi: 10.1093/brain/awt162. Epub 2013 Jul 18 [PubMed PMID: 23869106]
Level 3 (low-level) evidenceWeininger J, Roman E, Tierney P, Barry D, Gallagher H, Murphy P, Levins KJ, O'Keane V, O'Hanlon E, Roddy DW. Papez's Forgotten Tract: 80 Years of Unreconciled Findings Concerning the Thalamocingulate Tract. Frontiers in neuroanatomy. 2019:13():14. doi: 10.3389/fnana.2019.00014. Epub 2019 Feb 18 [PubMed PMID: 30833890]
Bubb EJ, Metzler-Baddeley C, Aggleton JP. The cingulum bundle: Anatomy, function, and dysfunction. Neuroscience and biobehavioral reviews. 2018 Sep:92():104-127. doi: 10.1016/j.neubiorev.2018.05.008. Epub 2018 May 16 [PubMed PMID: 29753752]
Rajmohan V, Mohandas E. The limbic system. Indian journal of psychiatry. 2007 Apr:49(2):132-9. doi: 10.4103/0019-5545.33264. Epub [PubMed PMID: 20711399]
Hayden BY, Platt ML. Neurons in anterior cingulate cortex multiplex information about reward and action. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010 Mar 3:30(9):3339-46. doi: 10.1523/JNEUROSCI.4874-09.2010. Epub [PubMed PMID: 20203193]
Level 3 (low-level) evidenceKelly AM, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Margulies DS, Castellanos FX, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral cortex (New York, N.Y. : 1991). 2009 Mar:19(3):640-57. doi: 10.1093/cercor/bhn117. Epub 2008 Jul 24 [PubMed PMID: 18653667]
Cilliers K, Page BJ. Review of the Anatomy of the Distal Anterior Cerebral Artery and Its Anomalies. Turkish neurosurgery. 2016:26(5):653-61. doi: 10.5137/1019-5149.JTN.14294-15.1. Epub [PubMed PMID: 27337235]
Pujol J, López A, Deus J, Cardoner N, Vallejo J, Capdevila A, Paus T. Anatomical variability of the anterior cingulate gyrus and basic dimensions of human personality. NeuroImage. 2002 Apr:15(4):847-55 [PubMed PMID: 11906225]
Steele JD, Christmas D, Eljamel MS, Matthews K. Anterior cingulotomy for major depression: clinical outcome and relationship to lesion characteristics. Biological psychiatry. 2008 Apr 1:63(7):670-7 [PubMed PMID: 17916331]
Level 2 (mid-level) evidenceCosgrove GR, Rauch SL. Stereotactic cingulotomy. Neurosurgery clinics of North America. 2003 Apr:14(2):225-35 [PubMed PMID: 12856490]
Starkweather CK, Bick SK, McHugh JM, Dougherty DD, Williams ZM. Lesion location and outcome following cingulotomy for obsessive-compulsive disorder. Journal of neurosurgery. 2022 Jan 1:136(1):221-230. doi: 10.3171/2020.11.JNS202211. Epub 2021 Jul 9 [PubMed PMID: 34243154]
Brown LT,Mikell CB,Youngerman BE,Zhang Y,McKhann GM 2nd,Sheth SA, Dorsal anterior cingulotomy and anterior capsulotomy for severe, refractory obsessive-compulsive disorder: a systematic review of observational studies. Journal of neurosurgery. 2016 Jan [PubMed PMID: 26252455]
Level 1 (high-level) evidenceBourne SK, Sheth SA, Neal J, Strong C, Mian MK, Cosgrove GR, Eskandar EN, Dougherty DD. Beneficial effect of subsequent lesion procedures after nonresponse to initial cingulotomy for severe, treatment-refractory obsessive-compulsive disorder. Neurosurgery. 2013 Feb:72(2):196-202; discussion 202. doi: 10.1227/NEU.0b013e31827b9c7c. Epub [PubMed PMID: 23147780]
Level 2 (mid-level) evidenceRück C, Karlsson A, Steele JD, Edman G, Meyerson BA, Ericson K, Nyman H, Asberg M, Svanborg P. Capsulotomy for obsessive-compulsive disorder: long-term follow-up of 25 patients. Archives of general psychiatry. 2008 Aug:65(8):914-21. doi: 10.1001/archpsyc.65.8.914. Epub [PubMed PMID: 18678796]
Tayebi Meybodi A, Tabani H, Benet A. Arachnoid and dural reflections. Handbook of clinical neurology. 2020:169():17-54. doi: 10.1016/B978-0-12-804280-9.00002-0. Epub [PubMed PMID: 32553288]
Leinonen V,Vanninen R,Rauramaa T, Raised intracranial pressure and brain edema. Handbook of clinical neurology. 2017; [PubMed PMID: 28987174]
Riveros Gilardi B, Muñoz López JI, Hernández Villegas AC, Garay Mora JA, Rico Rodríguez OC, Chávez Appendini R, De la Mora Malváez M, Higuera Calleja JA. Types of Cerebral Herniation and Their Imaging Features. Radiographics : a review publication of the Radiological Society of North America, Inc. 2019 Oct:39(6):1598-1610. doi: 10.1148/rg.2019190018. Epub [PubMed PMID: 31589570]
Johnson PL, Eckard DA, Chason DP, Brecheisen MA, Batnitzky S. Imaging of acquired cerebral herniations. Neuroimaging clinics of North America. 2002 May:12(2):217-28 [PubMed PMID: 12391633]
Byard RW. Patterns of cerebral and cerebellar herniation. Forensic science, medicine, and pathology. 2013 Jun:9(2):260-4. doi: 10.1007/s12024-012-9339-9. Epub 2012 Apr 29 [PubMed PMID: 22544455]
Level 3 (low-level) evidenceWang L,Hosakere M,Trein JC,Miller A,Ratnanather JT,Barch DM,Thompson PA,Qiu A,Gado MH,Miller MI,Csernansky JG, Abnormalities of cingulate gyrus neuroanatomy in schizophrenia. Schizophrenia research. 2007 Jul [PubMed PMID: 17433626]
Level 2 (mid-level) evidenceBaiano M, David A, Versace A, Churchill R, Balestrieri M, Brambilla P. Anterior cingulate volumes in schizophrenia: a systematic review and a meta-analysis of MRI studies. Schizophrenia research. 2007 Jul:93(1-3):1-12 [PubMed PMID: 17399954]
Level 1 (high-level) evidenceBenes FM, Bird ED. An analysis of the arrangement of neurons in the cingulate cortex of schizophrenic patients. Archives of general psychiatry. 1987 Jul:44(7):608-16 [PubMed PMID: 3606326]