Introduction
Clostridium is among the largest bacterial genera, belonging to the Firmicutes phylum.[1][2] Clostridia are gram-positive, endospore-forming, obligate anaerobic bacilli that form exotoxins. They are ubiquitous in the environment and can also be found in humans and animals. [2] Clostridium botulinum, Clostridium perfringens, Clostridium tetani, Clostridium difficile, and Clostridium sordellii are commonly encountered clostridial species.[2] This activity focuses on Clostridium botulinum, which produces botulinum neurotoxin, the most potent biologically-known substance that is the cause of botulism. Botulism is a neuroparalytic syndrome of reversible, symmetrical flaccid paralysis, and although rare, this infection can lead to respiratory failure and death. Due to its potential fatality and the fact that it can be mistaken for other similar syndromes, the diagnosis can be difficult or missed. [3] Clinicians should have a high index of suspicion for Clostridium botulinum infection when evaluating patients to diagnose and treat it promptly.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Clostridium botulinum belongs to the genus Clostridium, which is among the largest bacterial genera and belongs to the Firmicutes phylum.[1][2] Clostridia are gram-positive, spore-forming, obligate anaerobic bacilli that form exotoxins.[2] C botulinum is ubiquitous in the environment and is found as a saprophyte in soil, animal manure, vegetables, and sea mud.[4] The neurotoxin produced by this organism has likely evolved for it to acquire nutrition by killing its host. Botulism derives from the Latin "botulus," which means "sausage," because it was originally associated with contaminated sausages in Germany.[5]
C botulinum produces botulinum neurotoxin (BoNT), which is responsible for most cases of botulism. C botulinum produces 7 immunologically different toxins, and they are designated using letters from A to G.[3] Most of the cases of foodborne botulism worldwide are caused by BoNT type A, B, E, and F. Botulism is a potentially fatal paralytic illness that manifests as a descending flaccid, symmetrical paralysis and can lead to respiratory failure and death. Clostridial BoNT, considered the most potent neurotoxin, is a zinc metalloprotease that blocks the exocytosis of cholinergic neurotransmitters at the neuromuscular junctions, thus resulting in flaccid paralysis.[3][6] The exact lethal dose of BoNT is not known; a commonly cited estimated lethal dose of toxin type A is 0.09 to 0.15 μg intravenous and 0.8 to 0.9 μg inhaled. Lower doses have also been reported.[3]
Botulism can occur in 3 forms, depending on the route of entry: foodborne, wound botulism, infant botulism, adult intestinal botulism, and iatrogenic botulism. Endospores of C botulinum are ubiquitous in the environment, and ingestion of spores occurs through contact with the environment. They are routinely ingested and excreted from the human intestine without germination, and thus, there is no toxin production and no clinical disease. If the intestine is anatomically compromised or its microbiome is compromised, endospores may find an appropriate environment and germinate, causing intestinal botulism.[3][7]
When appropriate hygienic and sterilization conditions are implemented during the manufacturing of commercially processed products, botulism rarely ensues. Botulism can occur when there are deficiencies in the manufacturing of such products, which allows the food to be contaminated with already produced BoNT and/or C botulinum endospores, which can produce BoNT.[8] Metabolically dormant endospores of C botulinum, which are anaerobic, cause disease.[9] The endospores are ubiquitous and resistant to high temperatures, enzyme digestion, and pressure changes.[10] Thus, the presence of the endospore in foods, with conditions of poor sterilization and processing, leads to anaerobic environments that allow C botulinum to germinate and form BoNT.[9] Such conditions can occur during home canning, which leads to toxin-producing vegetative cells that intoxicate the food.[9]
Foodborne botulism occurs when preformed BoNT is ingested directly from food. The BoNT is produced by cells that originate from endospores that have germinated. Homemade canned food is the most common source of foodborne botulism; most cases are sporadic, and outbreaks are rare. In addition to the flaccid, symmetrical paralysis, gastrointestinal symptoms may also be noted. Wound botulism occurs when spores enter a wound, germinate, and produce toxins. The clinical presentation is similar to foodborne botulism but without the gastrointestinal symptoms.[6] Intestinal botulism occurs in patients with anatomically compromised intestines or dysbiosis when colonized with neurotoxigenic C botulinum; the organism can germinate and produce BoNT in their intestines. Intestinal botulism is called infant botulism if the person is under one year of age, and if the patient is older than one year of age, it is called adult intestinal botulism. Infant botulism from ingesting contaminated food (usually honey) with spores of C botulinum.[3][7] Clinical manifestations include the inability to suck and swallow, a weak voice, ptosis, a floppy neck, and extreme weakness and hence referred to as "floppy baby syndrome." This is a self-limiting condition, managed by supportive care and assisted feeding. Rarely, it can progress to generalized flaccidity, respiratory failure, and sudden death. Symptoms of all types of botulism are similar and involve symmetrical, flaccid paralysis, except for foodborne botulism, when gastrointestinal symptoms (eg, diarrhea) may occur before the neurological symptoms.[6]
Epidemiology
Generally, in countries with adequate resources and an excellent public health system, botulism is a notifiable disease, and data on incidence, trends, and mortality have been reported. Published data show that the incidence of human botulism has markedly decreased worldwide, as has mortality; this is most likely because of improved clinical recognition and diagnosis, treatment options, including the use of botulism antitoxin, and improved food safety measures and standards. A systematic review published in 2015 identified 197 outbreaks of foodborne botulism from 1920 to 2014.[11] The most commonly implicated cause of botulism were toxin types A, B, E, and F in 34%, 16%, 17%, and 1% of outbreaks, respectively.[11] Outbreaks were reported from 27 countries, and the majority of outbreaks, 55%, occurred in the United States, followed by Canada at 15%, Europe at 13%, Asia at 13%, Africa at 3% and Australia at 2%.[11] Most cases in the United States were due to home-canning of foods; in Europe, they were primarily due to commercial foods. Toxin A was the cause of 58% of the outbreaks in the United States, 45% in Europe, and 50% in Asia, whereas toxin type E was responsible for 50% of Canadian outbreaks. The incubation period was shorter in toxin type E botulism than in cases caused by toxin A; case-patients involving type A toxin had the highest mean percentage of mechanical ventilation, and mortality was lower for outbreaks in which all cases with toxin A and E botulism had received matching antitoxin (7.8% vs 53.9%; P < .001).[11] These findings are consistent with other reports.[12]
The United States Center for Disease Control and Prevention has been monitoring cases of botulism in the United States since 1973. From 2011 through 2015, an average of 162 annual cases were reported. These primarily included infant botulism at 71% to 88%, followed by foodborne botulism, wound botulism, and botulism of unknown origin. Botulism cases have remained relatively stable over the past 10 years except for rare, large outbreaks.[13][14] The mortality rate had decreased significantly from over 60% in the 1950s to only 3% in 2009, mostly likely due to appropriate detection, better supportive care, and the development of botulinum antitoxin.[15] Most deaths reported are due to cases of botulism without a known source, followed by foodborne, wound, and infant botulism, which comprise less than 1% of cases.[16]
A 2023 report from Canada has documented 55 cases of foodborne botulism from 2006 until 2021.[17] The mean annual incidence was 0.01 cases per 100,000 individuals. Botulinum toxin E was identified as the cause in 52% of cases, type A was responsible in 24%, type B in 16%, type F in 3%, and type AB in 1%. Seventy percent of cases required mechanical ventilation, and 7 deaths were reported. Type A toxin was responsible for longer hospital stays.[17]
Results from an epidemiological study from Taiwan reported 50 cases of botulism between 2003 and 2020, with an incidence ranging from 0 to 0.48 per 1000,000 from 2003 to 2020 and an overall decreasing trend.[8] Most of the patients were women (56%), were 50 years old or older, and lived in Taipei and northern Taiwan (44%). From 2010 to 2020, however, botulism in children youtnger than 20 showed an increasing trend, and most of the children were boys (66.7%). Infections mainly occurred during the spring and summer months (66.7%).[8] Data from 2015 and 2022 from the European Centre for Disease Prevention and Control report a stable number of cases of botulism in Europe at less than 0.1 cases per 100,000 individuals. Infant botulism, defined as children younger than 1, was the group with the highest rate of botulism.[18]
Pathophysiology
Humans can ingest spores that pass through the intestine without germinating; the toxin is produced only when spores germinate. Most of the cases of foodborne botulism worldwide are caused by BoNT type A, B, E, and F. Toxin type A causes the most severe botulism, and most patients with this toxin require mechanical ventilation.[19] Botulism is a potentially fatal paralytic illness that manifests as a descending flaccid, symmetrical paralysis and can lead to respiratory failure and death. Botulinum toxin can, however, also be used for therapeutic reasons because it causes flaccid paralysis; aesthetic and medical conditions that BoNT can treat include strabismus, blepharospasm, chronic migraines, torticollis, hyperhidrosis, and myoclonus.[20]
C botulinum produces botulinum neurotoxin (BoNT), a zinc-dependent protein of 150 kDa (100 kDa heavy chain and a 50 kDa light chain), and is responsible for most cases of botulism. C botulinum produces 7 immunologically different toxins, and they are designated using letters from A to G, some with subtypes (A, B, C1, C2, D, E, F, and G ) based on the immunological differences in the toxins they produce. All serotypes produce neurotoxin, except C2, which produces an enterotoxin.[3][20] BoNT types C and D are bacteriophage-coded. Botulinum neurotoxin differs from other exotoxins as it is produced intracellularly, is not secreted, and appears outside only after bacterial cell autolysis. The toxin is synthesized initially as a nontoxic protoxin; this requires trypsin or other proteolytic enzymes to convert it into an active form.[3] Clostridial BoNT, considered the most potent neurotoxin, is a zinc metalloprotease that blocks the exocytosis of cholinergic neurotransmitters at the neuromuscular junctions, thus resulting in flaccid paralysis.[3][6] The exact lethal dose of BoNT is not known; a commonly cited estimated lethal dose of toxin type A is 0.09 to 0.15 μg intravenously and 0.8 to 0.9 μg inhaled. Lower doses have also been reported.[3]
The BoNT is absorbed into the bloodstream from either a foodborne, wound, intestinal, or inhalational exposure and moves to the peripheral cholinergic nerve terminals, which include the neuromuscular junctions, postganglionic parasympathetic nerve endings, and peripheral ganglia.[19] The BoNT binds to the neuron, is internalized by endocytosis, and moves to the cytosol. Then it cleaves the proteins that release acetylcholine at the nerve junctions, and by inhibiting the release of acetylcholine, it blocks the transmission of the neurotransmitter across the junction, resulting in flaccid paralysis. BoNT does not cross the blood-brain barrier; the BoNT binds irreversibly to the nerve terminal, and recovery only occurs when new nerve terminals have sprouted. This process may take weeks to months.[19]
History and Physical
Botulism is a potentially life-threatening paralytic illness, and clinicians must, therefore, have a high index of suspicion when evaluating patients. Early clinical diagnosis of botulism is essential because the paralysis can quickly result in respiratory failure, and the antitoxin should be given as soon as possible. A complete travel, exposure, and risk factor history is essential to evaluate the possibility of ingestion or contamination of C botulinum spores or toxins. Such exposures include ingestion of improperly canned, preserved, or fermented foods, use of injectable drugs (particularly black-tar heroin), recent surgical procedures or injections, occurrence of contaminated wounds, ingestion of honey, any environmental exposure to soil containing C botulinum endospores, and having received a large dose of BoNT for therapeutic or cosmetic purposes.[3][21]
Symptoms of botulism can occur suddenly and usually appear 12 to 36 hours after exposure to the BoNT, although they can range from a few hours to a few days.[3] An extensive systematic review by Chatham-Stephens et al identified 233 studies and 171 patients with botulism with available data on foodborne and wound botulism clinical features from 1935 to 2015.[22] The median incubation period was 1 day, and the shortest reported was 2 hours.[22]
The clinical presentation of botulism is classic and follows a familiar pattern in most patients. Patients can appear weak and fatigued, and symptoms and signs usually start from the cranial nerves and then follow a descending, symmetrical paralysis of the torso and extremities with areflexia. Shortness of breath, tachypnea, or the inability to use respiratory muscles may be observed in patients with impending respiratory failure. Cranial nerve involvement can present as diplopia, ptosis, facial palsy, dysphagia, dysarthria, dysphonia, ophthalmoplegia with blurry vision, impaired gag reflex, and neck weakness. The most common signs are descending paralysis, ptosis, and ophthalmoplegia. The "4 Ds" are a mnemonic for cranial nerve paralysis: dysphagia, dysarthria, dry mouth, and diplopia.[22] Clinical criteria have been proposed by Rao et al to "trigger suspicion for botulism" so that rapid diagnosis and treatment can be performed.[19][23]
Nausea, vomiting, and abdominal pain are commonly reported in patients with foodborne botulism, and these symptoms can occur even before any neurological symptoms and signs appear. Constipation is a nearly universal eventual symptom.[3][22] Descending flaccid, symmetrical paralysis of voluntary muscles can follow the cranial nerve palsies, although in some cases, patients may only have cranial palsy-limited symptoms. Botulism usually descends, affecting neck, shoulders, torso, and proximal muscles before the distal extremities. This distribution is related to the toxins circulating through the body via the bloodstream after absorption.[19]
Evaluation
Clinicians should be aware that the clinical course of a patient with botulism can evolve rapidly, and botulism should be included in the differential diagnosis to provide immediate supportive care and administer antitoxin. Clinical criteria have been proposed by Rao et al to "trigger suspicion for botulism" so that rapid diagnosis and treatment can be performed.[19][23] Respiratory failure may occur not only because of paralysis of respiratory muscles but also because of cranial nerve paresis with pharyngeal collapse.[19] Botulism should be considered in any patient in whom Guillain-Barre or myasthenia gravis is suspected, and serial neurological examinations should be performed.[19] Isolated cases can be missed because the diagnosis may not be considered. Still, if the evaluation is performed with a high index of suspicion, the clinical diagnosis can be made, pending the laboratory results. If more than 2 patients have similar symptoms, the diagnosis of botulism is almost pathognomonic because the other diseases that may be confused with botulism do not produce outbreaks.[3] General laboratory tests, cerebrospinal fluid analysis, and neuroimaging are usually normal. Laboratory confirmation can be performed by detecting BoNT in serum, stool, or food or by culturing the organism. However, laboratory testing may take hours or days, so the clinical diagnosis through a complete history and physical examination is essential.
When clinicians see patients, a high index of suspicion is essential. Thinking of other syndromes that this condition may mimic and to be able to differentiate them as best as possible with whatever methods are available in most hospitals is crucial. Myasthenia gravis, Guillain-Barre, and cerebrovascular accidents are syndromes requiring special mention. Cerebrospinal fluid analysis is normal in botulism but abnormal in Guillain-Barre syndrome. The edrophonium test would be positive in myasthenia gravis but normal in botulism. Electromyography and nerve conduction studies could help differentiate botulism from other etiologies of motor neuron conduction diseases. However, not all healthcare systems have such equipment; these tests are operator-dependent and require expert interpretation. They also may not be positive early in the course of the disease.[19]
The diagnosis of botulism can be confirmed by detecting the BoNT in serum, feces, any other sample, and food. Identification of C botulinum in vomitus, gastric secretions, stool, or wound samples is diagnostic for infant botulism and adequate for adult botulism, always in conjunction with the clinical picture. The gold standard method to detect the BoNT is the mouse bioassay, which is available in only a few laboratories and requires having a population of mice on which to test. A specimen sample is injected into the peritoneum of a mouse, and the mouse is monitored for paralysis for up to 96 hours. However, paralysis is usually observed in the first 48 hours.[3][19] Other methods, but only available at reference laboratories, is the real-time polymerase chain reaction test that detects bont genes A to G and can identify clostridial species from cultures and the mass spectrometry method for detecting BoNT.[19]
Administrating antitoxin before the injection of the sample in the mouse bioassay can prevent paralysis in the mouse. The sensitivity of the mouse bioassay varies inversely with the time elapsed between the onset of symptoms and sample collection and has been reported to be as low as 33% to 44%.[3][24] When botulism, other than infant botulism, is suspected, samples should be obtained before the antitoxin is given because the antitoxin can neutralize circulating BoNT, and detection may be difficult. Generally, the presence of toxin is more diagnostic of botulism than the detection of C botulinum by Gram stain and culture. Detection of the organisms by culture can be adequate when considering the characteristic clinical picture. Every case of botulism is a public health emergency and should be reported to the public health department. The public health department should start an epidemiological investigation to identify the source of the infection and find other persons who may have been exposed.[3]
Treatment / Management
Evaluation, diagnosis, and treatment should be done rapidly to ensure the best possible outcomes for patients, especially since botulism can be a rapidly progressing disease. Supportive care and antitoxin administration are the quintessential components of treatment for botulism. Patients with acute-onset flaccid paralysis from botulism should be admitted to the hospital and intensive care unit and should be monitored regularly. If there is impending respiratory failure, intubation and mechanical ventilation may be necessary.[3]
Antitoxin should be administered right away, even with only a clinical diagnosis, and laboratory confirmation is not necessary, as it is a lengthy process, and immediate treatment is necessary. The antitoxin should be given within the first 24 hours of symptoms. When antitoxin is administered sooner rather than later, patient outcomes are improved because the antitoxin neutralizes unbound free BoNT.[19] If the botulism is caused by wound botulism, wounds should be cleaned and debrided promptly, and antibiotics should be administered, if necessary.[25][26]
Mortality from botulism has markedly decreased from 60% to 70% at the beginning of the century to the current rate of 3% to 5%. This is most likely due to many factors, including increased awareness and faster diagnosis, more evolved intensive care methods, including mechanical ventilation, and the administration of antitoxin.[19] The BoNT binds irreversibly to the presynaptic cholinergic nerve terminals, and recovery occurs when new axon terminals are generated. Supportive physical therapy and possibly long-term rehabilitation may be necessary to fully recover functionality.
Differential Diagnosis
The conditions that can mimic Clostridium botulism are as follows:
- Guillain-Barre syndrome
- Cerebral Vascular Accident
- Poliomyelitis
- Myasthenia gravis
- Amyotrophic lateral sclerosis
- Lambert Eaton syndrome
- Tick paralysis
- Acute intermittent porphyria
- Shellfish poisoning
Prognosis
Generally, when patients are evaluated, diagnosed, and treated promptly, the prognosis of botulism is excellent, and a full recovery is expected. Timely evaluation and diagnosis of patients with botulism is essential so they can receive supportive care and antitoxin quickly. If respiratory muscles are affected, intubation and mechanical ventilation may be necessary. A survival analysis from an outbreak of botulism in Thailand reported that from the 91 patients who were hospitalized with botulism, all of whom had received antitoxin, 42 required mechanical ventilation, the median time on the ventilator was 14 days, mechanical ventilation was associated with a shorter incubation and pupillary abnormalities, and there were no deaths.[27]
Almost all patients can survive, even without the antitoxin, as long as they receive supportive care, including mechanical ventilation.[19] Mortality from botulism has markedly decreased from 60% to 70% at the beginning of the century to the current rate of 3% to 5%. This decrease is most likely due to many factors, including increased awareness and faster diagnosis, more evolved intensive care methods, including mechanical ventilation, and the administration of antitoxin.[19]
Complications
Complications from Clostridium botulism infection can include the following:
- Nosocomial infections
- Urinary tract infection
- Thrombophlebitis
- Deep vein thrombosis
- Pressure sores
- Contractures
Consultations
Due to the potentially severe clinical syndrome caused by botulism with the need for hospitalization and the possibility of respiratory failure and death, it is essential to quickly make the diagnosis and treat patients. Treatment consists of supportive care, mechanical ventilation, if necessary, and the administration of antitoxin. Consulting medical specialties that are pertinent not only to the initial disease but also to the patient's projected clinical needs may be pertinent. Such medical consultations include neurologists, infectious disease specialists, and intensive care physicians. Other specialties that would be required include physical medicine and rehabilitation physicians who work in conjunction with physical therapists.
Deterrence and Patient Education
Proper canning, strict adherence to hygiene, and sterilization techniques are recommended. To avoid infant botulism, children younger than 2 should not be given honey.
Pearls and Other Issues
As most cases of botulism follow the consumption of inadequately canned or preserved food, control is achievable by proper canning and preservation. When an outbreak occurs, a prophylactic dose of antitoxin should be given intramuscularly to all who consumed the food article.
Enhancing Healthcare Team Outcomes
Patients with botulism are at high risk of rapid-onset flaccid paralysis and respiratory failure. Early identification and management of botulism cases are imperative in reducing morbidity and mortality. Caring for patients with botulism necessitates a collaborative approach among healthcare professionals to ensure patient-centered care and improve overall outcomes. Neurologists, emergency medicine physicians, critical care physicians, infectious disease physicians, advanced clinicians, nurses, pharmacists, and other healthcare professionals involved in the care of these patients should possess the essential clinical skills and knowledge to diagnose and manage botulism accurately.
A strategic approach is equally crucial, involving evidence-based strategies to optimize treatment plans and minimize adverse effects. Ethical considerations must guide decision-making, ensuring informed consent while respecting patient autonomy in treatment choices. Each healthcare professional must be aware of their responsibilities and contribute their unique expertise to the patient's care plan, fostering a multidisciplinary approach. Effective interprofessional communication is paramount, allowing seamless information exchange and collaborative decision-making among the team members. Care coordination plays a pivotal role in ensuring that the patient's journey from diagnosis to treatment and their follow-up is well-managed, minimizing errors and enhancing patient safety. By embracing these principles of skill, strategy, ethics, responsibilities, interprofessional communication, and care coordination, healthcare professionals can deliver patient-centered care, ultimately improving patient outcomes and enhancing team performance when managing botulism.
Media
(Click Image to Enlarge)
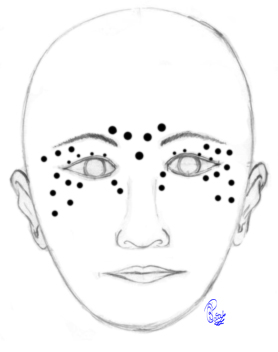
Botulinum Toxin Injections for Blepharospasm. Botulinum toxin injections may be administered at various sites, as depicted in the image. The dosage varies depending on the severity of the blepharospasm and apraxia of eyelid opening at each specific point. Pretarsal injections are administered to specifically counteract apraxia of eyelid opening. Injections just lateral to the lateral nasal wall aim to alleviate the squeezing of the nasalis muscle, which is observed in some patients. Injections into the corrugator and procerus muscles reduce the downward movement of the brow, consequently aiding eyelid control. Injections just below the brows provide a chemical lift to the brows, thereby improving the ability to open the eyelids. Caution is warranted to inject a minimal amount over the zygomaticus major and minor muscles to prevent the appearance of lower facial weakness following injections.
Contributed by BCK Patel, MD, FRCS
References
Dürre P. Physiology and Sporulation in Clostridium. Microbiology spectrum. 2014 Aug:2(4):TBS-0010-2012. doi: 10.1128/microbiolspec.TBS-0010-2012. Epub [PubMed PMID: 26104199]
Cruz-Morales P, Orellana CA, Moutafis G, Moonen G, Rincon G, Nielsen LK, Marcellin E. Revisiting the Evolution and Taxonomy of Clostridia, a Phylogenomic Update. Genome biology and evolution. 2019 Jul 1:11(7):2035-2044. doi: 10.1093/gbe/evz096. Epub [PubMed PMID: 31076745]
Sobel J. Botulism. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005 Oct 15:41(8):1167-73 [PubMed PMID: 16163636]
Dickson EC, Shevky E. BOTULISM. STUDIES ON THE MANNER IN WHICH THE TOXIN OF CLOSTRIDIUM BOTULINUM ACTS UPON THE BODY : II. THE EFFECT UPON THE VOLUNTARY NERVOUS SYSTEM. The Journal of experimental medicine. 1923 Sep 30:38(4):327-46 [PubMed PMID: 19868794]
Torrens JK. Clostridium botulinum was named because of association with "sausage poisoning. BMJ (Clinical research ed.). 1998 Jan 10:316(7125):151 [PubMed PMID: 9490126]
Rawson AM, Dempster AW, Humphreys CM, Minton NP. Pathogenicity and virulence of Clostridium botulinum. Virulence. 2023 Dec:14(1):2205251. doi: 10.1080/21505594.2023.2205251. Epub [PubMed PMID: 37157163]
Harris RA, Anniballi F, Austin JW. Adult Intestinal Toxemia Botulism. Toxins. 2020 Jan 24:12(2):. doi: 10.3390/toxins12020081. Epub 2020 Jan 24 [PubMed PMID: 31991691]
Chen BC,Huang YC,Huang SH,Yu PC,Wang BL,Lin FH,Chou YC,Hsieh CJ,Yu CP, Epidemiology and risk factors for notifiable Clostridium botulinum infections in Taiwan from 2003 to 2020. Medicine. 2022 Oct 21 [PubMed PMID: 36281180]
Shen A, Edwards AN, Sarker MR, Paredes-Sabja D. Sporulation and Germination in Clostridial Pathogens. Microbiology spectrum. 2019 Nov:7(6):. doi: 10.1128/microbiolspec.GPP3-0017-2018doi: 10.1128/microbiolspec.gpp3-0017-2018. Epub [PubMed PMID: 31858953]
Abecasis AB, Serrano M, Alves R, Quintais L, Pereira-Leal JB, Henriques AO. A genomic signature and the identification of new sporulation genes. Journal of bacteriology. 2013 May:195(9):2101-15. doi: 10.1128/JB.02110-12. Epub 2013 Feb 8 [PubMed PMID: 23396918]
Fleck-Derderian S, Shankar M, Rao AK, Chatham-Stephens K, Adjei S, Sobel J, Meltzer MI, Meaney-Delman D, Pillai SK. The Epidemiology of Foodborne Botulism Outbreaks: A Systematic Review. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017 Dec 27:66(suppl_1):S73-S81. doi: 10.1093/cid/cix846. Epub [PubMed PMID: 29293934]
Level 1 (high-level) evidenceWoodruff BA, Griffin PM, McCroskey LM, Smart JF, Wainwright RB, Bryant RG, Hutwagner LC, Hatheway CL. Clinical and laboratory comparison of botulism from toxin types A, B, and E in the United States, 1975-1988. The Journal of infectious diseases. 1992 Dec:166(6):1281-6 [PubMed PMID: 1431246]
Czerwiński M, Czarkowski MP, Kondej B. Foodborne botulism in Poland in 2016. Przeglad epidemiologiczny. 2018:72(2):149-155 [PubMed PMID: 30111083]
Rao AK, Lin NH, Jackson KA, Mody RK, Griffin PM. Clinical Characteristics and Ancillary Test Results Among Patients With Botulism-United States, 2002-2015. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017 Dec 27:66(suppl_1):S4-S10. doi: 10.1093/cid/cix935. Epub [PubMed PMID: 29293936]
Ni SA, Brady MF. Botulism Antitoxin. StatPearls. 2024 Jan:(): [PubMed PMID: 30521228]
Jackson KA, Mahon BE, Copeland J, Fagan RP. Botulism mortality in the USA, 1975-2009. The botulinum journal. 2015:3(1):6-17. doi: 10.1504/TBJ.2015.078132. Epub 2016 Aug 4 [PubMed PMID: 28603554]
Harris RA, Tchao C, Prystajecky N, Weedmark K, Tcholakov Y, Lefebvre M, Austin JW. Foodborne Botulism, Canada, 2006-2021(1). Emerging infectious diseases. 2023 Sep:29(9):1730-7. doi: 10.3201/eid2909.230409. Epub [PubMed PMID: 37610295]
Peñuelas M, Guerrero-Vadillo M, Valdezate S, Zamora MJ, Leon-Gomez I, Flores-Cuéllar Á, Carrasco G, Díaz-García O, Varela C. Botulism in Spain: Epidemiology and Outcomes of Antitoxin Treatment, 1997-2019. Toxins. 2022 Dec 20:15(1):. doi: 10.3390/toxins15010002. Epub 2022 Dec 20 [PubMed PMID: 36668823]
Rao AK, Sobel J, Chatham-Stephens K, Luquez C. Clinical Guidelines for Diagnosis and Treatment of Botulism, 2021. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2021 May 7:70(2):1-30. doi: 10.15585/mmwr.rr7002a1. Epub 2021 May 7 [PubMed PMID: 33956777]
Nigam PK, Nigam A. Botulinum toxin. Indian journal of dermatology. 2010:55(1):8-14. doi: 10.4103/0019-5154.60343. Epub [PubMed PMID: 20418969]
Raza N, Dhital S, Espinoza VE, Jariwal R, Chiu CW, Valdez M, Heidari A, Petersen G, Sabetian K. Wound Botulism in Black Tar Heroin Injecting Users: A Case Series. Journal of investigative medicine high impact case reports. 2021 Jan-Dec:9():23247096211028078. doi: 10.1177/23247096211028078. Epub [PubMed PMID: 34259080]
Level 2 (mid-level) evidenceChatham-Stephens K, Fleck-Derderian S, Johnson SD, Sobel J, Rao AK, Meaney-Delman D. Clinical Features of Foodborne and Wound Botulism: A Systematic Review of the Literature, 1932-2015. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017 Dec 27:66(suppl_1):S11-S16. doi: 10.1093/cid/cix811. Epub [PubMed PMID: 29293923]
Level 1 (high-level) evidenceRao AK, Lin NH, Griese SE, Chatham-Stephens K, Badell ML, Sobel J. Clinical Criteria to Trigger Suspicion for Botulism: An Evidence-Based Tool to Facilitate Timely Recognition of Suspected Cases During Sporadic Events and Outbreaks. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017 Dec 27:66(suppl_1):S38-S42. doi: 10.1093/cid/cix814. Epub [PubMed PMID: 29293926]
Level 3 (low-level) evidenceLe Maréchal C, Fourour S, Ballan V, Rouxel S, Souillard R, Chemaly M. Detection of Clostridium botulinum group III in environmental samples from farms by real-time PCR using four commercial DNA extraction kits. BMC research notes. 2018 Jul 4:11(1):441. doi: 10.1186/s13104-018-3549-5. Epub 2018 Jul 4 [PubMed PMID: 29973253]
Przedpelski A, Tepp WH, Zuverink M, Johnson EA, Pellet S, Barbieri JT. Enhancing toxin-based vaccines against botulism. Vaccine. 2018 Feb 1:36(6):827-832. doi: 10.1016/j.vaccine.2017.12.064. Epub 2018 Jan 4 [PubMed PMID: 29307477]
Sobel J, Rao AK. Making the Best of the Evidence: Toward National Clinical Guidelines for Botulism. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017 Dec 27:66(suppl_1):S1-S3. doi: 10.1093/cid/cix829. Epub [PubMed PMID: 29293933]
Witoonpanich R, Vichayanrat E, Tantisiriwit K, Wongtanate M, Sucharitchan N, Oranrigsupak P, Chuesuwan A, Nakarawat W, Tima A, Suwatcharangkoon S, Ingsathit A, Rattanasiri S, Wananukul W. Survival analysis for respiratory failure in patients with food-borne botulism. Clinical toxicology (Philadelphia, Pa.). 2010 Mar:48(3):177-83. doi: 10.3109/15563651003596113. Epub [PubMed PMID: 20184431]
