Definition/Introduction
The cornea is a smooth, clear structure at the front of the eye. It functions to (1) shield the eye from foreign substances and (2) help control visual focus.[1][2] To focus light, the cornea must be clear; therefore, it has no blood vessels to impede light refraction. Tears and the aqueous humor of the eye nourish it (fluid in the anterior part of the eye between the cornea and the pupil and iris).[1]
The cornea is composed of 5 layers: epithelium, Bowman’s layer, stroma, Descemet’s membrane, and endothelium (Figure 1A).[1] The epithelium is the outermost layer of the cornea (light blue, Figure 1A). It provides a smooth surface that absorbs both oxygen from the environment and nutrients from tears to supply the other layers of the cornea. It also functions as a barrier to foreign substances. Bowman’s layer (deep to the epithelium) is comprised of collagen to provide strength and structure. If injured, this layer can scar and impede vision (dark blue, Figure 1A). The stroma is the thickest layer of the cornea. Most of this layer is water with small amounts of collagen (gray, Figure 1A). Deep to the stroma, another strong layer of collagen, Descemet’s membrane, provides a secondary layer against injuries and infections. This layer is created by the deeper endothelial cells and can regenerate if injured (orange, Figure 1A). The deepest layer is the endothelium (pink, Figure 1A). The endothelium serves as a pump to remove excess fluid out of the stroma. The fluid typically leaks from inside the eye into the stroma. Without the endothelial pump, the stroma swells. Because endothelium cells do not regenerate if damaged, damage can cause blindness. The only therapy for endothelial damage is a corneal transplant.[1]
The cornea of the human eye is the most densely innervated part of the body, 300 to 600 times more sensitive than skin.[3] It receives sensory innervation from the long ciliary branches of the ophthalmic division of the trigeminal nerve (V).[2] Branches from these nerves form nerve plexuses running parallel to the surface of the cornea between the epithelium and Bowman’s layers.[4][5] Sensory nerve fibers within the cornea include myelinated A-beta and A-gamma nerves and unmyelinated C fibers.[6][2]
Corneal Reflex
The cornea is the first substance irritants or foreign objects will touch when they come in contact with the eye. Contact with the cornea initiates 2 reflexes: blink reflex (corneal reflex) and tear production. The corneal blink reflex is caused by a loop between the trigeminal sensory nerves and the facial motor (VII) nerve innervation of the orbicularis oculi muscles. The reflex activates when a sensory stimulus contacts either free nerve endings or mechanoreceptors within the epithelium of the cornea. Sensory information transmits through the ophthalmic division of the trigeminal nerve (V) to synapse within the spinal trigeminal nucleus in the brainstem (blue pathway, Figure 1B). The contacted nerve within the spinal trigeminal nucleus then projects to the facial nucleus and synapses with the facial nerve (purple pathway, Figure 1B). The facial nerve exits the facial nucleus, wraps around the abducens nucleus, and exits the skull at the stylomastoid foramen. After exiting the skull, the facial nerve travels medially over the surface of the face to activate the orbicularis oculi muscle. Contraction of this muscle causes a blink movement (i.e., eye closure; red pathway; Figure 1B).
There are 2 stages to the blink reflex, early and late. A-beta fibers activate the initial movement of the eyelid (early response) on the ipsilateral side. Other fiber types activate the late stage. The late-stage reflex stimulates facial nerves bilaterally so that both eyes blink. Input from the secondary motor system (e.g., interpositus nucleus of the cerebellum, red nucleus, and reticular activating system) can modulate this late stage reflex.[7][8][9][10][11][12][13][6]
The motor output of each blink can vary in non-pathological conditions. Many factors can influence the blink response. One factor is the duration and intensity of the sensory stimulus.[9] Secondary factors include a variety of brainstem and cortical circuits that synapse directly or indirectly with the trigeminal spinal and facial nuclei. These other circuits modulate the strength of blink activity based on conditioning.[14][15]
The blink response causes the eyelid to close. This system works together with a secondary circuit pathway through the oculomotor nucleus to re-open the eyelid. This secondary pathway is activated by the same touch pathway used by the blink response (Trigeminal V1). To facilitate this response, V1 nerves have secondary inputs within the trigeminal spinal nucleus onto nerves that project to the oculomotor nucleus. These spinal nucleus/oculomotor neurons synapse onto oculomotor neurons that then activate the levator palpebrae. The oculomotor pathway initiates the eyelid to re-open after it closes in the blink response. It works in conjunction with the facial motor pathway.[9]
Tear Production
Touching the cornea can also activate circuits that initiate tear production. This reflex circuit follows the same pathway as the blink reflex except that the parasympathetic nerves within the facial VII nerve innervates the lacrimal and meibomian “tarsal” glands instead of muscle (green circuit; Figure 1B).[16] The lacrimal gland produces tears. The Meibomian gland produces an oily film that helps to prevent evaporation of tears over the eye.[17] To reach these glands, parasympathetic fibers travel through the zygomatic branch of the facial VII nerve. They synapse in the pterygopalatine ganglion and then continue as the lacrimal nerve.
Tears have several functions.[16]
- Form smooth optical surface
- Keep cornea surface moist
- Wash away debris or irritants
- Have antibacterial properties that help to prevent infection
The corneal blink reflex is not the only reflex that can trigger tear production. Certain visual and taste reflexes can also stimulate these glands. Bright lights trigger visual reflexes. Specific chemicals that may be specifically noxious if they entered the eye (e.g., peppery, spicy, or hot foods) activate taste reflexes. Both systems contact parasympathetics that generate tear production. [16]
Issues of Concern
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Issues of Concern
Several disorders and environmental substances can influence the corneal blink reflex by altering the anatomy of the trigeminal nerve within the cornea. Glaucoma, for example, is a disorder that induces increased pressure in the eye. As this disorder progresses, trigeminal nerves in the cornea bend. These bends correlate with progressive ocular symptomology and decreased corneal blink reflex sensitivity.[2] Research into glaucoma treatments shows that patients prescribed topical medications that include benzalkonium chloride have a much higher prevalence of bending than patient-prescribed preservative-free medications.[18][19][20] Physicians should take care when prescribing topical medications for the eye because their choice can significantly impact the prognosis of their patients.
Diabetes is another disorder that impacts corneal health, resulting in peripheral neuropathy in many patients. The unmyelinated C fibers and small A-gamma nerve fibers are most commonly affected. Research has shown that as the disease progresses, there are decreases in the density of nerve plexuses, decreased nerve branching, and increases in bending of the nerve fibers.[21][22] These anatomical changes result in a loss of pain and temperature sensation for the eye which leads to decreases in the late phase of the corneal reflex bilaterally.[23][24][25]
A disorder that influences the tear production circuits is Bogorad’s syndrome, “Crocodile tears syndrome.” This syndrome is resultant from improper connections that form during nerve regeneration in patients recovering from Bell’s palsy (i.e., damage to the facial nerve, VII). During recovery, efferent fibers from the superior salivary nucleus synapse with parasympathetic fibers of the facial nerve. This causes the patient to produce tears during salivation (e.g., when smelling or eating foods).[26]
Clinical Significance
Dysfunction of the corneal “blink” reflex can occur from damage at any point in the pathway (i.e., central or peripheral nervous system). Peripherally damage causes ipsilateral reflex deficits while central dysfunctions have bilateral deficits.
Peripherally, damage to either the trigeminal nerve (V) or facial nerve (VII) nerve will disrupt the corneal blink circuit. Physicians diagnose damage to peripheral nerves by examining either the early and late stages of the blink reflex or secondary symptoms of the nerve damage.[27][9][6] When examining the stages of the blink reflex, patients with trigeminal nerve (V) lesions often have early stage reflex deficits on the ipsilateral side, while patients with facial nerve (VII) lesions show ipsilateral deficits for both early and late stages.[28][6]
When examining secondary symptoms of nerve damage, physicians perform tests for motor and sensory function. The trigeminal nerve (V1) causes a lack of sensation for the forehead. To test V1 function, have the patient close their eyes and indicate when they feel a touch to different regions of the face. Patients with V1 damage do not feel or identify touch on the affected side of the forehead.
Facial nerve damage causes ipsilateral paresis of the muscles of facial expression. This results in drooping of the mouth and flattening of the forehead and nasolabial fold on the ipsilateral side.[29] To access motor activity of the face, ask the patient to smile or make other facial expressions. Patients with facial nerve damage cannot move the muscles of facial expression on the affected side.
Central nervous system dysfunction will cause deficits in the blink reflex bilaterally. Symptoms will present as dysfunction in the late stage but not the early stage of the blink reflex. A common lesion that influences the blink response is Wallenberg syndrome (i.e., lateral medulla lesion). The spinal trigeminal nucleus is located within the lateral medulla. Disruption of this area region interrupts the blink reflex pathway and results in a lack of late-stage reflexes bilaterally with corneal stimulation on the affected side.[30][6]
Damage to the reticular activating system, red nucleus, or cerebellum will also influence the corneal blink reflex.[6] These regions influence facial nerve signals and can impact the strength or speed of the late stage blink response bilaterally.[6][10][13][11][7][8][12][9]
Media
(Click Image to Enlarge)
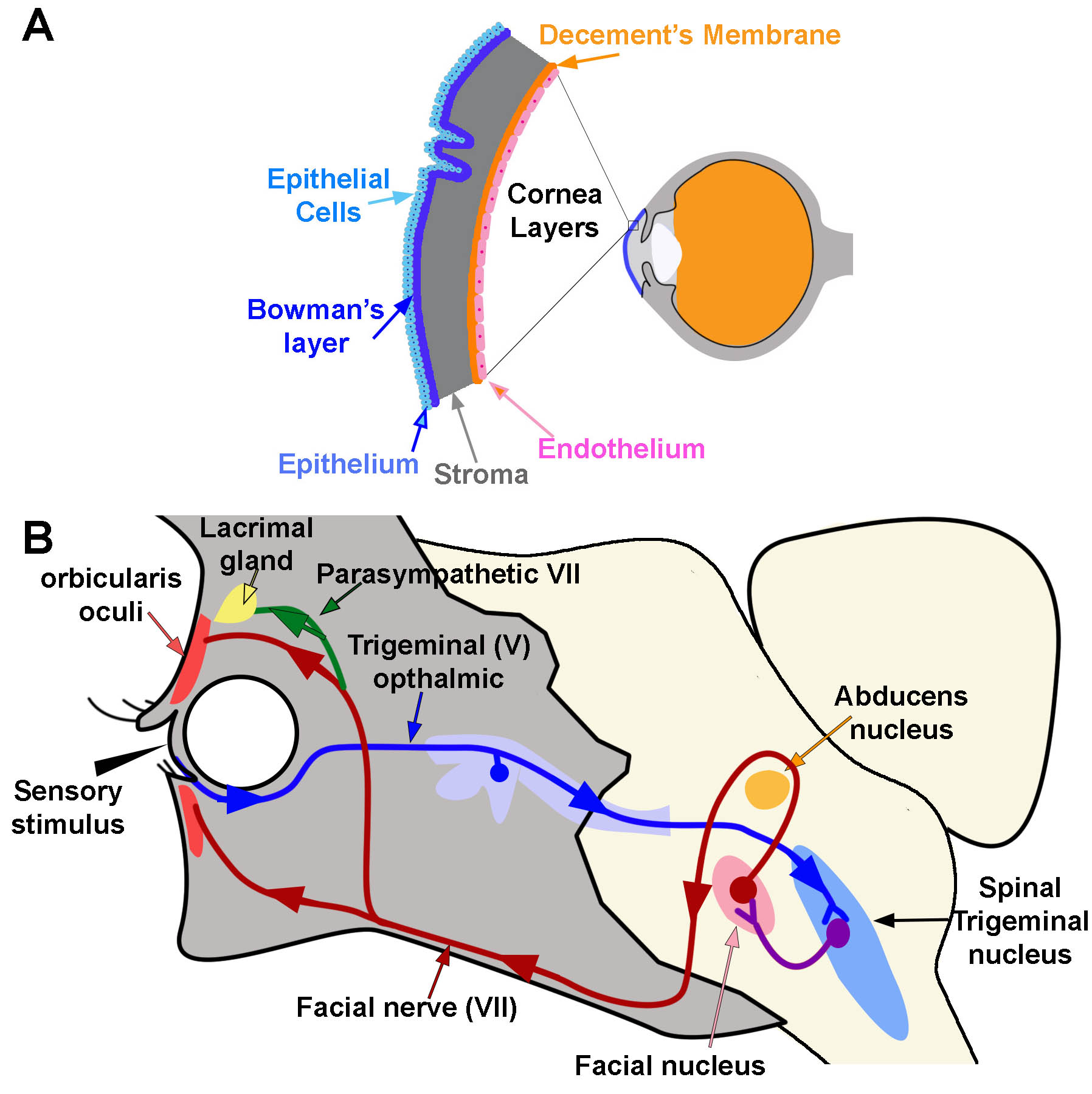
Corneal Reflex. A) Diagram of the location and layers of the cornea. B) Corneal Reflex. The blink reflex circuit is shown in blue, purple, and red. Arrows indicate the direction of flow. The tear production reflex utilizes the same pathways as the blink reflex. However it activates the lacrimal gland via parasympathetic branches (green line) Contributed and Created by Dr. Diana Peterson
References
Ludwig PE, Lopez MJ, Sevensma KE. Anatomy, Head and Neck, Eye Cornea. StatPearls. 2023 Jan:(): [PubMed PMID: 29262108]
Yang AY, Chow J, Liu J. Corneal Innervation and Sensation: The Eye and Beyond. The Yale journal of biology and medicine. 2018 Mar:91(1):13-21 [PubMed PMID: 29599653]
ZANDER E, WEDDELL G. Observations on the innervation of the cornea. Journal of anatomy. 1951 Jan:85(1):68-99 [PubMed PMID: 14814019]
Müller LJ, Marfurt CF, Kruse F, Tervo TM. Corneal nerves: structure, contents and function. Experimental eye research. 2003 May:76(5):521-42 [PubMed PMID: 12697417]
Level 3 (low-level) evidenceCruzat A, Qazi Y, Hamrah P. In Vivo Confocal Microscopy of Corneal Nerves in Health and Disease. The ocular surface. 2017 Jan:15(1):15-47. doi: 10.1016/j.jtos.2016.09.004. Epub 2016 Oct 19 [PubMed PMID: 27771327]
Casale R, Frazzitta G, Fundarò C, Balbi P, Del Rosso A, Bertinotti L, Matucci-Cerinic M. Blink reflex discloses CNS dysfunction in neurologically asymptomatic patients with systemic sclerosis. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2004 Aug:115(8):1917-20 [PubMed PMID: 15261870]
Fanardjian VV, Manvelyan LR. Peculiarities of cerebellar excitation of facial nucleus motoneurons. Neuroscience letters. 1984 Aug 31:49(3):265-70 [PubMed PMID: 6493608]
Level 3 (low-level) evidenceHolstege G, van Ham JJ, Tan J. Afferent projections to the orbicularis oculi motoneuronal cell group. An autoradiographical tracing study in the cat. Brain research. 1986 May 28:374(2):306-20 [PubMed PMID: 3719340]
Level 3 (low-level) evidenceManning KA, Evinger C. Different forms of blinks and their two-stage control. Experimental brain research. 1986:64(3):579-88 [PubMed PMID: 3803493]
Level 3 (low-level) evidenceWikgren J, Korhonen T. Interpositus nucleus inactivation reduces unconditioned response amplitude after paired but not explicitly unpaired treatment in rabbit eyeblink conditioning. Neuroscience letters. 2001 Aug 10:308(3):181-4 [PubMed PMID: 11479018]
Level 3 (low-level) evidenceDelgado-García JM, Gruart A. The role of interpositus nucleus in eyelid conditioned responses. Cerebellum (London, England). 2002 Dec:1(4):289-308 [PubMed PMID: 12879967]
Level 3 (low-level) evidenceMorcuende S, Delgado-Garcia JM, Ugolini G. Neuronal premotor networks involved in eyelid responses: retrograde transneuronal tracing with rabies virus from the orbicularis oculi muscle in the rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002 Oct 15:22(20):8808-18 [PubMed PMID: 12388587]
Level 3 (low-level) evidenceWikgren J, Ruusuvirta T, Korhonen T. Reflex facilitation during eyeblink conditioning and subsequent interpositus nucleus inactivation in the rabbit (Oryctolagus cuniculus). Behavioral neuroscience. 2002 Dec:116(6):1052-8 [PubMed PMID: 12492303]
Level 3 (low-level) evidenceFreeman JH, Steinmetz AB. Neural circuitry and plasticity mechanisms underlying delay eyeblink conditioning. Learning & memory (Cold Spring Harbor, N.Y.). 2011:18(10):666-77. doi: 10.1101/lm.2023011. Epub 2011 Oct 3 [PubMed PMID: 21969489]
Level 3 (low-level) evidenceDelgado-García JM, Gruart A. Building new motor responses: eyelid conditioning revisited. Trends in neurosciences. 2006 Jun:29(6):330-8 [PubMed PMID: 16713636]
Level 3 (low-level) evidenceShaheen BS, Bakir M, Jain S. Corneal nerves in health and disease. Survey of ophthalmology. 2014 May-Jun:59(3):263-85. doi: 10.1016/j.survophthal.2013.09.002. Epub 2014 Jan 23 [PubMed PMID: 24461367]
Level 3 (low-level) evidenceBron AJ, Tiffany JM, Gouveia SM, Yokoi N, Voon LW. Functional aspects of the tear film lipid layer. Experimental eye research. 2004 Mar:78(3):347-60 [PubMed PMID: 15106912]
Skalicky SE, Goldberg I, McCluskey P. Ocular surface disease and quality of life in patients with glaucoma. American journal of ophthalmology. 2012 Jan:153(1):1-9.e2. doi: 10.1016/j.ajo.2011.05.033. Epub 2011 Aug 26 [PubMed PMID: 21872203]
Level 2 (mid-level) evidenceFechtner RD, Godfrey DG, Budenz D, Stewart JA, Stewart WC, Jasek MC. Prevalence of ocular surface complaints in patients with glaucoma using topical intraocular pressure-lowering medications. Cornea. 2010 Jun:29(6):618-21. doi: 10.1097/ICO.0b013e3181c325b2. Epub [PubMed PMID: 20386433]
Rossi GC, Blini M, Scudeller L, Ricciardelli G, Depolo L, Amisano A, Bossolesi L, Pasinetti GM, Bianchi PE. Effect of preservative-free tafluprost on keratocytes, sub-basal nerves, and endothelium: a single-blind one-year confocal study on naïve or treated glaucoma and hypertensive patients versus a control group. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2013 Nov:29(9):821-5. doi: 10.1089/jop.2013.0069. Epub 2013 Aug 14 [PubMed PMID: 23944905]
Level 2 (mid-level) evidenceKallinikos P, Berhanu M, O'Donnell C, Boulton AJ, Efron N, Malik RA. Corneal nerve tortuosity in diabetic patients with neuropathy. Investigative ophthalmology & visual science. 2004 Feb:45(2):418-22 [PubMed PMID: 14744880]
Chang PY, Carrel H, Huang JS, Wang IJ, Hou YC, Chen WL, Wang JY, Hu FR. Decreased density of corneal basal epithelium and subbasal corneal nerve bundle changes in patients with diabetic retinopathy. American journal of ophthalmology. 2006 Sep:142(3):488-90 [PubMed PMID: 16935596]
Level 2 (mid-level) evidenceRosenberg ME, Tervo TM, Immonen IJ, Müller LJ, Grönhagen-Riska C, Vesaluoma MH. Corneal structure and sensitivity in type 1 diabetes mellitus. Investigative ophthalmology & visual science. 2000 Sep:41(10):2915-21 [PubMed PMID: 10967045]
Petropoulos IN, Green P, Chan AW, Alam U, Fadavi H, Marshall A, Asghar O, Efron N, Tavakoli M, Malik RA. Corneal confocal microscopy detects neuropathy in patients with type 1 diabetes without retinopathy or microalbuminuria. PloS one. 2015:10(4):e0123517. doi: 10.1371/journal.pone.0123517. Epub 2015 Apr 8 [PubMed PMID: 25853247]
Bitirgen G, Ozkagnici A, Malik RA, Kerimoglu H. Corneal nerve fibre damage precedes diabetic retinopathy in patients with type 2 diabetes mellitus. Diabetic medicine : a journal of the British Diabetic Association. 2014 Apr:31(4):431-8. doi: 10.1111/dme.12324. Epub 2013 Nov 5 [PubMed PMID: 24117485]
Level 2 (mid-level) evidenceModi P, Arsiwalla T. Crocodile Tears Syndrome. StatPearls. 2023 Jan:(): [PubMed PMID: 30247828]
Kimura J, Powers JM, Van Allen MW. Reflex response of orbicularis oculi muscle to supraorbital nerve stimulation. Study in normal subjects and in peripheral facial paresis. Archives of neurology. 1969 Aug:21(2):193-9 [PubMed PMID: 5797352]
Cruccu G, Deuschl G. The clinical use of brainstem reflexes and hand-muscle reflexes. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2000 Mar:111(3):371-87 [PubMed PMID: 10699396]
Portela RC, Miller AC. Antivirals With Corticosteroids for the Treatment of Acute Bell's Palsy. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2019 Mar:26(3):342-344. doi: 10.1111/acem.13563. Epub 2018 Oct 23 [PubMed PMID: 30182458]
Ongerboer de Visser BW, Kuypers HG. Late blink reflex changes in lateral medullary lesions. An electrophysiological and neuro-anatomical study of Wallenberg's Syndrome. Brain : a journal of neurology. 1978 Jun:101(2):285-94 [PubMed PMID: 667600]