Introduction
Crohn disease is a form of inflammatory bowel disease (IBD) like ulcerative colitis, though Crohn disease often presents more subtly. Crohn disease is an immunologically mediated inflammatory gastrointestinal condition, with pathology involving the entire thickness of the bowel wall.
The condition may involve any part of the gastrointestinal tract. Minnesota data show that 19% of patients present with stricturing or fistulizing disease within 90 days of diagnosis. About half of all patients experience an intestinal complication, such as fistulae, phlegmons, strictures, and abscesses, within 20 years of diagnosis. Population-based studies from Northern Europe and Minnesota suggest that Crohn disease presents with ileal, ileocolonic, or colonic involvement each third of the time, with disease migration occurring in only 6% to 14% of patients.[1] Pathology in the upper gastrointestinal tract, ileal, or ileocolonic region portends a greater stricturing and fistulizing risk compared to colonic involvement.[2] Crohn disease may also have extraintestinal manifestations, which often involve the eyes, skin, liver, and joints.
The disease runs a chronic and often progressive course. Frequent symptoms include diarrhea, abdominal pain, nausea, or vomiting. Weight loss, fever, and fatigue are systemic manifestations of this condition. Without treatment, longstanding inflammation can produce debilitating complications. Early diagnosis and management can help optimize quality of life and outcomes for patients with Crohn disease.[3][4][5][6][7]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
IBD's exact etiology is unknown. However, substantial evidence suggests that the condition may result from an inappropriate immune response to environmental antigens like drugs, toxins, infections, or intestinal microbes in a genetically susceptible host. Large-scale genome studies have identified over 200 IBD-associated genes and more than 71 Crohn disease–susceptibility loci.[8][9][10]
Genetic variants are associated with certain Crohn disease phenotypes. For example, NOD2/CARD15 mutations have been detected in patients with Crohn disease presenting with ileal involvement and increased severity at a younger age. These individuals often require surgical intervention. Genotyping is expected to provide prognostic information on disease severity in the future. Currently, genetic testing remains mostly a research tool.[11]
Epidemiology
Crohn disease is most commonly seen in North America, Northern Europe, and New Zealand. The condition has a bimodal distribution, with the onset occurring most frequently between ages 15 and 30 and 40 and 60. Crohn disease is more prominent in urban than rural areas. The condition has a high incidence in Northern Europeans and people of Jewish descent (incidence 3.2 per 1000 individuals). Prevalence in Asians, Africans, and South Americans is low.[12] However, recent studies have shown a significant increase in incidence in rapidly industrializing areas of Asia, Africa, and Australasia.[13]
Pathophysiology
Crohn disease is multifactorial. Genetic, infectious, immunological, environmental, and dietary factors contribute to the condition's development. The excessive immune response arises from innate and acquired mechanisms involving intestinal macrophages, neutrophils, and helper T-cells (Th), promoting proinflammatory mediators like tumor necrosis factor-α (TNF-α). Th1 and Th17 are crucial mediators in the Crohn disease inflammatory cascade. Colonic Crohn lesions were found to have high levels of cytokines like interferon-γ and interleukins (ILs) 2, 12, and 18.[14][15][16]
Inflammation in the initial stages of the illness gives rise to nonspecific symptoms such as fever and malaise. Diarrhea and abdominal pain arise from intestinal injury. Right lower quadrant (RLQ) pain may manifest due to ileocecal involvement. Terminal ileal damage can give rise to malabsorption and vitamin deficiencies. Anemia can arise from either vitamin B12 insufficiency or fecal blood loss, depending on which intestinal area is most involved. Extraction of inflammation can cause dysfunction of the surrounding organs due to fistulae formation.
Extraintestinal manifestations of Crohn disease are mostly due to systemic inflammation and include arthritis, uveitis, pericholangitis, and renal disorders. These conditions may appear before the intestinal manifestations. Systemic amyloidosis is a rare, late sequela.
Anatomy of the Small and Large Intestines
The small and large intestines comprise most of the gastrointestinal tract. The mucosal surfaces in these gut regions are designed for maximal absorption—of nutrients in the small bowel and water in the large bowel. Thus, more environmental antigens can enter the bloodstream through the gut than the skin and pulmonary tract.
The small intestine is typically 6 meters long and divided into the duodenum, jejunum, and ileum. The duodenum is retroperitoneal, while the jejunum and ileum are intraperitoneal. The ileum terminates in the ileocecal valve.
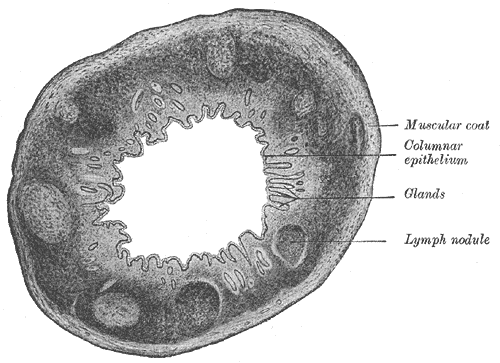
The mucosal surface of the small bowel consists of columnar absorptive epithelium and goblet, endocrine, and Paneth cells. Columnar cells occupying most of the small intestinal mucosa have numerous villi for absorbing nutrients. Under the microscope, these villi resemble a brush. Thus, this part of the small bowel luminal surface is called the "brush border." Each villus' core houses a portion of the lamina propria, which contains blood and lymph vessels, leukocytes, fibroblasts, and smooth muscle cells. The crypts of Lieberkuhn (or simply "crypts") are the furrows between the villi's bases extending as deep as the muscularis mucosa. The normal villus-to-crypt height is 4 to 5.1:1.
Goblet, endocrine, and Paneth cells are scattered between the columnar cells and may be found in the crypts. Goblet cells secrete mucin for gut protection and lubrication. Gut endocrine cells secrete various peptides that regulate the digestive process. Paneth cells produce antimicrobial proteins like defensins. The crypts also contain stem cells, which replace damaged or sloughing luminal cells.
In the duodenum, the submucosa contains Brenner glands. These glands secrete mucus, bicarbonate, glycoproteins, and pepsinogen II. Microscopically, Brenner glands resemble pyloric mucous glands.
The muscularis mucosa contains smooth muscle sheets anchoring the absorptive villi and crypts. The smooth muscle cells in this area facilitate villous folding and unfolding to maximize the gut absorptive surface.
The large intestine (colon) is normally about 1.5 meters long and comprises the cecum and the ascending, transverse, and descending colon. The sigmoid colon extends from the pelvic brim, traverses within the peritoneum, and becomes the rectum at the S3 vertebral level. The distal rectum is extraperitoneal.
The colon's mucosal surface is lined mainly by columnar absorptive cells with shorter villi than small intestinal epithelial cells. The colonic crypts also contain goblet, endocrine, Paneth, and stem cells.
Both small and large intestinal mucosae have great regenerative capacities, with complete cell turnover occurring within a week. Thus, these gut segments have remarkable reparative abilities but are also prone to cytotoxic damage from cancer therapies.
Bacteria colonize the small and large bowel mucosae. Colonization is highest at the ileum. Nonpathogenic gram-negative bacterial strains, eg, nonpathogenic E coli, typically comprise the intestinal flora.
Mucosal and submucosal lymphoid tissue nodules are scattered throughout the intestines. In the ileum, these nodules form the Peyer patches. M cells in the surface epithelium covering the lymph nodules can engulf antigens intact from the intestinal lumen to the antigen-presenting cells beneath the mucosal surface. Antigen-presenting cells consist of macrophages and dendritic cells. T lymphocytes, activated B cells, and plasma cells are scattered in the lamina propria. Immunoglobulins are also scattered in the lamina propria, but the most abundant is immunoglobulin A.
The outer muscular layers of the intestines are responsible for peristalsis. The myenteric plexus regulates movements in these layers. The serosal layer consists primarily of collagen and elastic fibers. A layer of mesothelial cells covers the serosa, secreting a serous fluid that lubricates the outer intestinal surfaces (see Image. Large Intestine Transverse Section).
Histopathology
Crohn disease's characteristic transmural inflammation can occur in the entire gastrointestinal tract from the mouth to the perianal area. However, the condition most frequently affects the terminal ileum and right colon.
The initial lesion starts as an infiltrate around an intestinal crypt. Ulceration then develops, initially involving only the superficial mucosa but later growing into the deeper layers. Noncaseating granulomas develop with continuing inflammation, spreading toward the intestinal wall. Granuloma formation is seen in up to 33% of patients with Crohn disease, but their absence does not exclude the diagnosis.
The classic mucosal cobblestone appearance with skip lesions develops along the length of the bowel and intervening areas of normal mucosa. Resolution of acute episodes results in scarring in previously inflamed intestinal areas.[17] Repeated inflammation-and-scarring cycles can lead to stricture formation and bowel obstruction. Inflammation can spread to adjacent organs, which may manifest as enterovesicular, enteroenteral, enterocutaneous, and enterovaginal fistulae.
History and Physical
History
Crohn disease manifests variably, depending on the area involved. Patients initially report recurrent episodes of mild abdominal pain, diarrhea, flatulence, and fever with intervening asymptomatic periods lasting weeks to months. Emotional or physical stress often precipitates the "flare" or "flare-up" symptoms. More serious, localizing signs start to appear after repeated flare-ups. A family history of IBD may or may not be reported.
Ileocolic involvement
Patients with ileocolitis typically report recurrent RLQ pain and diarrhea. The pain may be described as colicky and relieved by defecation. Crohn disease may present similarly to acute appendicitis, which also typically has fever, diarrhea, and RLQ pain as symptoms. An inflammatory RLQ mass may also be palpated in patients with CD.
A low-grade fever is more typical of Crohn disease. A different pathology, such as an intraabdominal abscess, must be suspected in the presence of a high-grade fever. Weight loss often develops due to diarrhea and fear of eating.
Years of recurrent inflammation typically lead to fibrostenosis and stricture formation in the ileocecal area. Diarrhea is later replaced by chronic bowel obstruction. Ileocecal wall thinning eventually occurs, leading to microperforation and fistula formation into nearby organs. Dysuria, pneumaturia, and recurrent urinary tract infections are often due to enterovesical fistulae. Enterocutaneous fistulae may manifest as surgical scar drainage, as surgical scars are relatively weaker areas than intact skin. Dyspareunia and feculent vaginal discharge may signify an enterovaginal fistula.
Jejunoileal disease
Involvement of these small bowel regions often causes malabsorption and steatorrhea. Nutritional deficiencies consequently arise, compounded by poor eating. Nutrients that may be lost to chronic diarrhea include iron, albumin, calcium, magnesium, fat-soluble vitamins (A, D, E, and K), niacin, folic acid, vitamin B12, and trace minerals like zinc, selenium, and copper. Patients may present with symptoms attributable to insufficient levels of specific nutrients. Individuals with jejunoileal pathology may also develop fistulae and electrolyte imbalance.
Colitis with perianal involvement
More characteristic of this condition are manifestations like hematochezia, pain on defecation, reduced rectal wall elasticity, incontinence, anorectal pain and fistulae, perirectal abscesses, anal strictures, and hemorrhoidal tags. Bowel obstruction may result from colonic stricturing, which may warrant surgery. Fistulization into the stomach may cause feculent emesis. Fistulization into the small bowel may manifest as bacterial overgrowth and malabsorption. Rectovaginal fistula can also develop in women with this condition.
Gastroduodenal pathology
Upper gastrointestinal tract symptoms are more common in patients with this condition than in the other forms of Crohn disease. Such symptoms include emesis, nausea, and epigastric pain. Gastric ulcer diagnostic workups are likely to reveal the absence of H pylori. Upper gastrointestinal obstruction is common. Children may develop esophageal involvement.
Extraintestinal manifestations
Crohn disease is associated with extraintestinal manifestations involving various organs. These conditions include the following:[18]
- Eyes: episcleritis, scleritis, uveitis
- Mouth: stomatitis, aphthous ulcers
- Liver: gallstones, cholangitis, primary sclerosing cholangitis
- Kidneys: nephrolithiasis, hydronephrosis, urinary tract infections
- Joints: axial (ankylosing spondylitis) or peripheral (knees, ankles, wrists, elbows) arthritis
- Skin: erythema nodosum and pyoderma gangrenosum
Crohn disease also induces a hypercoagulable state, increasing the risk of thromboembolic disease.[19] Decreased mobility in hospitalized patients may result in deep vein thrombosis, stroke, or pulmonary embolism.
Physical Examination
A thorough physical examination with a detailed abdominal assessment is crucial for patients with abdominal complaints. General assessment in individuals with Crohn disease may reveal signs of malnutrition like pallor and low weight. Vital signs assessment of these patients may reveal fever, tachycardia, and hypotension.
Abdominal inspection may reveal enterocutaneous fistulae in patients with advanced disease. Hyperactive bowel sounds may indicate inflammation or obstruction, while absent bowel sounds may suggest ileus or severe inflammation. Palpation may reveal tenderness localizing to the intestinal area involved. Guarding is a sign of peritonitis in individuals with intraabdominal perforation or abscess. Individuals with advanced perianal disease may have perianal fistulae or hemorrhoids, necessitating a digital rectal examination.
The skin, eyes, oral cavity, and joints must be examined for extraintestinal manifestations of Crohn disease. Liver involvement may manifest with right upper quadrant tenderness and jaundice. Renal damage must be suspected in the presence of flank tenderness and genitourinary abnormalities like genital fistulae and perineal skin tags. Female patients with gynecological complaints must undergo a pelvic examination.
Neurologic deficits may be elicited in patients with vitamin deficiencies. Diminished sensation, muscle atrophy, and gait abnormalities may be observed.
Overall, a thorough physical examination of patients with Crohn disease can help identify active inflammation, assess disease severity, detect complications, and guide further diagnostic evaluation and management.
Evaluation
Laboratory Tests
Stool tests to rule out infections include culture and sensitivities, ovum and parasites, and C. difficile toxins. Stool calprotectin can detect active Crohn disease and is used for monitoring disease activity.[20][21][22][23]
Blood tests, including a complete blood count and a metabolic panel, may reveal anemia (due to vitamin B12 or iron deficiency) and liver disease. Electrolytes may be deranged due to diarrhea. Increased creatinine, blood urea nitrogen, and liver enzymes may indicate renal and liver involvement, respectively. Urinalysis may reveal bacteriuria and leukocyturia. Specific nutrient deficiencies, eg, iron and calcium, may be documented by taking serum levels if the test results can be used to correct the problem.
Special serologic tests, such as antineutrophil cytoplasmic and anti-Saccharomyces cerevisiae antibodies, are not routinely indicated to distinguish Crohn disease from ulcerative colitis. C-reactive protein (CRP) or erythrocyte sedimentary rate elevation may reflect inflammation severity.[24][25]
Imaging
Plain x-rays should be ordered if bowel obstruction is suspected. Small bowel follow-through is often used to assess terminal ileal involvement. This modality can also detect fistulas. The classic string sign due to stricture formation or spasm is often seen.
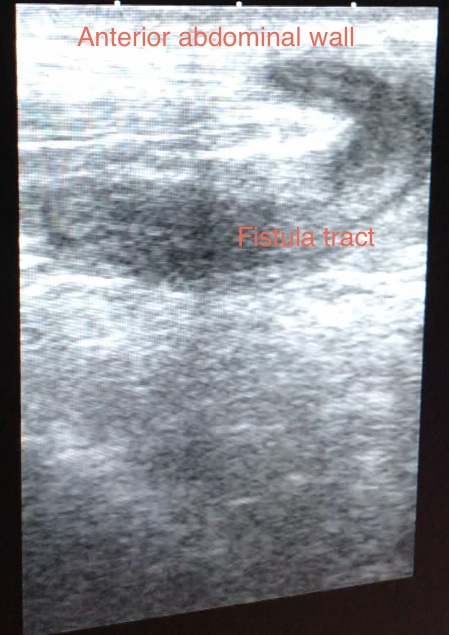
Gastroenterologists may perform a quick abdominal ultrasound during flare-ups or monitoring of treatment response (see Image. Periumbilical Fistula Ultrasonography). Ultrasound does not pose a radiation risk and is widely available, though the image may not have good resolution. Features that may be appreciated using this modality include fistulae, free intraperitoneal fluid, abscess formation, and increased superior mesenteric artery flow. The superior mesenteric artery often has increased flow volume during active disease that may be documented by Doppler ultrasound.
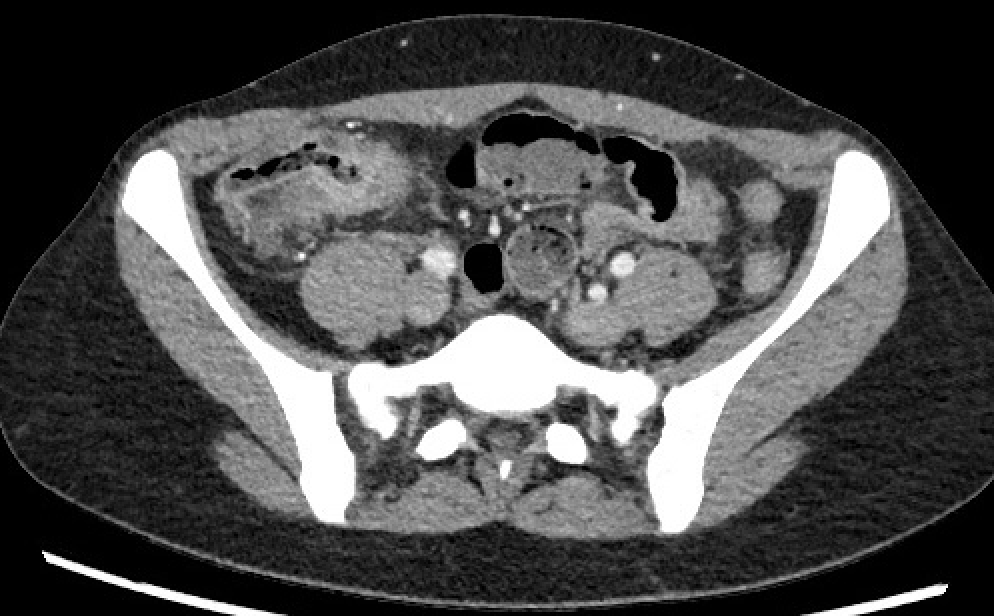
Abdominal and pelvic computed tomography (CT), magnetic resonance imaging (MRI), or enterography (MRE) can detect abscesses, strictures, and fistulization (see Image. Crohn Disease on Computed Tomography). Both give clearer images of the diseased intestine. However, MRI can provide more detail when investigating fistulizing disease. Additionally, MRI is preferable over CT in pediatric populations, as it emanates little ionizing radiation.
The bowel mucosa may be visualized by upper endoscopy and colonoscopy. Additionally, these modalities permit the assessment of the extent of bowel inflammation and tissue sample collection for disease confirmation. Endoscopic evaluation is also useful for assessing treatment response.
Video capsule endoscopy (VCE) can visualize the small bowel in patients with Crohn disease when regular endoscopy or colonoscopy cannot reach these areas. However, caution is advised in using VCE in the setting of known stricturing disease due to the potential for capsule retention within the strictured segment. Capsule endoscopy can only detect mucosal changes. In contrast, MRI can detect transmural inflammation and other complications.
Miscellaneous Tests
Crohn disease treatments suppress the immune system. The patient's vaccination history should thus be known before initiating the regimen. Vaccines to inquire about include tetanus, diphtheria, pertussis, human papillomavirus, influenza, pneumococcal, hepatitis A, hepatitis B, measles, mumps, rubella, varicella-zoster virus, and severe acute respiratory syndrome coronavirus 2.
Obtain a baseline tuberculosis screen, particularly in patients initiating anti-TNF biologics. Screening for latent tuberculosis may include a combination of history, tuberculin skin test, interferon-γ release assay, and chest radiography.[26]
Baseline thiopurine methyltransferase (TPMT) levels should be checked before deciding on treatment options. Low TPMT levels may increase the risk of side effects, while very high levels may decrease the effectiveness of prescribed treatment.
Treatment / Management
Medical Management
Medical treatment of Crohn disease is typically divided into the induction and maintenance phases. During induction, inflammation control is ideally achieved within 3 months. The maintenance phase aims to prolong the asymptomatic phase.
The choice of medical therapy is based on the patient's risk profile and disease severity.
- Mild-to-moderate disease can be treated by oral mesalamine, immunomodulators like thiopurines (6-mercaptopurine, azathioprine), methotrexate, and steroids.
- Mesalamines (5-ASAs) and sulfasalazine (5-ASA with a sulfapyridine moiety) exert an anti-inflammatory effect. The use of 5-ASAs is not as well-established in Crohn disease as in ulcerative colitis. While these medications may be tried in mild colonic Crohn disease, use in small bowel disease is not effective.[27]
- Corticosteroids are primarily used to induce Crohn disease flare remission and stabilize the condition until immunomodulator or biologic therapy takes effect, particularly in moderate-to-severe Crohn disease. However, prolonged corticosteroid use, eg, for maintenance, is avoided due to chronic side effects like osteoporosis, osteonecrosis, and adrenal insufficiency.
- Immunomodulators are steroid-sparing drugs effective in the maintenance therapy of moderate Crohn disease. These medications have a relatively slow action onset—between 8 to 12 weeks—and are thus not used for active Crohn disease induction remission. However, immunomodulators may be initially combined with steroids.[28][29][30][31] Dosages for Crohn disease are azathioprine 1.5 to 2.5 mg/kg/day, 6-mercaptopurine 0.75 to 1.5 mg/kg day, and methotrexate (MTX) 15 to 25 mg once weekly. TPMT is the main enzyme that inactivates toxic thiopurine metabolites. A low TPMT level can increase the risk of side effects, while a high level can reduce treatment potency.[32] MTX is teratogenic and may affect spermatogenesis. Thus, women of childbearing age and men should be counseled to use contraception within 3 months of MTX use.
- Moderate-to-severe disease (including fistulizing disease) is best treated with combined immunomodulators and biologics or biologics alone.
- Corticosteroids are primarily used to induce remission in moderate-to-severe Crohn disease and should be used sparingly to avoid side effects.
- Immunomodulators serve as adjuncts that reduce immunogenicity against biologics. Notably, combination therapy with an immunomodulator and anti-TNF is more effective than monotherapy with either medication.[33]
- Anti-TNF agents (infliximab, adalimumab, and certolizumab pegol) block the downstream effects of the TNF inflammatory cascade. These agents are efficacious in steroid-resistant or immunomodulator-refractory Crohn disease. The effect onset is relatively rapid, with clinical benefit seen within 2 weeks of initiation. Anti-TNF drugs are effective in treating fistulizing disease and reducing the risk of postsurgical endoscopic recurrence.[34] However, caution is advised when using these agents in patients with a history of demyelinating disease, congestive heart failure, and malignancies like lymphoma.[35] Recent data demonstrate no associated increase in adverse maternal-fetal outcomes in pregnant patients exposed to anti-TNF therapy.[36]
- Leukocyte trafficking agents selectively inhibit an adhesion protein (integrin α4β7) on a memory T cell subset, preventing the T cells' binding to gut mucosal cells. This action mechanism produces gut-specific anti-inflammatory effects. Vedolizumab is the agent in this class used in Crohn disease. This drug was found to be more effective than placebo in inducing and maintaining remission in Crohn disease, with or without immunomodulators.[37] However, the time to clinical effect may be slower (around 10 weeks) than anti-TNF agents, particularly in patients previously on anti-TNF therapy.[38] Gut-specific actions limit vedolizumab's toxicity. Thus, this drug's side effect profile is relatively favorable.
- IL-12/23 agents (ustekinumab, risankizumab) are efficacious in patients who failed prior corticosteroid, immunomodulator, or anti-TNF treatment.[39][40][41] Ustekinumab is a nonselective IL-12/23 inhibitor, while risankizumab is a selective IL-23 inhibitor. The side effect profile of these agents is relatively favorable according to safety surveillance data in psoriasis patients.[42]
- JAK inhibitors target Janus kinases that contribute to abnormal immune responses. Upadacitinib is the first FDA-approved oral selective JAK inhibitor therapy for Crohn disease. This medication is efficacious in patients who previously failed conventional and biologic Crohn disease therapies.[43] The onset of clinical effects is relatively rapid at 2 weeks. However, this drug increases Herpes Zoster risk. Thus, the shingles vaccine is recommended before treatment initiation. Use of upadacitinib during pregnancy is not advised due to findings of teratogenicity in animal studies.[44]
In 2021, the American Gastroenterology Association (AGA) published perianal and fistulizing Crohn disease management guidelines strongly recommending infliximab over no treatment for inducing and maintaining disease remission. This biologic is the only medication with dedicated randomized controlled trial (RCT) data demonstrating efficacy.[45] The AGA also strongly recommended combining biologic agents with an antibiotic instead of using biologic monotherapy for inducing fistula healing. This recommendation was based on 2 RCTs showing that anti-TNF agents combined with ciprofloxacin were more efficacious in inducing fistula remission than anti-TNF monotherapy.
Surgical Management
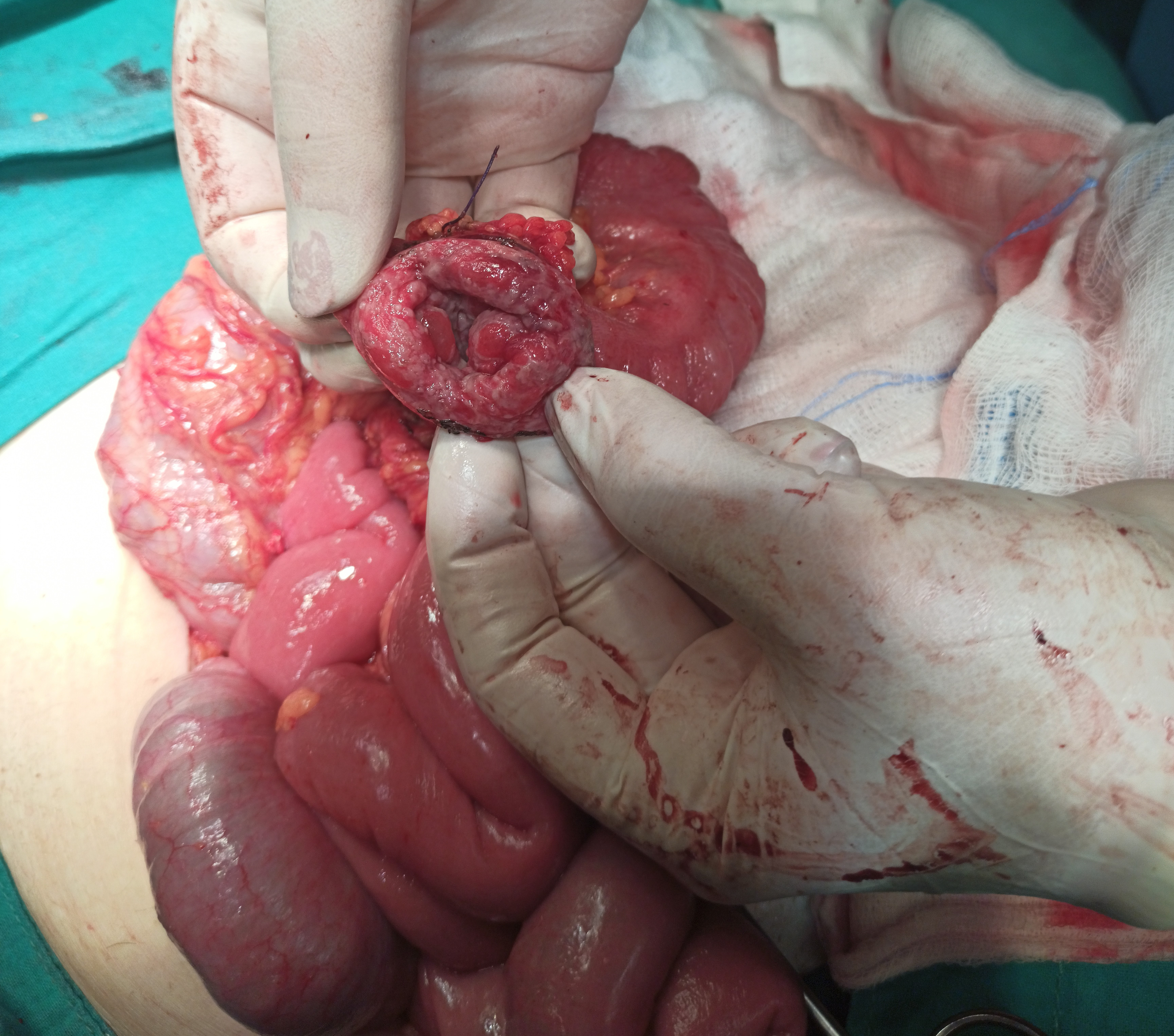
Surgery is warranted when Crohn disease complications arise, such as bowel obstruction from fibrostenotic strictures, fistulization despite appropriate medical therapy, recurrent abscesses, perforated bowel, dysplasia, cancer, or medically refractory disease. Surgical resection and stricturoplasty are options for managing fibrostenotic disease, the most common surgical indication (see Image. Subtotal Colectomy in Severe Crohn Colitis). Short segment strictures may be considered for endoscopic dilation.
Fistulotomy may be performed for simple fistulas, defined as superficial or low transsphincteric fistulas without associated proctitis. Complex fistulas may warrant chronic seton placement. Mesenchymal stem cell injection into the fistula pathway to reduce inflammation shows promise and is undergoing investigation.[46](B3)
The American Society of Colon and Rectal Surgeons recommends total colectomy with ileorectal anastomosis or total proctocolectomy for patients with Crohn colitis with dysplasia not amenable to endoscopic resection. Other recommended indications are multifocal dysplasia and colorectal cancer. The recommendation is based on the increased metachronous colorectal cancer risk (14-40%) and high multifocal dysplasia rates in patients with Crohn's colitis who underwent colectomy for low- or high-grade dysplasia.[47][48][49](A1)
A diverting ileostomy rather than primary anastomosis is usually considered when performing ileocolectomy in Crohn disease patients with multiple risk factors for an anastomotic leak. Such risk factors include smoking, steroid use, and weight loss.[47] (A1)
Residual postsurgical disease or high postsurgical recurrence risk warrants postoperative biologic therapy, eg, with an anti-TNF agent, 2 to 4 weeks after surgery if postoperative infections have been ruled out.[47] (A1)
Treatment Monitoring
The AGA recommended in 2023 that monitoring Crohn disease treatment response should be based on the presence of clinical symptoms and biomarker levels.[50] Biomarkers may be taken every 6 to 12 months in patients in remission and more frequently in those requiring therapy titration.(A1)
Suggested biomarkers are fecal calprotectin and serum CRP. Targets should be less than 150 μg/g for calprotectin and less than 5 mg/L for CRP to rule out active inflammation and avoid routine endoscopic evaluation of disease activity.
The AGA further recommended the following:
- Endoscopic or radiologic confirmation of disease remission within 3 years of symptomatic remission
- Biomarkers every 2 to 4 months to assess therapeutic response during active disease
A follow-up endoscopy should be performed 6 to 12 months after symptom resolution to document mucosal healing.
A fecal calprotectin of less than 50 μg/g is sufficient to rule out disease recurrence in patients who have remained asymptomatic 12 months after surgical remission. Routine endoscopic assessment is not required in these patients. The same biomarker and target may be used in asymptomatic individuals with low postoperative recurrence risk or higher postoperative recurrence risk but already on prophylactic medication. High-risk patients not on prophylactic medication should undergo endoscopic evaluation for disease activity assessment.
Dietary Management
Diet remains an adjunctive therapy. Dietitian input and nutritional supplementation are highly recommended during Crohn disease treatment, as symptomatic patients are at risk for malnutrition and micronutrient deficiencies. For example, individuals with a history of terminal ileum inflammation or resection are at higher risk for vitamin B12 deficiency.[51][52][53][54](B3)
Data predominantly from the pediatric population show that certain dietary therapies, such as elemental and semielemental diets, effectively reduce mucosal inflammation.[55] However, these diets have been largely unsuccessful in adults due to poor adherence. Benefits are also not durable due to inflammation recurrence after resuming a nonrestrictive diet.
Differential Diagnosis
The differential diagnoses to consider when assessing patients with possible Crohn disease are listed below. Documentation of travel and exposure history and judicious use of laboratory, imaging, and histologic tests help differentiate the above diagnoses.
- Infectious enteritis and terminal ileitis
- Coccidioides
- Histoplasma
- Salmonella
- Tuberculosis
- Yersinia
- Infectious colitis
- Amebiasis
- Campylobacter
- C.difficile
- Cytomegalovirus
- E.coli
- Salmonella
- Shigella
- Noninfectious
- Behcet disease
- Common variable immunodeficiency
- Diverticulitis
- Drug-induced colitis, eg, from nonsteroidal anti-inflammatory drugs and immunotherapy
- Ischemic colitis
- Sarcoidosis
- Segmental colitis associated with diverticulosis
- Small vessel vasculitis
- Solitary rectal ulcer syndrome
Prognosis
High-risk patients with moderate-to-severe Crohn disease often have the following characteristics:
- Relatively younger age, ie, younger than 30 years
- History of active or recent tobacco consumption
- Elevated CRP or fecal calprotectin levels
- Deep ulcers on colonoscopy
- Involvement of long bowel segments
- Perianal disease
- Extraintestinal manifestations
- History of bowel resections [56][57]
Without immunomodulator or biologic therapy, up to 50% of patients may become steroid-dependent or treatment-resistant, with a cumulative incidence of abdominal surgery of 46.6% 10 years after diagnosis.[58] The postsurgical endoscopic recurrence risk at or above the surgical anastomosis is around 90%. Risk factors for early recurrence include cigarette smoking, short duration between Crohn disease diagnosis to the first surgery, need for multiple resections, and penetrating disease.[59] Surveillance endoscopy is recommended in 6 to 12 months once a patient has had surgery. Ileocolonoscopy should be repeated in 1 to 3 years without endoscopic recurrence.
Prior meta-analyses show a slight mortality risk in patients with Crohn disease, with a standardized pooled mortality ratio of 1.4 to 1.5 compared to the general population. This outcome is attributable to gastrointestinal disease, gastrointestinal cancer, and pulmonary disease.[60][61] Current corticosteroid use is further associated with increased mortality.[62]
Complications
Crohn disease is a systemic illness with many intestinal and extraintestinal manifestations. Some of the complications associated with this condition are listed below. Early detection and treatment adherence can reduce the risk of these complications.
- Stricture formation
- Fistulae and abscesses
- Colorectal carcinoma
- Ankylosing spondylitis
- Episcleritis, iritis
- Erythema nodosum, pyoderma gangrenosum
- Nephrolithiasis
- Cholelithiasis
- Anemia
- Hypercoagulable state
- Osteoporosis
- Osteonecrosis
- Macro and micronutrient deficiencies
- Infections
Deterrence and Patient Education
Preventing Crohn disease is challenging because the exact cause of this condition remains unknown. However, the strategies below may help reduce the risk of developing Crohn disease or mitigate its severity:
- Genetic counseling: Individuals with a family history of Crohn disease may benefit from genetic counseling to understand their risk of developing the condition and discuss potential preventive measures or screening options.
- Early detection and treatment of symptoms: Being vigilant about recognizing and promptly addressing symptoms suggestive of Crohn disease, such as persistent abdominal pain, diarrhea, rectal bleeding, unexplained weight loss, and fatigue, may help prevent complications and improve long-term outcomes. Seeking medical attention promptly for early signs of Crohn disease is crucial for initiating appropriate treatment and disease management strategies.
- Dietary modifications: No specific diet has been proven to prevent Crohn disease, but some dietary factors may influence disease risk or exacerbate symptoms in susceptible individuals. Controlling sugar intake and avoiding foods that trigger the symptoms may help prevent flare-ups.
- Avoiding environmental triggers: Environmental factors that may contribute to Crohn disease development or exacerbation and thus must be avoided include cigarette smoke, air pollution, medications like nonsteroidal anti-inflammatory drugs, and infections.
Secondary prevention consists of measures that help avoid complications after a Crohn disease diagnosis. The following steps may help:
- Medication adherence: Compliance with prescribed treatment regimens is essential for managing symptoms, reducing inflammation, preventing disease flares, and minimizing the risk of complications. When counseling patients, taking medications as directed, attending regular follow-up appointments, and promptly communicating symptom changes must be emphasized.
- Cancer screening: Patients with Crohn disease are prone to malignancies, which may include skin and gastrointestinal tumors. Patients should undergo skin cancer screening irrespective of the regimen they received. More frequent screening colonoscopy, ie, every 1 to 3 years, is recommended to detect colon cancer early in patients with inflammation involving at least a third of the colon.
- Osteoporosis prevention: Steroid treatment and Crohn disease–related vitamin deficiencies make patients susceptible to osteoporosis. Bone density should be regularly assessed to allow timely osteoporosis recognition and management.
- Infection prevention: Immunosuppressive therapy makes patients with Crohn disease vulnerable to infections. Vaccination against lung pathogens, such as pneumococcus, H influenzae, and influenza virus, is recommended. The decision to vaccinate against other microbial agents must be based on patient and geographic factors.
Individual responses to these measures may vary, so not all may be applicable or effective for everyone. Further research is needed to better understand the underlying causes of Crohn disease and identify more targeted preventive and treatment approaches.
Enhancing Healthcare Team Outcomes
Crohn disease is a serious chronic inflammatory disorder that poses various diagnostic and management challenges. This condition's potential to cause multiorgan damage makes an interprofessional care approach imperative. The interprofessional team caring for patients with Crohn disease should include the following:
- Primary care physicians are usually the first to evaluate patients with mild abdominal complaints, prescribe initial treatment, and refer the patient to specialists for further evaluation and management.
- Internists may lend expertise in diagnosing recurrent abdominal symptoms associated with systemic manifestations like fever and weight loss. Internists may prescribe additional treatments and refer the patient for further specialized care.
- Gastroenterologists are specialists who can perform diagnostic and therapeutic endoscopies, prescribe more targeted therapies, and monitor the disease course.
- Colorectal surgeons may be involved if patients present with complications like fistulae and intestinal obstruction.
- Dietitians educate the patient on the appropriate diet to avoid malnutrition and micronutrient deficiencies.
- Nurses: administer treatments, educate the postsurgical patient on treating stomas and surgical wounds, monitor patient progress, and coordinate care.
- Pharmacists educate the patient regarding their prescribed medical regimens, particularly the benefits, adverse effects, and cruciality of compliance.
- Rheumatologists, neurologists, ophthalmologists, hepatologists, nephrologists, dermatologists, and otorhinolaryngologists are specialists who may be consulted to manage Crohn associated extraintestinal problems.
- Mental health providers may lend their expertise to patients who develop depression and severe anxiety due to their symptoms and possible complications.
The prognosis for most patients with Crohn disease is guarded, and the quality of life is often poor.[63] An interprofessional team approach can optimize outcomes within the condition's limitations.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ. The natural history of adult Crohn's disease in population-based cohorts. The American journal of gastroenterology. 2010 Feb:105(2):289-97. doi: 10.1038/ajg.2009.579. Epub 2009 Oct 27 [PubMed PMID: 19861953]
Thia KT, Sandborn WJ, Harmsen WS, Zinsmeister AR, Loftus EV Jr. Risk factors associated with progression to intestinal complications of Crohn's disease in a population-based cohort. Gastroenterology. 2010 Oct:139(4):1147-55. doi: 10.1053/j.gastro.2010.06.070. Epub 2010 Jul 14 [PubMed PMID: 20637205]
Level 2 (mid-level) evidenceLightner AL, McKenna NP, Alsughayer A, Loftus EV Jr, Raffals LE, Faubion WA, Moir C. Anti-TNF biologic therapy does not increase postoperative morbidity in pediatric Crohn's patients. Journal of pediatric surgery. 2019 Oct:54(10):2162-2165. doi: 10.1016/j.jpedsurg.2019.01.006. Epub 2019 Jan 18 [PubMed PMID: 30773391]
Marazuela García P, López-Frías López-Jurado A, Vicente Bártulos A. Acute abdominal pain in patients with Crohn's disease: what urgent imaging tests should be done? Radiologia. 2019 Jul-Aug:61(4):333-336. doi: 10.1016/j.rx.2018.12.003. Epub 2019 Feb 14 [PubMed PMID: 30772003]
Aksan A, Farrag K, Stein J. An update on the evaluation and management of iron deficiency anemia in inflammatory bowel disease. Expert review of gastroenterology & hepatology. 2019 Feb:13(2):95-97. doi: 10.1080/17474124.2019.1553618. Epub 2018 Dec 7 [PubMed PMID: 30791779]
Hwang JH, Yu CS. Depression and resilience in ulcerative colitis and Crohn's disease patients with ostomy. International wound journal. 2019 Mar:16 Suppl 1(Suppl 1):62-70. doi: 10.1111/iwj.13076. Epub [PubMed PMID: 30793856]
Khan S, Rupniewska E, Neighbors M, Singer D, Chiarappa J, Obando C. Real-world evidence on adherence, persistence, switching and dose escalation with biologics in adult inflammatory bowel disease in the United States: A systematic review. Journal of clinical pharmacy and therapeutics. 2019 Aug:44(4):495-507. doi: 10.1111/jcpt.12830. Epub 2019 Mar 14 [PubMed PMID: 30873648]
Level 1 (high-level) evidenceLiu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, Ripke S, Lee JC, Jostins L, Shah T, Abedian S, Cheon JH, Cho J, Dayani NE, Franke L, Fuyuno Y, Hart A, Juyal RC, Juyal G, Kim WH, Morris AP, Poustchi H, Newman WG, Midha V, Orchard TR, Vahedi H, Sood A, Sung JY, Malekzadeh R, Westra HJ, Yamazaki K, Yang SK, International Multiple Sclerosis Genetics Consortium, International IBD Genetics Consortium, Barrett JC, Alizadeh BZ, Parkes M, Bk T, Daly MJ, Kubo M, Anderson CA, Weersma RK. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nature genetics. 2015 Sep:47(9):979-986. doi: 10.1038/ng.3359. Epub 2015 Jul 20 [PubMed PMID: 26192919]
Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, Anderson CA, Bis JC, Bumpstead S, Ellinghaus D, Festen EM, Georges M, Green T, Haritunians T, Jostins L, Latiano A, Mathew CG, Montgomery GW, Prescott NJ, Raychaudhuri S, Rotter JI, Schumm P, Sharma Y, Simms LA, Taylor KD, Whiteman D, Wijmenga C, Baldassano RN, Barclay M, Bayless TM, Brand S, Büning C, Cohen A, Colombel JF, Cottone M, Stronati L, Denson T, De Vos M, D'Inca R, Dubinsky M, Edwards C, Florin T, Franchimont D, Gearry R, Glas J, Van Gossum A, Guthery SL, Halfvarson J, Verspaget HW, Hugot JP, Karban A, Laukens D, Lawrance I, Lemann M, Levine A, Libioulle C, Louis E, Mowat C, Newman W, Panés J, Phillips A, Proctor DD, Regueiro M, Russell R, Rutgeerts P, Sanderson J, Sans M, Seibold F, Steinhart AH, Stokkers PC, Torkvist L, Kullak-Ublick G, Wilson D, Walters T, Targan SR, Brant SR, Rioux JD, D'Amato M, Weersma RK, Kugathasan S, Griffiths AM, Mansfield JC, Vermeire S, Duerr RH, Silverberg MS, Satsangi J, Schreiber S, Cho JH, Annese V, Hakonarson H, Daly MJ, Parkes M. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nature genetics. 2010 Dec:42(12):1118-25. doi: 10.1038/ng.717. Epub [PubMed PMID: 21102463]
Level 1 (high-level) evidenceLee HS, Oh H, Yang SK, Baek J, Jung S, Hong M, Kim KM, Shin HD, Kim KJ, Park SH, Ye BD, Han B, Song K. X Chromosome-wide Association Study Identifies a Susceptibility Locus for Inflammatory Bowel Disease in Koreans. Journal of Crohn's & colitis. 2017 Jul 1:11(7):820-830. doi: 10.1093/ecco-jcc/jjx023. Epub [PubMed PMID: 28333213]
Zaidi D, Wine E. Regulation of Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells (NF-κβ) in Inflammatory Bowel Diseases. Frontiers in pediatrics. 2018:6():317. doi: 10.3389/fped.2018.00317. Epub 2018 Oct 30 [PubMed PMID: 30425977]
Ghersin I, Khteeb N, Katz LH, Daher S, Shamir R, Assa A. Trends in the epidemiology of inflammatory bowel disease among Jewish Israeli adolescents: a population-based study. Alimentary pharmacology & therapeutics. 2019 Mar:49(5):556-563. doi: 10.1111/apt.15160. Epub 2019 Jan 27 [PubMed PMID: 30687945]
Coward S, Clement F, Benchimol EI, Bernstein CN, Avina-Zubieta JA, Bitton A, Carroll MW, Hazlewood G, Jacobson K, Jelinski S, Deardon R, Jones JL, Kuenzig ME, Leddin D, McBrien KA, Murthy SK, Nguyen GC, Otley AR, Panaccione R, Rezaie A, Rosenfeld G, Peña-Sánchez JN, Singh H, Targownik LE, Kaplan GG. Past and Future Burden of Inflammatory Bowel Diseases Based on Modeling of Population-Based Data. Gastroenterology. 2019 Apr:156(5):1345-1353.e4. doi: 10.1053/j.gastro.2019.01.002. Epub 2019 Jan 10 [PubMed PMID: 30639677]
Gálvez J. Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN inflammation. 2014:2014():928461. doi: 10.1155/2014/928461. Epub 2014 Mar 25 [PubMed PMID: 25101191]
Targan SR. Biology of inflammation in Crohn's disease: mechanisms of action of anti-TNF-a therapy. Canadian journal of gastroenterology = Journal canadien de gastroenterologie. 2000 Sep:14 Suppl C():13C-16C [PubMed PMID: 11023555]
Level 3 (low-level) evidenceKanai T, Watanabe M, Okazawa A, Nakamaru K, Okamoto M, Naganuma M, Ishii H, Ikeda M, Kurimoto M, Hibi T. Interleukin 18 is a potent proliferative factor for intestinal mucosal lymphocytes in Crohn's disease. Gastroenterology. 2000 Dec:119(6):1514-23 [PubMed PMID: 11113073]
Greuter T, Piller A, Fournier N, Safroneeva E, Straumann A, Biedermann L, Godat S, Nydegger A, Scharl M, Rogler G, Vavricka SR, Schoepfer AM, Swiss IBD Cohort Study Group. Upper Gastrointestinal Tract Involvement in Crohn's Disease: Frequency, Risk Factors, and Disease Course. Journal of Crohn's & colitis. 2018 Nov 28:12(12):1399-1409. doi: 10.1093/ecco-jcc/jjy121. Epub [PubMed PMID: 30165603]
Fumery M, Pariente B, Sarter H, Savoye G, Spyckerelle C, Djeddi D, Mouterde O, Bouguen G, Ley D, Peneau A, Dupas JL, Turck D, Gower-Rousseau C, Epimad Group. Long-term outcome of pediatric-onset Crohn's disease: A population-based cohort study. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2019 Apr:51(4):496-502. doi: 10.1016/j.dld.2018.11.033. Epub 2018 Dec 23 [PubMed PMID: 30611597]
Owczarek D, Cibor D, Głowacki MK, Rodacki T, Mach T. Inflammatory bowel disease: epidemiology, pathology and risk factors for hypercoagulability. World journal of gastroenterology. 2014 Jan 7:20(1):53-63. doi: 10.3748/wjg.v20.i1.53. Epub [PubMed PMID: 24415858]
Fadeeva NA, Korneeva IA, Knyazev OV, Parfenov AI. Biomarkers of inflammatory bowel disease activity. Terapevticheskii arkhiv. 2018 Dec 30:90(12):107-111. doi: 10.26442/00403660.2018.12.000018. Epub [PubMed PMID: 30701842]
Parfenov AI, Knyazev OV, Kagramanova AV, Fadeeva NA. Personalized medicine in the treatment of inflammatory bowel diseases. Terapevticheskii arkhiv. 2018 Feb 15:90(2):4-11. doi: 10.26442/terarkh20189024-11. Epub [PubMed PMID: 30701765]
Kedia S, Das P, Madhusudhan KS, Dattagupta S, Sharma R, Sahni P, Makharia G, Ahuja V. Differentiating Crohn's disease from intestinal tuberculosis. World journal of gastroenterology. 2019 Jan 28:25(4):418-432. doi: 10.3748/wjg.v25.i4.418. Epub [PubMed PMID: 30700939]
Moon JS. Clinical Aspects and Treatments for Pediatric Inflammatory Bowel Diseases. Pediatric gastroenterology, hepatology & nutrition. 2019 Jan:22(1):50-56. doi: 10.5223/pghn.2019.22.1.50. Epub 2019 Jan 10 [PubMed PMID: 30671373]
Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: Management of Crohn's Disease in Adults. The American journal of gastroenterology. 2018 Apr:113(4):481-517. doi: 10.1038/ajg.2018.27. Epub 2018 Mar 27 [PubMed PMID: 29610508]
Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T, Adamina M, Armuzzi A, Bachmann O, Bager P, Biancone L, Bokemeyer B, Bossuyt P, Burisch J, Collins P, El-Hussuna A, Ellul P, Frei-Lanter C, Furfaro F, Gingert C, Gionchetti P, Gomollon F, González-Lorenzo M, Gordon H, Hlavaty T, Juillerat P, Katsanos K, Kopylov U, Krustins E, Lytras T, Maaser C, Magro F, Marshall JK, Myrelid P, Pellino G, Rosa I, Sabino J, Savarino E, Spinelli A, Stassen L, Uzzan M, Vavricka S, Verstockt B, Warusavitarne J, Zmora O, Fiorino G. ECCO Guidelines on Therapeutics in Crohn's Disease: Medical Treatment. Journal of Crohn's & colitis. 2020 Jan 1:14(1):4-22. doi: 10.1093/ecco-jcc/jjz180. Epub [PubMed PMID: 31711158]
Rahier JF, Magro F, Abreu C, Armuzzi A, Ben-Horin S, Chowers Y, Cottone M, de Ridder L, Doherty G, Ehehalt R, Esteve M, Katsanos K, Lees CW, Macmahon E, Moreels T, Reinisch W, Tilg H, Tremblay L, Veereman-Wauters G, Viget N, Yazdanpanah Y, Eliakim R, Colombel JF, European Crohn's and Colitis Organisation (ECCO). Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. Journal of Crohn's & colitis. 2014 Jun:8(6):443-68. doi: 10.1016/j.crohns.2013.12.013. Epub 2014 Mar 6 [PubMed PMID: 24613021]
Level 3 (low-level) evidenceFord AC, Kane SV, Khan KJ, Achkar JP, Talley NJ, Marshall JK, Moayyedi P. Efficacy of 5-aminosalicylates in Crohn's disease: systematic review and meta-analysis. The American journal of gastroenterology. 2011 Apr:106(4):617-29. doi: 10.1038/ajg.2011.71. Epub 2011 Mar 15 [PubMed PMID: 21407190]
Level 1 (high-level) evidenceChande N, Patton PH, Tsoulis DJ, Thomas BS, MacDonald JK. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn's disease. The Cochrane database of systematic reviews. 2015 Oct 30:2015(10):CD000067. doi: 10.1002/14651858.CD000067.pub3. Epub 2015 Oct 30 [PubMed PMID: 26517527]
Level 1 (high-level) evidenceChande N, Tsoulis DJ, MacDonald JK. Azathioprine or 6-mercaptopurine for induction of remission in Crohn's disease. The Cochrane database of systematic reviews. 2013 Apr 30:(4):CD000545. doi: 10.1002/14651858.CD000545.pub4. Epub 2013 Apr 30 [PubMed PMID: 23633304]
Level 1 (high-level) evidenceMcDonald JW, Wang Y, Tsoulis DJ, MacDonald JK, Feagan BG. Methotrexate for induction of remission in refractory Crohn's disease. The Cochrane database of systematic reviews. 2014 Aug 6:2014(8):CD003459. doi: 10.1002/14651858.CD003459.pub4. Epub 2014 Aug 6 [PubMed PMID: 25099640]
Level 1 (high-level) evidenceFeagan BG, Fedorak RN, Irvine EJ, Wild G, Sutherland L, Steinhart AH, Greenberg GR, Koval J, Wong CJ, Hopkins M, Hanauer SB, McDonald JW. A comparison of methotrexate with placebo for the maintenance of remission in Crohn's disease. North American Crohn's Study Group Investigators. The New England journal of medicine. 2000 Jun 1:342(22):1627-32 [PubMed PMID: 10833208]
Richard VS, Al-Ismail D, Salamat A. Should we test TPMT enzyme levels before starting azathioprine? Hematology (Amsterdam, Netherlands). 2007 Aug:12(4):359-60 [PubMed PMID: 17654066]
Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P, SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. The New England journal of medicine. 2010 Apr 15:362(15):1383-95. doi: 10.1056/NEJMoa0904492. Epub [PubMed PMID: 20393175]
Kawalec P, Mikrut A, Wiśniewska N, Pilc A. Tumor necrosis factor-α antibodies (infliximab, adalimumab and certolizumab) in Crohn's disease: systematic review and meta-analysis. Archives of medical science : AMS. 2013 Oct 31:9(5):765-79. doi: 10.5114/aoms.2013.38670. Epub 2013 Nov 5 [PubMed PMID: 24273556]
Level 1 (high-level) evidenceVan Assche G, Lewis JD, Lichtenstein GR, Loftus EV, Ouyang Q, Panes J, Siegel CA, Sandborn WJ, Travis SP, Colombel JF. The London position statement of the World Congress of Gastroenterology on Biological Therapy for IBD with the European Crohn's and Colitis Organisation: safety. The American journal of gastroenterology. 2011 Sep:106(9):1594-602; quiz 1593, 1603. doi: 10.1038/ajg.2011.211. Epub 2011 Aug 16 [PubMed PMID: 21844919]
De Felice KM, Kane S. Safety of anti-TNF agents in pregnancy. The Journal of allergy and clinical immunology. 2021 Sep:148(3):661-667. doi: 10.1016/j.jaci.2021.07.005. Epub [PubMed PMID: 34489011]
Hui S, Sinopoulou V, Gordon M, Aali G, Krishna A, Ding NS, Boyapati RK. Vedolizumab for induction and maintenance of remission in Crohn's disease. The Cochrane database of systematic reviews. 2023 Jul 17:7(7):CD013611. doi: 10.1002/14651858.CD013611.pub2. Epub 2023 Jul 17 [PubMed PMID: 37458279]
Level 1 (high-level) evidenceHazlewood GS, Rezaie A, Borman M, Panaccione R, Ghosh S, Seow CH, Kuenzig E, Tomlinson G, Siegel CA, Melmed GY, Kaplan GG. Comparative effectiveness of immunosuppressants and biologics for inducing and maintaining remission in Crohn's disease: a network meta-analysis. Gastroenterology. 2015 Feb:148(2):344-54.e5; quiz e14-5. doi: 10.1053/j.gastro.2014.10.011. Epub 2014 Oct 16 [PubMed PMID: 25448924]
Level 1 (high-level) evidenceFeagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, Blank MA, Johanns J, Gao LL, Miao Y, Adedokun OJ, Sands BE, Hanauer SB, Vermeire S, Targan S, Ghosh S, de Villiers WJ, Colombel JF, Tulassay Z, Seidler U, Salzberg BA, Desreumaux P, Lee SD, Loftus EV Jr, Dieleman LA, Katz S, Rutgeerts P, UNITI–IM-UNITI Study Group. Ustekinumab as Induction and Maintenance Therapy for Crohn's Disease. The New England journal of medicine. 2016 Nov 17:375(20):1946-1960 [PubMed PMID: 27959607]
D'Haens G, Panaccione R, Baert F, Bossuyt P, Colombel JF, Danese S, Dubinsky M, Feagan BG, Hisamatsu T, Lim A, Lindsay JO, Loftus EV Jr, Panés J, Peyrin-Biroulet L, Ran Z, Rubin DT, Sandborn WJ, Schreiber S, Neimark E, Song A, Kligys K, Pang Y, Pivorunas V, Berg S, Duan WR, Huang B, Kalabic J, Liao X, Robinson A, Wallace K, Ferrante M. Risankizumab as induction therapy for Crohn's disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet (London, England). 2022 May 28:399(10340):2015-2030. doi: 10.1016/S0140-6736(22)00467-6. Epub [PubMed PMID: 35644154]
Ferrante M, Panaccione R, Baert F, Bossuyt P, Colombel JF, Danese S, Dubinsky M, Feagan BG, Hisamatsu T, Lim A, Lindsay JO, Loftus EV Jr, Panés J, Peyrin-Biroulet L, Ran Z, Rubin DT, Sandborn WJ, Schreiber S, Neimark E, Song A, Kligys K, Pang Y, Pivorunas V, Berg S, Duan WR, Huang B, Kalabic J, Liao X, Robinson A, Wallace K, D'Haens G. Risankizumab as maintenance therapy for moderately to severely active Crohn's disease: results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial. Lancet (London, England). 2022 May 28:399(10340):2031-2046. doi: 10.1016/S0140-6736(22)00466-4. Epub [PubMed PMID: 35644155]
Level 1 (high-level) evidencePapp K, Gottlieb AB, Naldi L, Pariser D, Ho V, Goyal K, Fakharzadeh S, Chevrier M, Calabro S, Langholff W, Krueger G. Safety Surveillance for Ustekinumab and Other Psoriasis Treatments From the Psoriasis Longitudinal Assessment and Registry (PSOLAR). Journal of drugs in dermatology : JDD. 2015 Jul:14(7):706-14 [PubMed PMID: 26151787]
Loftus EV Jr, Panés J, Lacerda AP, Peyrin-Biroulet L, D'Haens G, Panaccione R, Reinisch W, Louis E, Chen M, Nakase H, Begun J, Boland BS, Phillips C, Mohamed MF, Liu J, Geng Z, Feng T, Dubcenco E, Colombel JF. Upadacitinib Induction and Maintenance Therapy for Crohn's Disease. The New England journal of medicine. 2023 May 25:388(21):1966-1980. doi: 10.1056/NEJMoa2212728. Epub [PubMed PMID: 37224198]
Akiyama S, Steinberg JM, Kobayashi M, Suzuki H, Tsuchiya K. Pregnancy and medications for inflammatory bowel disease: An updated narrative review. World journal of clinical cases. 2023 Mar 16:11(8):1730-1740. doi: 10.12998/wjcc.v11.i8.1730. Epub [PubMed PMID: 36969991]
Level 3 (low-level) evidenceSingh S, Proctor D, Scott FI, Falck-Ytter Y, Feuerstein JD. AGA Technical Review on the Medical Management of Moderate to Severe Luminal and Perianal Fistulizing Crohn's Disease. Gastroenterology. 2021 Jun:160(7):2512-2556.e9. doi: 10.1053/j.gastro.2021.04.023. Epub [PubMed PMID: 34051985]
Wang X, Shen B. Advances in Perianal Disease Associated with Crohn's Disease-Evolving Approaches. Gastrointestinal endoscopy clinics of North America. 2019 Jul:29(3):515-530. doi: 10.1016/j.giec.2019.02.011. Epub [PubMed PMID: 31078250]
Level 3 (low-level) evidenceLightner AL, Vogel JD, Carmichael JC, Keller DS, Shah SA, Mahadevan U, Kane SV, Paquette IM, Steele SR, Feingold DL. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Surgical Management of Crohn's Disease. Diseases of the colon and rectum. 2020 Aug:63(8):1028-1052. doi: 10.1097/DCR.0000000000001716. Epub [PubMed PMID: 32692069]
Level 1 (high-level) evidenceMaser EA, Sachar DB, Kruse D, Harpaz N, Ullman T, Bauer JJ. High rates of metachronous colon cancer or dysplasia after segmental resection or subtotal colectomy in Crohn's colitis. Inflammatory bowel diseases. 2013 Aug:19(9):1827-32. doi: 10.1097/MIB.0b013e318289c166. Epub [PubMed PMID: 23669402]
Derikx LAAP, Nissen LHC, Smits LJT, Shen B, Hoentjen F. Risk of Neoplasia After Colectomy in Patients With Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2016 Jun:14(6):798-806.e20. doi: 10.1016/j.cgh.2015.08.042. Epub 2015 Sep 25 [PubMed PMID: 26407752]
Level 1 (high-level) evidenceAnanthakrishnan AN, Adler J, Chachu KA, Nguyen NH, Siddique SM, Weiss JM, Sultan S, Velayos FS, Cohen BL, Singh S, AGA Clinical Guidelines Committee. Electronic address: clinicalpractice@gastro.org. AGA Clinical Practice Guideline on the Role of Biomarkers for the Management of Crohn's Disease. Gastroenterology. 2023 Dec:165(6):1367-1399. doi: 10.1053/j.gastro.2023.09.029. Epub [PubMed PMID: 37981354]
Level 1 (high-level) evidenceBrown SR, Fearnhead NS, Faiz OD, Abercrombie JF, Acheson AG, Arnott RG, Clark SK, Clifford S, Davies RJ, Davies MM, Douie WJP, Dunlop MG, Epstein JC, Evans MD, George BD, Guy RJ, Hargest R, Hawthorne AB, Hill J, Hughes GW, Limdi JK, Maxwell-Armstrong CA, O'Connell PR, Pinkney TD, Pipe J, Sagar PM, Singh B, Soop M, Terry H, Torkington J, Verjee A, Walsh CJ, Warusavitarne JH, Williams AB, Williams GL, Wilson RG, ACPGBI IBD Surgery Consensus Collaboration. The Association of Coloproctology of Great Britain and Ireland consensus guidelines in surgery for inflammatory bowel disease. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2018 Dec:20 Suppl 8():3-117. doi: 10.1111/codi.14448. Epub [PubMed PMID: 30508274]
Level 3 (low-level) evidencede Kloet LC, Schagen SEE, van den Berg A, Clement-de Boers A, Houdijk MECAM, van der Kaay DCM. [Growth failure as a symptom of inflammatory bowel disease]. Nederlands tijdschrift voor geneeskunde. 2018 Nov 19:162():. pii: D2515. Epub 2018 Nov 19 [PubMed PMID: 30500117]
Rodríguez-Lago I, Ferreiro-Iglesias R, Nos P, Gisbert JP, en representación del Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU). Management of acute severe ulcerative colitis in Spain: A nationwide clinical practice survey. Gastroenterologia y hepatologia. 2019 Feb:42(2):90-101. doi: 10.1016/j.gastrohep.2018.09.002. Epub 2018 Oct 4 [PubMed PMID: 30293913]
Level 3 (low-level) evidenceAmbruzs JM, Larsen CP. Renal Manifestations of Inflammatory Bowel Disease. Rheumatic diseases clinics of North America. 2018 Nov:44(4):699-714. doi: 10.1016/j.rdc.2018.06.007. Epub 2018 Sep 7 [PubMed PMID: 30274631]
Lewis JD, Abreu MT. Diet as a Trigger or Therapy for Inflammatory Bowel Diseases. Gastroenterology. 2017 Feb:152(2):398-414.e6. doi: 10.1053/j.gastro.2016.10.019. Epub 2016 Oct 25 [PubMed PMID: 27793606]
Peyrin-Biroulet L, Panés J, Sandborn WJ, Vermeire S, Danese S, Feagan BG, Colombel JF, Hanauer SB, Rycroft B. Defining Disease Severity in Inflammatory Bowel Diseases: Current and Future Directions. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association. 2016 Mar:14(3):348-354.e17. doi: 10.1016/j.cgh.2015.06.001. Epub 2015 Jun 11 [PubMed PMID: 26071941]
Level 3 (low-level) evidenceSandborn WJ. Crohn's disease evaluation and treatment: clinical decision tool. Gastroenterology. 2014 Sep:147(3):702-5. doi: 10.1053/j.gastro.2014.07.022. Epub 2014 Jul 18 [PubMed PMID: 25046160]
Frolkis AD, Dykeman J, Negrón ME, Debruyn J, Jette N, Fiest KM, Frolkis T, Barkema HW, Rioux KP, Panaccione R, Ghosh S, Wiebe S, Kaplan GG. Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta-analysis of population-based studies. Gastroenterology. 2013 Nov:145(5):996-1006. doi: 10.1053/j.gastro.2013.07.041. Epub 2013 Jul 27 [PubMed PMID: 23896172]
Level 1 (high-level) evidenceDe Cruz P, Kamm MA, Prideaux L, Allen PB, Desmond PV. Postoperative recurrent luminal Crohn's disease: a systematic review. Inflammatory bowel diseases. 2012 Apr:18(4):758-77. doi: 10.1002/ibd.21825. Epub 2011 Aug 9 [PubMed PMID: 21830279]
Level 1 (high-level) evidenceCanavan C, Abrams KR, Mayberry JF. Meta-analysis: mortality in Crohn's disease. Alimentary pharmacology & therapeutics. 2007 Apr 15:25(8):861-70 [PubMed PMID: 17402989]
Level 1 (high-level) evidenceBewtra M, Kaiser LM, TenHave T, Lewis JD. Crohn's disease and ulcerative colitis are associated with elevated standardized mortality ratios: a meta-analysis. Inflammatory bowel diseases. 2013 Mar:19(3):599-613. doi: 10.1097/MIB.0b013e31827f27ae. Epub [PubMed PMID: 23388544]
Level 1 (high-level) evidenceLewis JD, Gelfand JM, Troxel AB, Forde KA, Newcomb C, Kim H, Margolis DJ, Strom BL. Immunosuppressant medications and mortality in inflammatory bowel disease. The American journal of gastroenterology. 2008 Jun:103(6):1428-35; quiz 1436. doi: 10.1111/j.1572-0241.2008.01836.x. Epub 2008 May 20 [PubMed PMID: 18494836]
Inokuchi T, Takahashi S, Hiraoka S, Toyokawa T, Takagi S, Takemoto K, Miyaike J, Fujimoto T, Higashi R, Morito Y, Nawa T, Suzuki S, Nishimura M, Inoue M, Kato J, Okada H. Long-term outcomes of patients with Crohn's disease who received infliximab or adalimumab as the first-line biologics. Journal of gastroenterology and hepatology. 2019 Aug:34(8):1329-1336. doi: 10.1111/jgh.14624. Epub 2019 Feb 27 [PubMed PMID: 30724387]