Indications
In 1962, there was a need for new antiarrhythmic drugs other than quinidine and procainamide, the main available antiarrhythmic agents available at that time. From more than 500 compounds synthesized for the research program of new antiarrhythmic agents, disopyramide is the selected agent. The chemical structures of disopyramide resemble the synthetic muscarinic antagonist, lachesine, which explained its anticholinergic property.[1]
Despite rarely used now for heart rhythm abnormalities because of the availability of newer drugs that provided better efficacy and favorable side effect profiles, disopyramide is still the drug of choice for vagally mediated atrial fibrillation such as sleep-induced or atrial fibrillation in athlete groups.[2] The effectivity of disopyramide in these conditions is due to its anticholinergic activity that abolished parasympathetic tone.[3]
It is also a third-line antiarrhythmic agent for a patient with coronary artery disease. Moreover, in a patient with left ventricular hypertrophy, there is impaired depolarization, which can induce torsade de pointes. Hence, antiarrhythmics that prolong QT interval are avoided, but if sotalol or amiodarone either fail or are inappropriate, disopyramide can be an alternative.[4] In a patient with atrial fibrillation and hypertrophic obstructive cardiomyopathy (HOCM), disopyramide is the selected agent other than amiodarone because it can decrease the left ventricular outflow tract (LVOT) gradient (off-label use).[4][5] Data from the multicenter study of safety and efficacy of disopyramide in obstructive cardiomyopathy showed that disopyramide significantly decreases (P<0.0001) the LVOT gradient from 75+/- 33 to 40+/-32 mmHg in 78 patients (66% of the study subjects) and improves New York Heart Association functional class (NYHA FC) from 23+/-07 to 17+/-06( P<0.0001).[5] When using disopyramide in combination non-dihydropyridine calcium channel blocker or beta-blocker, they can effectively prevent the recurrence of AF in HCOM patients.[2]
A high symptom burden can be present in patients with a ventricular premature beat (VPB) or premature ventricular complexes (PVC). Disopyramide can be used for patients without structural heart disease can be treated with, albeit their efficacy is lower compared to ablation.[6] Additionally, based on a randomized, double-blind placebo-controlled one year follow up study, disopyramide (n=44) is effective in maintaining sinus rhythm after electro cardioversion for atrial fibrillation compared to placebo (n= 46), and it is significantly different at one-month follow-up (70% vs. 39%) and persist after twelve months (54% vs. 30%).[7]
Mechanism of Action
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Mechanism of Action
According to the Vaughan Williams classification, antiarrhythmic drugs classify into five main classes based on their mechanism of action. The class I antiarrhythmic works mainly by blocking the sodium ion channels, which are responsible for the fast inward depolarizing current of the cardiomyocytes. This classification then is grouped again according to their effect on the length of the action potential of the cardiomyocyte to Ia., Ib, and 1c, with the type Ic. is the strongest. The type Ib is the weakest with respect to its binding affinity to the sodium channels.[8] Disopyramide is an antiarrhythmic drug that belongs to class Ia. As with other class I antiarrhythmic drugs, such as quinidine, ajmaline, and procainamide, the mechanism of action of disopyramide is to lengthen the action potential duration (APD) of cardiomyocytes, reflected by the rightward shift of the action potential curve. It also lowers the rate of diastolic depolarization (phase 4) in cells with augmented automaticity and the upstroke velocity (phase 1).[9] Therefore, disopyramide decreases myocardial excitability and conduction velocity
Compared to the ischemic myocardium, the normally perfused adjacent myocardia display a minimal difference in refractoriness due to APD lengthening hence disopyramide's protective effect from the reentry phenomenon. On the contrary, although theoretically useful to prevent reentry phenomenon from ischemic myocardia, a multicenter, double-blind, randomized study involving 1985 subjects (n treatment = 995, n placebo= 990 ) evaluating oral disopyramide for patients admitted due to myocardial infarction (MI) found that there is no decrease in mortality rate.[10] There was another single-center randomized study involving 199 subjects (n treatment =100, n placebo = 99) evaluating oral and intravenous disopyramide versus placebo after MI found that prophylactic disopyramide does not reduce mortality after myocardial infarction.[11] Furthermore, based on a systematic review and meta-analysis involving 44 clinical trials, according to the pooled analysis, disopyramide can increase mortality, although it is hard to translate this evidence to other populations due to the short follow-up duration of these clinical trials and that they involved mainly healthy subjects. Therefore, disopyramide is not used prophylactically for rhythm disturbances due to MI.[12]
Disopyramide and other class I.A. antiarrhythmics also block the rapid component of the delayed rectifier potassium current when blocking the sodium channel resulting in QT interval prolongation.[13] Another effect displayed by disopyramide is AV node blocking with the prerequisite that the vagal tone has already diminished by atropine. The heart will exhibit delay of av conduction resulting in bradycardia.
Administration
Disopyramide is an orally administered drug in the form of capsules. There are two types of capsule formulations contrasting with their drug delivery system property: the immediate-release and extended-release disopyramide. When using the standard release capsules, 200 to 300 mg of disopyramide is given as an initial dose, and 100 to 150 mg of disopyramide is given every 6 hours. Fewer initial dose and maintenance dose are needed for extended-release capsules because of the formulation's extended half-life, and consequently, its duration of actions. However, extended-release capsules are usually avoided if the clinician wants to achieve a rapid drug peak concentration.
To promote less variation in peak and trough serum levels, disopyramide should be administered around the clock (4 times per day; 12-6-12- 6, not 9-1-5-9). The controlled release capsules should not be broken nor chewed.
Concerning its side effects, disopyramide must be started when the patient is under in-hospital treatment except for patients without heart disease who have a normal QT interval.[4] Furthermore, In HOCM patients, initiation of disopyramide in the outpatient setting is safe, and the subsequent sudden cardiac death is low.[14]
Adverse Effects
The main actions of disopyramide, i.e., sodium channel blocking and anticholinergic properties, contribute to its side effects. The maximum rate of depolarization (MRD) decreases by preventing sodium channel entry, hence the reduced contractility of the myocardia. The accompanying rapid component of delayed rectifier potassium channel blocking mentioned previously (see "Mechanism of Action") also contributes to the increase of QT interval. Because of these effects, disopyramide is excluded as a rhythm control treatment option for patients with overt heart failure (with systemic congestion) and long QT syndromes.[13] Regarding its anticholinergic side effect, disopyramide can cause urinary retention. Therefore, caution is advised, especially when using this agent for the elderly population in which benign prostate hypertrophy is common. Other anticholinergic side effects of disopyramide usage are xerostomia, constipation, and glaucoma. Accordingly, to circumvent disopyramide's anticholinergic effects, pyridostigmine could be used.[15]
Although rare (<1%), some have reported in case reports adverse events such as agranulocytosis, cholestatic jaundice, malignant arrhythmia, and other electrocardiographic abnormalities.[16][17][18]
Contraindications
Several conditions stated below prevent the use of disopyramide as an antiarrhythmic drug:[2][19]
- Overt heart failure; disopyramide is known to have negative inotropic effects resulting in decreased heart ejection fraction.
- Second or third-degree AV block without a pacemaker present; In a heart that is free from the influence of vagal tone, disopyramide can worsen the current AV conduction status.
- Long QT syndromes: disopyramide and other class Ia antiarrhythmics can prolong the QT interval.
- Hypersensitivity to the drugs itself or its components
Monitoring
The cytochrome CYP3A4 is responsible for the liver metabolism of disopyramide. It can be induced or inhibited by various substances. Hence, these substances should be avoided or used cautiously to prevent the unpredictable modification of disopyramide pharmacokinetics. Listed below are several substances that are known to induce and inhibit CYP3A4.[2]
CYP3A4 inducers (increase disopyramide metabolism, reducing the disopyramide's plasma half-life):
- Rifampin
- Phenobarbital
- Phenytoin
CYP3A4 inhibitors (decrease disopyramide metabolism, increasing the disopyramide's plasma half-life):
- Verapamil
- Diltiazem
- Ketoconazole
- Macrolide antibiotics
- Protease-inhibitors
- Grapefruit juice
If any of these substances will be used concurrently with disopyramide, monitoring disopyramide's plasma concentration is warranted to prevent adverse consequences.
In patients with severe renal dysfunction (renal clearance of < 8mL/min), disopyramide's half-life is longer, and the duration ranges from 14 to 43 hours.[20] Whereas disopyramide's plasma half-life in normal healthy adults ranges from 6 to 8 hours.[21] Therefore, drug dose modification is warranted. This information is acquirable through the manufacturer's labeling. Moreover, in patients with hepatic impairment and heart failure, disopyramide's half-life is also increased.[17] Additionally, impairment of liver function can decrease alpha-1-acid-glycoprotein, a protein that binds disopyramide, causing free disopyramide concentrations to rise.[22]
Toxicity
The safe ranges for therapeutic disopyramide concentrations are from 2 to 5 mg/mL. Of note, in a patient with cirrhosis, lower concentrations are preferable due to increase free form.[23] When concentration is exceeding 9mg/mL, signs and symptoms of disopyramide toxicities will ensue.[24][25][26]
Due to relatively small volume distribution (Vd), low protein binding at toxic concentrations, and low intrinsic clearance, extracorporeal drug removal techniques (hemoperfusion/ hemodialysis) can be useful to remove disopyramide from the circulation in the setting of intoxication.[27]
Generally, the initial priority is to decontaminate the patient's gastrointestinal system with lavage, repeat doses of oral activated charcoals, and cathartic. These are done even when several hours have passed since the first ingestion because it can significantly delay the absorption of class Ia antiarrhythmics. Followed by admission to the intensive care unit for continuous electrocardiographic monitoring. Hemodialysis/hemoperfusion can be useful if the patient has ingested large doses or with high drug concentration or in the setting of circulatory collapse or renal insufficiency. Symptomatic drugs should be given accordingly. For seizures, administration of diazepam, phenytoin, or phenobarbital can be useful. In hypotensive patients, fluid challenge and intravenous inotropic and vasopressor agents should be used, and mechanical supports such as intra-aortic balloon pumps or cardiopulmonary bypass are taken into consideration in refractory cardiogenic shock. Lastly, in the setting of arrhythmia, whether it is bradyarrhythmia or ventricular tachycardia (torsade de pointes), the appropriate medication should be given.[17]
Although only based on theoretical considerations, NaHCO3 50 mmol can be given intravenously and repeated every 5 to 10 minutes. The main goal is to maintain the arterial pH at 7.4 to 7.5. The drug reversal mechanism possibly multifactorial, such as increasing blood pH, increasing blood plasma sodium concentration, and lowering plasma potassium concentration.[17]
Enhancing Healthcare Team Outcomes
With a wide variety of drugs available to treat heart rhythm abnormalities and substantial information regarding them, a newly graduated physician or junior doctor can become overwhelmed regarding which agent to use, especially among the older agents. This is why antiarrhythmic therapy should utilize the efforts of an interprofessional healthcare team. With help from a pharmacist, a doctor can choose whether disopyramide is indicated or contraindicated to use based on the patient's unique characteristics. When disopyramide has already been administered, a coordinative effort between the clinician and a nurse is necessary. A nurse can notify the ordering clinician regarding the patient's response to the drug, whether other agents are needed or not, and when side effects/adverse events prevent its use. These are just a few examples of why a collaborative approach of the interprofessional healthcare team is needed. The use of disopyramide is already approved by several guidelines (HRA/AHA/ACC/EHRS), and its indicated as a third-line agent for the treatment of life-threatening arrhythmia (VT/VF) and atrial fibrillation, especially sleep-induced or vagal induced.[2][13][19] [Level 5] It can also be used in HOCM with a combination of beta-blocker.[5] [Level 3]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
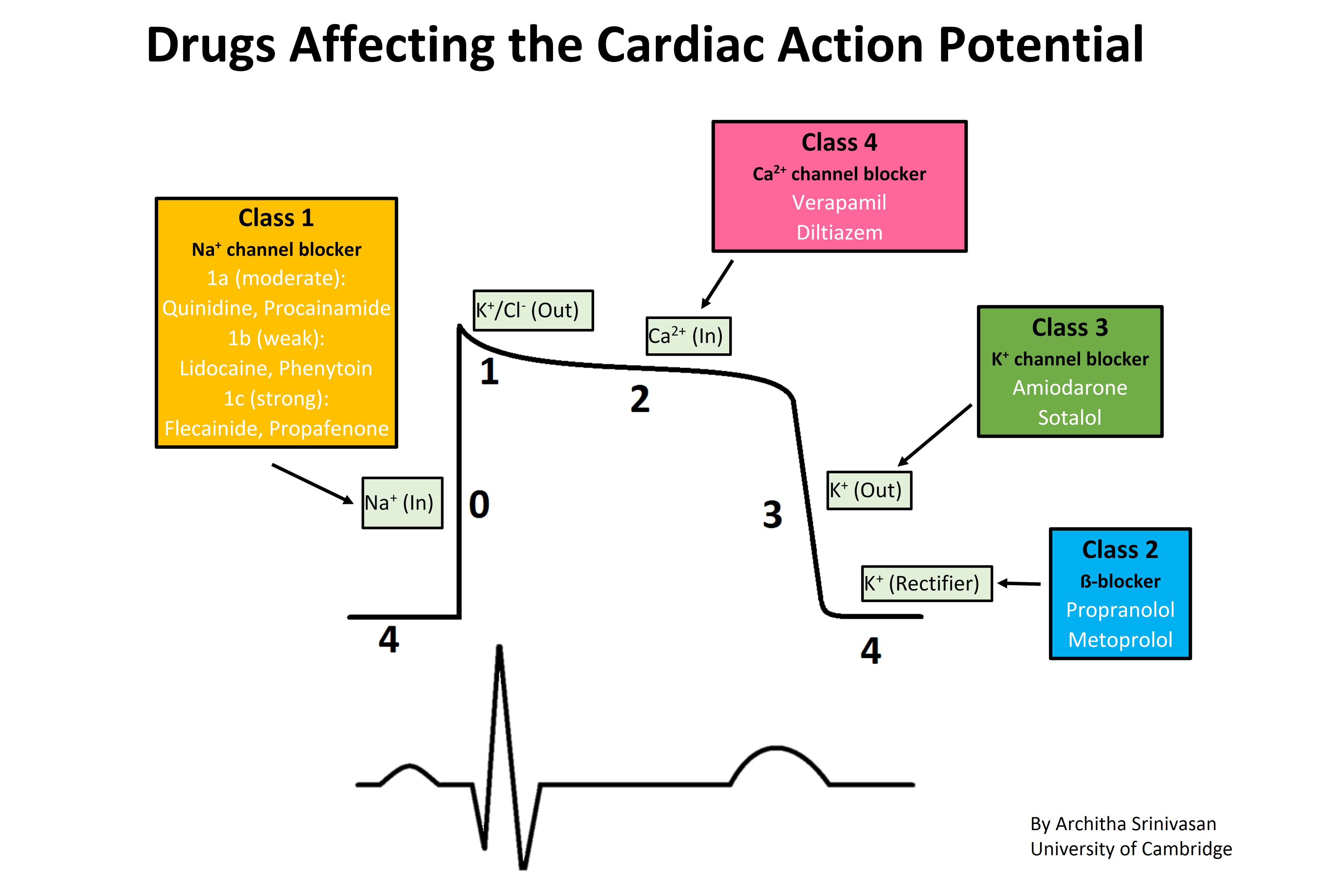
Drugs affecting the cardiac action potential. The sharp rise in voltage ("0") corresponds to the influx of sodium ions, whereas the two decays ("1" and "3", respectively) correspond to the sodium-channel inactivation and the repolarizing efflux of potassium ions. The characteristic plateau ("2") results from the opening of voltage-sensitive calcium channels. Contributed by Wikimedia User: Architha Srinivasan (CC BY-SA 4.0 https://creativecommons.org/licenses/by-sa/4.0/deed.en)
References
MOKLER CM, VAN ARMAN CG. Pharmacology of a new antiarrhythmic agent, gamma-diisopropyl-amino-alpha-phenyl-alpha-(2-pyridyl)-butyramide (SC-7031). The Journal of pharmacology and experimental therapeutics. 1962 Apr:136():114-24 [PubMed PMID: 14475124]
January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW, American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Journal of the American College of Cardiology. 2014 Dec 2:64(21):e1-76. doi: 10.1016/j.jacc.2014.03.022. Epub 2014 Mar 28 [PubMed PMID: 24685669]
Level 1 (high-level) evidenceFuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL, American College of Cardiology/American Heart Association Task Force on Practice Guidelines, European Society of Cardiology Committee for Practice Guidelines, European Heart Rhythm Association, Heart Rhythm Society. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006 Aug 15:114(7):e257-354 [PubMed PMID: 16908781]
Level 1 (high-level) evidenceFuster V, Rydén LE, Asinger RW, Cannom DS, Crijns HJ, Frye RL, Halperin JL, Kay GN, Klein WW, Lévy S, McNamara RL, Prystowsky EN, Wann LS, Wyse DG, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC Jr, Klein WW, Alonso-Garcia A, Blomström-Lundqvist C, de Backer G, Flather M, Hradec J, Oto A, Parkhomenko A, Silber S, Torbicki A, American College of Cardiology/American Heart Association Task Force on Practice Guidelines, European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation), North American Society of Pacing and Electrophysiology. ACC/AHA/ESC Guidelines for the Management of Patients With Atrial Fibrillation: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation) Developed in Collaboration With the North American Society of Pacing and Electrophysiology. Circulation. 2001 Oct 23:104(17):2118-50 [PubMed PMID: 11673357]
Level 1 (high-level) evidenceSherrid MV, Barac I, McKenna WJ, Elliott PM, Dickie S, Chojnowska L, Casey S, Maron BJ. Multicenter study of the efficacy and safety of disopyramide in obstructive hypertrophic cardiomyopathy. Journal of the American College of Cardiology. 2005 Apr 19:45(8):1251-8 [PubMed PMID: 15837258]
Level 2 (mid-level) evidenceDan GA, Martinez-Rubio A, Agewall S, Boriani G, Borggrefe M, Gaita F, van Gelder I, Gorenek B, Kaski JC, Kjeldsen K, Lip GYH, Merkely B, Okumura K, Piccini JP, Potpara T, Poulsen BK, Saba M, Savelieva I, Tamargo JL, Wolpert C, ESC Scientific Document Group. Antiarrhythmic drugs-clinical use and clinical decision making: a consensus document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) Working Group on Cardiovascular Pharmacology, endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and International Society of Cardiovascular Pharmacotherapy (ISCP). Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2018 May 1:20(5):731-732an. doi: 10.1093/europace/eux373. Epub [PubMed PMID: 29438514]
Level 3 (low-level) evidenceKarlson BW, Torstensson I, Abjörn C, Jansson SO, Peterson LE. Disopyramide in the maintenance of sinus rhythm after electroconversion of atrial fibrillation. A placebo-controlled one-year follow-up study. European heart journal. 1988 Mar:9(3):284-90 [PubMed PMID: 3289932]
Level 1 (high-level) evidenceVaughan Williams EM. A classification of antiarrhythmic actions reassessed after a decade of new drugs. Journal of clinical pharmacology. 1984 Apr:24(4):129-47 [PubMed PMID: 6144698]
Level 3 (low-level) evidenceKus T, Sasyniuk BI. Electrophysiological actions of disopyramide phosphate on canine ventricular muscle and purkinje fibers. Circulation research. 1975 Dec:37(6):844-54 [PubMed PMID: 1192576]
Level 3 (low-level) evidence. Oral disopyramide after admission to hospital with suspected acute myocardial infarction. U. K. Rythmodan Multicentre Study Group. Postgraduate medical journal. 1984 Feb:60(700):98-107 [PubMed PMID: 6369290]
Level 1 (high-level) evidenceNicholls DP, Haybyrne T, Barnes PC. Intravenous and oral disopyramide after myocardial infarction. Lancet (London, England). 1980 Nov 1:2(8201):936-8 [PubMed PMID: 6107587]
Level 1 (high-level) evidenceLafuente-Lafuente C, Valembois L, Bergmann JF, Belmin J. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. The Cochrane database of systematic reviews. 2015 Mar 28:(3):CD005049. doi: 10.1002/14651858.CD005049.pub4. Epub 2015 Mar 28 [PubMed PMID: 25820938]
Level 1 (high-level) evidencePriori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez-Madrid A, Nikolaou N, Norekvål TM, Spaulding C, Van Veldhuisen DJ, ESC Scientific Document Group. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). European heart journal. 2015 Nov 1:36(41):2793-2867. doi: 10.1093/eurheartj/ehv316. Epub 2015 Aug 29 [PubMed PMID: 26320108]
Adler A, Fourey D, Weissler-Snir A, Hindieh W, Chan RH, Gollob MH, Rakowski H. Safety of Outpatient Initiation of Disopyramide for Obstructive Hypertrophic Cardiomyopathy Patients. Journal of the American Heart Association. 2017 May 26:6(6):. doi: 10.1161/JAHA.116.005152. Epub 2017 May 26 [PubMed PMID: 28550094]
Sherrid MV, Arabadjian M. A primer of disopyramide treatment of obstructive hypertrophic cardiomyopathy. Progress in cardiovascular diseases. 2012 May-Jun:54(6):483-92. doi: 10.1016/j.pcad.2012.04.003. Epub [PubMed PMID: 22687589]
Conrad ME, Cumbie WG, Thrasher DR, Carpenter JT. Agranulocytosis associated with disopyramide therapy. JAMA. 1978 Oct 20:240(17):1857-8 [PubMed PMID: 691193]
Level 3 (low-level) evidenceKim SY, Benowitz NL. Poisoning due to class IA antiarrhythmic drugs. Quinidine, procainamide and disopyramide. Drug safety. 1990 Nov-Dec:5(6):393-420 [PubMed PMID: 2285495]
Level 3 (low-level) evidenceEdmonds ME, Hayler AM. A case of intra-hepatic cholestasis after disopyramide therapy. European journal of clinical pharmacology. 1980 Oct:18(3):285-6 [PubMed PMID: 7439249]
Level 3 (low-level) evidenceKirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2016 Nov:18(11):1609-1678 [PubMed PMID: 27567465]
Whiting B, Elliott HL. Disopyramide in renal impairment. Lancet (London, England). 1977 Dec 24-31:2(8052-8053):1363 [PubMed PMID: 74774]
Level 3 (low-level) evidenceBryson SM, Whiting B, Lawrence JR. Disopyramide serum and pharmacologic effect kinetics applied to the assessment of bioavailability. British journal of clinical pharmacology. 1978 Nov:6(5):409-19 [PubMed PMID: 728284]
Level 1 (high-level) evidenceBonde J, Graudal NA, Pedersen LE, Balsløv S, Angelo HR, Svendsen TL, Kampmann JP. Kinetics of disopyramide in decreased hepatic function. European journal of clinical pharmacology. 1986:31(1):73-7 [PubMed PMID: 3780831]
Echizen H, Saima S, Umeda N, Ishizaki T. Protein binding of disopyramide in liver cirrhosis and in nephrotic syndrome. Clinical pharmacology and therapeutics. 1986 Sep:40(3):274-80 [PubMed PMID: 3742934]
Gosselin B, Mathieu D, Chopin C, Wattel F, Dupuis B, Haguenoer JM, Desprez M. Acute intoxication with diisopyramide: clinical and experimental study by hemoperfusion an Amberlite XAD 4 resin. Clinical toxicology. 1980 Oct:17(3):439-49 [PubMed PMID: 7449357]
Level 3 (low-level) evidenceHayler AM, Holt DW, Volans GN. Fatal overdosage with disopyramide. Lancet (London, England). 1978 May 6:1(8071):968-9 [PubMed PMID: 76895]
Level 3 (low-level) evidenceSathyavagiswaran L. Fatal disopyramide intoxication from suicidal/accidental overdose. Journal of forensic sciences. 1987 Nov:32(6):1813-8 [PubMed PMID: 3323413]
Level 3 (low-level) evidenceHinderling PH, Garrett ER. Pharmacokinetics of the antiarrhythmic disopyramide in healthy humans. Journal of pharmacokinetics and biopharmaceutics. 1976 Jun:4(3):199-230 [PubMed PMID: 978389]