Introduction
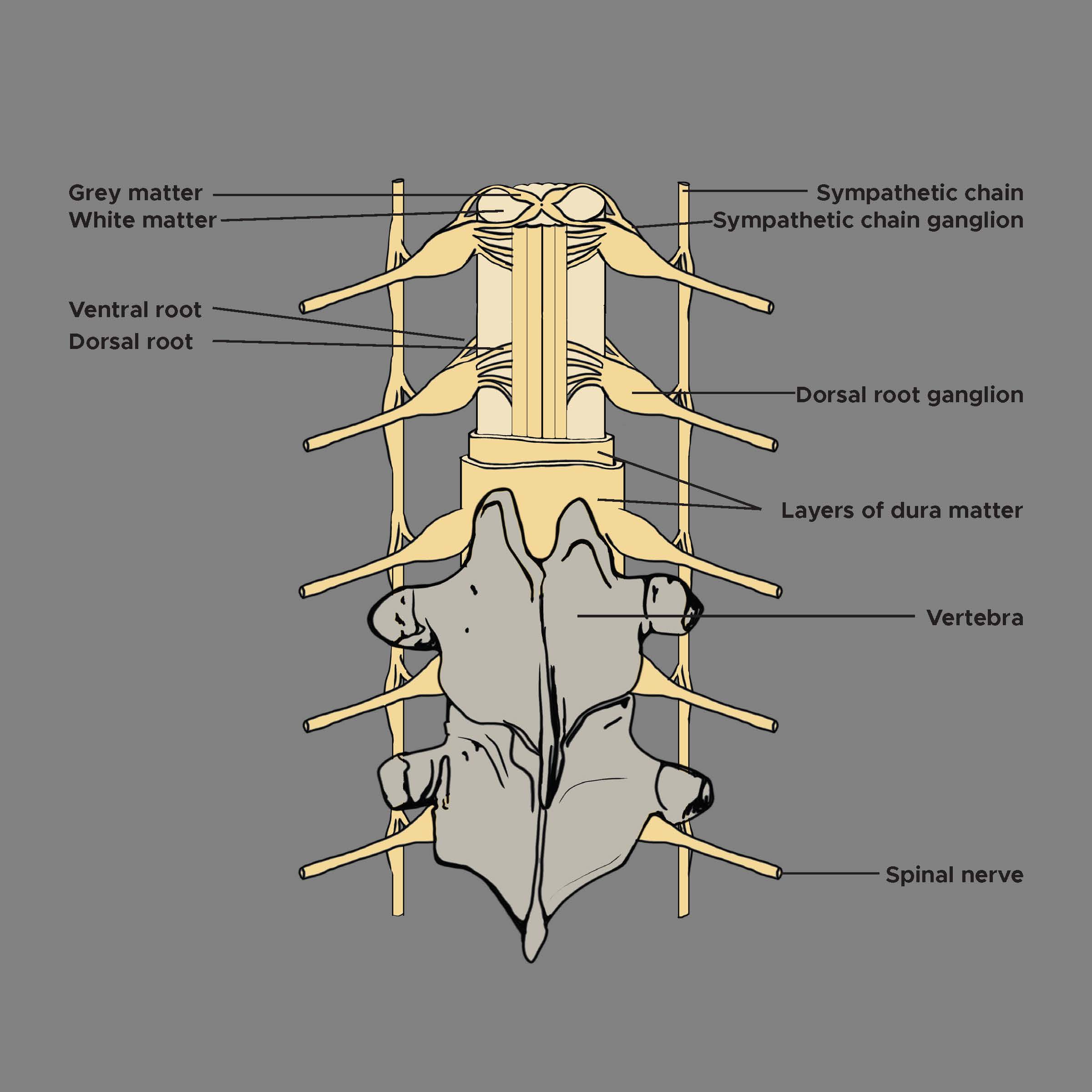
Dorsal nerve roots carry sensory neural signals to the central nervous system (CNS) from the peripheral nervous system (PNS). Anatomically, a dorsal root ganglion (DRG) emerges from the dorsal root of the spinal nerves (see Image. Dorsal Root Ganglion and Proximal Nerve Roots in the Spinal Foramen). They carry sensory messages from various receptors (ie, pain and temperature) at the periphery toward the central nervous system for a response. The role of DRG in chronic pain has been well established. The earliest technique of anesthetic infiltration of DRG was reported in 1949.[1] The DRG has been the focus of numerous interventions, including dorsal rhizotomy or gangliectomy, dorsal root entry zone (DREZ) lesioning (an adjacent related neural target), conventional radiofrequency denervation, pulsed radiofrequency, and steroid injection.[2]
Over the last decade, the DRG has been recognized as a viable option for neuromodulation therapy; electrical stimulation of primary sensory neuron somata is also considered a viable option in treating chronic pain.[3][4] Additionally, it is noted that DRG is an active participant in peripheral processes, including PAF injury, inflammation, and neuropathic pain development.[5] Properly understanding the significance and functioning of the DRG can help improve the diagnosis and treatment of clinical outcomes.
Structure and Function
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Structure and Function
Structure
The DRG participates in sensory transduction and modulation, including pain transmission.[6] The DRG, located within the dural sheath, is a bilateral structure found at every vertebral level and housed within fixed bony vertebral structures (neuroforamen) as it spans the transition from the spinal cord and vertebral column to the periphery.[7] The DRG (about the size of a tiny peanut) is an enlargement of the dorsal root that houses somata (cell bodies) of primary sensory neurons (PSNs); up to 15,000 neurons are present in each DRG at limb-innervating segmental levels. Somata diameters range from 20 to 150 μm. They can be categorized based on histologic staining of neurofilament density as "large-light" neurons (generally A-neurons, relaying non-noxious information) or "small-dark" neurons (generally C-neurons, relaying painful signals).[8]
The axons of these neurons are bundled into roots/nerves that contain a mix of fibers with varied excitability, including low-threshold mechanosensory fibers, higher-threshold Aβ nociceptors, and Aδ fibers. Aβ, Aδ, and C fibers carry peripheral sensation information to their respective soma in the DRG. Myelinated Aδ fibers have a relatively high velocity to carry acute nociceptive details (temperature, mechanical, and chemical-induced) to the DRG. Unmyelinated C fibers have a smaller diameter and slower conduction velocity. They also carry nociceptive input to the DRG but contribute to the more diffuse and more profound secondary pain after an injury. In addition, DRGs have a large population of glial cells; each DRG has approximately eightfold more glia than neurons.[9] Satellite glial cells are a specialized form of glia in the DRG that envelop each PSN to create an independent functional unit. They are physically separated from other PSN somata.[9][10]
DRG neurons are pseudounipolar; a single axon projects from the cell body and bifurcates at the T-junction. The peripheral portion of the axon extends to receptor endings in the periphery and is responsible for afferent signaling. The central part of the axon extends into the central nervous system (CNS). It shows considerable axonal arborizations into the spinal cord, terminating in synapses at ipsilateral or contralateral wide dynamic range neurons, inhibitory interneuron networks, and other targets in the dorsal horn.[11] In turn, other DRG fibers traverse the length of the dorsal columns to reach the dorsal column nuclei in the brainstem. These fibers—typically large-diameter central axons of Aβ primary sensory neurons—comprise the dorsal columns and are most commonly recruited in spinal cord stimulation (SCS).[12] Thus, a single PSN can span dramatically large anatomy.[8] Also, DRGs are intimately connected with the sympathetic chain via rami communicantes nerves.[13] The white rami communicantes nerve also serves as a conduit for discogenic afferents, conveying intrinsic spinal pain signals to the DRG.[14]
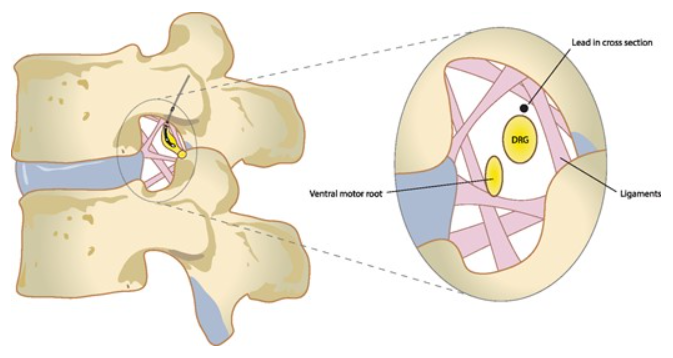
The 31 right and left spinal nerve pairs in humans form from afferent sensory dorsal axons (the dorsal root) and motor ventral efferent axons (the ventral root). The DRG is consistently positioned under the vertebral pedicle at the thoracic and lumbar levels. The use of magnetic resonance imaging (MRI) in asymptomatic subjects identifies the DRG in the foramen in 92% of L1, 98% of L2, 100% of L3, and L4, and 95% of L5, with the remainder of DRGs located in intraspinal or extraforaminal (lateral) regions (see Image. Dorsal Root Ganglion, Vertebrae, Grey and White Matter).[15] Another MRI study in healthy volunteers corroborated that 97.8 to 100% of L1-L4 DRGs are located in the foramen, with a small percentage in extraforaminal sites. At L5, most DRGs (94.3%) were found in the foramen, with the remainder (5.7%) in the intraspinal region.[16] Confirmatory findings exist in the form of cadaveric studies.[16] Distinct from thoracic and lumbar DRGs, those located in the sacrum have been either intracanalar (medial to the medial border of the sacral pedicle) or intraforaminal (lateral to the medial border of the sacral pedicle).
Function
The role of peripheral sensory neurons is to conduct action potentials from peripheral sensory neurons to central terminals for transmission to the central nervous system. Action potentials are characterized by ionic currents across excitatory membranes. Sodium depolarization is followed by potassium repolarization and increased intracellular calcium, a second messenger for development, excitability, neurotransmitter release, gene expression, and cell death.[6] Action potentials are normally generated in peripheral sensory endings in response to peripheral stimuli.[17][18][19] Axons of neurons transduce signals, while cell bodies support metabolism and act as gatekeepers (or de novo generators) of signal transmission from the periphery to the central nervous system. Action potentials generated by peripheral sensory nerves can cause depolarization of DRG cell bodies.[20] DRG-T connections normally impede the propagation of peripherally generated action potentials, acting as filters.[21][22]
DRG neurons have specialized membrane properties and are separated from each other within the ganglia. Each is encased in a layer of satellite glial cells with supporting functions. Almost all her DRG neurons undergo subthreshold excitation while activating other cell bodies. This is sometimes referred to as DRG "cross-depolarization" (or "cross-excitation"). Up to 90% of her DRG neurons depolarize when stimulation is applied to the axons of neighboring DRG neurons that share the same ganglion.[21] The receiving field and axonal branching of DRG neurons are highly detailed. One-third of the gelatinous neurons receive input from up to 4 different dorsal roots. At the spinal nerve level (before the DRG), C and Aδ fibers innervate a single skin area before diverging and then converge again on a single gelatinous neuron.[23]
Embryology
Along with the rest of the nervous system, the embryological onset of dorsal root ganglia occurs when the neural tube has formed, and neural crest cells arise at the margins of the neural tube. Neural crest cells differentiate into the peripheral nervous system components, the dorsal root ganglia. These neural crest cells migrate to various locations and differentiate into different cell types within the embryo. DRG cells develop at about 4 weeks postconception from the neural crest migration and migrate ventrally immediately after.[24]
During the seventh to eighth weeks, early bipolar neurons begin to appear. At about 11 weeks, unipolar neurons with a single broad process and well-developed organelles start to form. The onset of reflex responsiveness from the skin of the upper limb correlates with the appearance of (pseudo) unipolar neurons.[20] Because the dorsal root ganglion arises from neural crest cells rather than the neural tube, it can be considered the gray matter of the spinal cord migrates to the periphery of the spinal cord. Studies suggest that for neural crest cells to survive and potentially differentiate into the DRG, they require a signal from the CNS as early as the first hours after initiating the migration.[24]
Blood Supply and Lymphatics
Arterial Supply
The blood supply to the DRG is via 2 interconnected superficial and deep arterial plexuses. These plexuses arise from arteries descending from the radicular branches of the segmental arteries.[25] Muscular sphincters along the arterioles regulate blood flow to the DRG to meet the varying functional and metabolic demands. The DRG is an exception to the otherwise restricted permeability of the peripheral nervous system. While most of the PNS has a low permeability between blood and nervous tissue, like the blood-brain barrier, the DRG exhibits high permeability using its loose blood-nerve interface.[25] This vascular organization provides the human DRG with a robust blood supply, serving neurons with long processes with the required high-energy demand critical for maintaining the production and transport of receptors, ion channels, cytoskeletal and transport proteins. Additionally, its high permeability may have clinical implications, as this renders it susceptible to low- and high-molecular-weight neurotoxicants and toxic metabolites in drug-induced neuropathy.[26]
Venous Supply
Peri-ganglionic venous plexuses drain predominantly from the dorsal side of DRG into intervertebral veins.[26]
Nerves
DRG neurons are pseudo-unipolar neurons with a single axon that bifurcates into 2 separate branches, resulting in a distal and proximal process. Dorsal root neurons make up the spinal nerves when conjoined with ventral root neurons.[27]
Physiologic Variants
Understanding the physiologic DRG variants in humans has been limited, though there are several proposed rat models to explain the phenomena. Variants in the voltage-gated ion channels SCN9A, Na(v)1.7, and Troy are common sites of current DRG investigation in rats. Some variants considerably influenced the excitability of DRG neurons. For instance, the Na(v)1.7 variant 1739V was identified in some patients with autonomic dysfunction and neuropathic pain to impair slow inactivation within dorsal root ganglion neurons hyperexcitable.[28]
Surgical Considerations
Since the introduction of the gate control theory, the treatment of chronic pain has undergone many progressions. New treatment targets are continually identified, including the dorsal root ganglion, a relatively novel neural target.
Several current methods exist to reduce neuropathic pain directed at the DRG. These include:
- Ablation or modulation of the DRG using continuous thermal radiofrequency
- Pulsed radiofrequency
- Electrical DRG neurostimulator technologies
- Modification of DRG cellular function using viral vectors and gene silencing
- Dorsal root ganglionectomy
Of these techniques, DRG radiofrequency ablation is the most common therapeutic choice in favor of its non-surgical, minimally invasive approach. It is also beneficial because it can target inaccessible areas such as the lower back and foot.[29]
Studies have shown ganglionectomy, an irreversible neurosurgical technique, to be less effective in long-term pain reduction than radiofrequency and other neuromodulation techniques. While it can help treat dermatomal segmental pain, it can produce pain in other unwanted areas.[30]
Clinical Significance
Role of DRG in Neuropathic Pain
DRGs are involved in developing and maintaining neuropathic pain.[27] After the injury, DRGs undergo dramatic changes in phenotype and function, making them the source of pain signals to the brain.[31] After peripheral afferent nerve injury, an immune cascade involving leukocytes, macrophages, T cells, glial cells, and Schwann cells is initiated.[27] Increased numbers of major histocompatibility complex class II T lymphocytes and macrophages are seen in DRGs of injured peripheral nerves several months after injury. Infiltration of these inflammatory cells likely results in the prolonged release of excitatory cytokines, contributing to persistent pain despite the resolution of the original injury.[32] Glial cells also respond to peripheral nerve injury by proliferating and releasing inflammatory mediators.[9] Peripheral axotomy causes increased expression of neurotrophic factors in satellite glial cells surrounding sensory neuron somata in the DRG. These neutrophils within the DRG can induce persistent mechanical allodynia and cause neuropathic pain after peripheral nerve injury.[27][33][27]
The functional consequence of these changes is the sensitization and hyperexcitability of DRG neurons, leading to neuropathic pain.[27][30][27][34] After chronic contractile injury of peripheral axons, low-threshold voltage-gated calcium currents are significantly reduced. Loss of this inward calcium current and reduced extracellular shift of potassium may contribute to post-injury hyperexcitability.[27] Additionally, allodynia may be associated with elevated norepinephrine levels.[35]
Normal DRG neurons generate sinusoidal oscillation patterns through voltage-sensitive mechanisms, increasing frequency after nerve injury. When these oscillations reach a threshold, action potentials are generated. Upregulation of transmembrane sodium ion channels and increased sodium ion transport plays a significant role in the increased oscillations and, thus, the ectopic discharge associated with chronic neuropathic pain.[36] It has also been shown that DRG injury increases the number of Aβ fibers terminating in the dorsal horn of the spinal cord.[37]
Spontaneous action potentials are generated in the DRG after peripheral nerve injury and damage. However, they do not occur in lesions proximal to the DRG.[38] These action potentials can originate within the DRG from cell bodies and axons.[39] Many ectopic discharges originate from Aβ fibers, conveying tactile and vibratory sensations.[40] After nerve injury, electrophysiological changes occur that enable these fibers to transmit pain. These Aβ fibers may contribute significantly to central sensitization.
DRG as a Target in Neuromodulation of Pain
DRG stimulation is a clinically effective intervention. First reported in 2013, DRG stimulation was an effective treatment for chronic, intractable trunk and extremity pain, reducing pain in an average of 70% of subjects in a 4-week feasibility study and reducing drug use.[41] A multicenter, prospective study demonstrated sustained pain relief at 6 and 12 months, with individualized coverage of difficult-to-treat areas and no difference in paresthesia intensity with postural changes.[3][4][3] These results were subsequently replicated in a prospective multicenter study in patients with complex regional pain syndrome (CRPS).[42]
Multiple reports have suggested stimulation of DRG is likely an ideal treatment for pain in areas such as the foot and the groin.[3][43][44][45][46] These sites are more difficult to target with spinal cord stimulation (SCS) because the relevant dorsal column fibers are inaccessible to epidural stimulation and may require higher stimulation amplitudes that preferentially generate painful nerve root activation.[47] In DRG stimulation, recruitment at the somata avoids these issues. Additionally, DRG stimulation has shown benefits in treating disease states that have been underserved by traditional SCS, such as axial low back pain and discogenic pain, phantom limb pain, post-herpetic neuralgia, CRPS/causalgia, diabetic peripheral neuropathy, and perineal pain.[2][14][48][49][50][2][51][52] DRG stimulation can treat pain in various locations across the body as long as paresthesia coverage of the painful regions can be achieved.[13]
In a landmark randomized controlled trial of DRG stimulation vs. conventional SCS (the ACCURATE study), outcomes with DRG stimulation were statistically superior to those with SCS after 3 months of treatment, and pain relief and superiority were sustained through 12 months. Additionally, this trial demonstrated that DRG stimulation, compared with traditional tonic dorsal column SCS, provides greater specificity of stimulation for painful areas, less stimulation intensity with postural variation, and the ability to deliver paresthesia-free analgesia in some subjects.[2] Recently, excellent pain relief outcomes with DRG stimulation, durable through 3 years, have been reported.[53]
Outcomes with DRG stimulation are often compared with those with spinal cord stimulation (SCS), a treatment modality that has rightly earned a place in advanced treatment when conservative medical management has failed.[54] Its appeal has expanded as implantation techniques and neuromodulation technology have advanced. However, SCS may be effective only in a limited range of conditions and can provide incomplete relief.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
SORESI AL. Control of intractable pain by spinal ganglia block. American journal of surgery. 1949 Jan:77(1):72-8 [PubMed PMID: 18122204]
Deer TR, Levy RM, Kramer J, Poree L, Amirdelfan K, Grigsby E, Staats P, Burton AW, Burgher AH, Obray J, Scowcroft J, Golovac S, Kapural L, Paicius R, Kim C, Pope J, Yearwood T, Samuel S, McRoberts WP, Cassim H, Netherton M, Miller N, Schaufele M, Tavel E, Davis T, Davis K, Johnson L, Mekhail N. Dorsal root ganglion stimulation yielded higher treatment success rate for complex regional pain syndrome and causalgia at 3 and 12 months: a randomized comparative trial. Pain. 2017 Apr:158(4):669-681. doi: 10.1097/j.pain.0000000000000814. Epub [PubMed PMID: 28030470]
Level 2 (mid-level) evidenceLiem L, Russo M, Huygen FJ, Van Buyten JP, Smet I, Verrills P, Cousins M, Brooker C, Levy R, Deer T, Kramer J. One-year outcomes of spinal cord stimulation of the dorsal root ganglion in the treatment of chronic neuropathic pain. Neuromodulation : journal of the International Neuromodulation Society. 2015 Jan:18(1):41-8; discussion 48-9. doi: 10.1111/ner.12228. Epub 2014 Aug 21 [PubMed PMID: 25145467]
Liem L, Russo M, Huygen FJ, Van Buyten JP, Smet I, Verrills P, Cousins M, Brooker C, Levy R, Deer T, Kramer J. A multicenter, prospective trial to assess the safety and performance of the spinal modulation dorsal root ganglion neurostimulator system in the treatment of chronic pain. Neuromodulation : journal of the International Neuromodulation Society. 2013 Sep-Oct:16(5):471-82; discussion 482. doi: 10.1111/ner.12072. Epub 2013 May 13 [PubMed PMID: 23668228]
Noh MC, Mikler B, Joy T, Smith PA. Time Course of Inflammation in Dorsal Root Ganglia Correlates with Differential Reversibility of Mechanical Allodynia. Neuroscience. 2020 Jan 21:428():199-216. doi: 10.1016/j.neuroscience.2019.12.040. Epub 2020 Jan 7 [PubMed PMID: 31918012]
Hogan QH. Labat lecture: the primary sensory neuron: where it is, what it does, and why it matters. Regional anesthesia and pain medicine. 2010 May-Jun:35(3):306-11. doi: 10.1097/AAP.0b013e3181d2375e. Epub [PubMed PMID: 20460965]
BRIERLEY JB. The penetration of particulate matter from the cerebrospinal fluid into the spinal ganglia, peripheral nerves, and perivascular spaces of the central nervous system. Journal of neurology, neurosurgery, and psychiatry. 1950 Aug:13(3):203-15 [PubMed PMID: 14774743]
Devor M. Unexplained peculiarities of the dorsal root ganglion. Pain. 1999 Aug:Suppl 6():S27-S35. doi: 10.1016/S0304-3959(99)00135-9. Epub [PubMed PMID: 10491970]
Level 3 (low-level) evidenceZnaor L, Lovrić S, Hogan Q, Sapunar D. Association of neural inflammation with hyperalgesia following spinal nerve ligation. Croatian medical journal. 2007 Feb:48(1):35-42 [PubMed PMID: 17309137]
Level 3 (low-level) evidenceHanani M. Satellite glial cells in sensory ganglia: from form to function. Brain research. Brain research reviews. 2005 Jun:48(3):457-76 [PubMed PMID: 15914252]
Level 3 (low-level) evidenceLeijnse JN, D'Herde K. Revisiting the segmental organization of the human spinal cord. Journal of anatomy. 2016 Sep:229(3):384-93. doi: 10.1111/joa.12493. Epub 2016 May 12 [PubMed PMID: 27173936]
Oakley JC, Prager JP. Spinal cord stimulation: mechanisms of action. Spine. 2002 Nov 15:27(22):2574-83 [PubMed PMID: 12435996]
Level 3 (low-level) evidenceHunter CW, Sayed D, Lubenow T, Davis T, Carlson J, Rowe J, Justiz R, McJunkin T, Deer T, Mehta P, Falowski S, Kapural L, Pope J, Mekhail N. DRG FOCUS: A Multicenter Study Evaluating Dorsal Root Ganglion Stimulation and Predictors for Trial Success. Neuromodulation : journal of the International Neuromodulation Society. 2019 Jan:22(1):61-79. doi: 10.1111/ner.12796. Epub 2018 Aug 7 [PubMed PMID: 30085382]
Level 2 (mid-level) evidenceHuygen F, Liem L, Cusack W, Kramer J. Stimulation of the L2-L3 Dorsal Root Ganglia Induces Effective Pain Relief in the Low Back. Pain practice : the official journal of World Institute of Pain. 2018 Feb:18(2):205-213. doi: 10.1111/papr.12591. Epub 2017 Dec 6 [PubMed PMID: 28486758]
Hasegawa T, Mikawa Y, Watanabe R, An HS. Morphometric analysis of the lumbosacral nerve roots and dorsal root ganglia by magnetic resonance imaging. Spine. 1996 May 1:21(9):1005-9 [PubMed PMID: 8724082]
Ebraheim NA, Lu J. Morphometric evaluation of the sacral dorsal root ganglia. A cadaveric study. Surgical and radiologic anatomy : SRA. 1998:20(2):105-8 [PubMed PMID: 9658528]
Lirk P, Poroli M, Rigaud M, Fuchs A, Fillip P, Huang CY, Ljubkovic M, Sapunar D, Hogan Q. Modulators of calcium influx regulate membrane excitability in rat dorsal root ganglion neurons. Anesthesia and analgesia. 2008 Aug:107(2):673-85. doi: 10.1213/ane.0b013e31817b7a73. Epub [PubMed PMID: 18633052]
Level 3 (low-level) evidenceRush AM, Dib-Hajj SD, Liu S, Cummins TR, Black JA, Waxman SG. A single sodium channel mutation produces hyper- or hypoexcitability in different types of neurons. Proceedings of the National Academy of Sciences of the United States of America. 2006 May 23:103(21):8245-50 [PubMed PMID: 16702558]
Level 3 (low-level) evidenceKovalsky Y, Amir R, Devor M. Simulation in sensory neurons reveals a key role for delayed Na+ current in subthreshold oscillations and ectopic discharge: implications for neuropathic pain. Journal of neurophysiology. 2009 Sep:102(3):1430-42. doi: 10.1152/jn.00005.2009. Epub 2009 Jul 1 [PubMed PMID: 19571204]
Level 3 (low-level) evidenceTeillet MA, Kalcheim C, Le Douarin NM. Formation of the dorsal root ganglia in the avian embryo: segmental origin and migratory behavior of neural crest progenitor cells. Developmental biology. 1987 Apr:120(2):329-47 [PubMed PMID: 3549390]
Level 3 (low-level) evidenceUtzschneider D, Kocsis J, Devor M. Mutual excitation among dorsal root ganglion neurons in the rat. Neuroscience letters. 1992 Oct 26:146(1):53-6 [PubMed PMID: 1475049]
Level 3 (low-level) evidenceKent AR, Min X, Hogan QH, Kramer JM. Mechanisms of Dorsal Root Ganglion Stimulation in Pain Suppression: A Computational Modeling Analysis. Neuromodulation : journal of the International Neuromodulation Society. 2018 Apr:21(3):234-246. doi: 10.1111/ner.12754. Epub 2018 Jan 29 [PubMed PMID: 29377442]
Pinto V, Szûcs P, Derkach VA, Safronov BV. Monosynaptic convergence of C- and Adelta-afferent fibres from different segmental dorsal roots on to single substantia gelatinosa neurones in the rat spinal cord. The Journal of physiology. 2008 Sep 1:586(17):4165-77. doi: 10.1113/jphysiol.2008.154898. Epub 2008 Jul 17 [PubMed PMID: 18635648]
Level 3 (low-level) evidenceKalcheim C, Le Douarin NM. Requirement of a neural tube signal for the differentiation of neural crest cells into dorsal root ganglia. Developmental biology. 1986 Aug:116(2):451-66 [PubMed PMID: 3525281]
Level 3 (low-level) evidenceParke WW, Whalen JL. The vascular pattern of the human dorsal root ganglion and its probable bearing on a compartment syndrome. Spine. 2002 Feb 15:27(4):347-52 [PubMed PMID: 11840098]
Level 3 (low-level) evidenceHaberberger RV, Barry C, Dominguez N, Matusica D. Human Dorsal Root Ganglia. Frontiers in cellular neuroscience. 2019:13():271. doi: 10.3389/fncel.2019.00271. Epub 2019 Jun 19 [PubMed PMID: 31293388]
Krames ES. The role of the dorsal root ganglion in the development of neuropathic pain. Pain medicine (Malden, Mass.). 2014 Oct:15(10):1669-85. doi: 10.1111/pme.12413. Epub 2014 Mar 18 [PubMed PMID: 24641192]
Level 3 (low-level) evidenceHan C, Hoeijmakers JG, Liu S, Gerrits MM, te Morsche RH, Lauria G, Dib-Hajj SD, Drenth JP, Faber CG, Merkies IS, Waxman SG. Functional profiles of SCN9A variants in dorsal root ganglion neurons and superior cervical ganglion neurons correlate with autonomic symptoms in small fibre neuropathy. Brain : a journal of neurology. 2012 Sep:135(Pt 9):2613-28. doi: 10.1093/brain/aws187. Epub 2012 Jul 22 [PubMed PMID: 22826602]
Level 3 (low-level) evidencePope JE, Deer TR, Kramer J. A systematic review: current and future directions of dorsal root ganglion therapeutics to treat chronic pain. Pain medicine (Malden, Mass.). 2013 Oct:14(10):1477-96. doi: 10.1111/pme.12171. Epub 2013 Jun 26 [PubMed PMID: 23802747]
Level 1 (high-level) evidenceLiem L, van Dongen E, Huygen FJ, Staats P, Kramer J. The Dorsal Root Ganglion as a Therapeutic Target for Chronic Pain. Regional anesthesia and pain medicine. 2016 Jul-Aug:41(4):511-9. doi: 10.1097/AAP.0000000000000408. Epub [PubMed PMID: 27224659]
Liu CN, Wall PD, Ben-Dor E, Michaelis M, Amir R, Devor M. Tactile allodynia in the absence of C-fiber activation: altered firing properties of DRG neurons following spinal nerve injury. Pain. 2000 Apr:85(3):503-521. doi: 10.1016/S0304-3959(00)00251-7. Epub [PubMed PMID: 10781925]
Level 3 (low-level) evidenceHu P, McLachlan EM. Distinct functional types of macrophage in dorsal root ganglia and spinal nerves proximal to sciatic and spinal nerve transections in the rat. Experimental neurology. 2003 Dec:184(2):590-605 [PubMed PMID: 14769352]
Level 3 (low-level) evidenceSapunar D, Kostic S, Banozic A, Puljak L. Dorsal root ganglion - a potential new therapeutic target for neuropathic pain. Journal of pain research. 2012:5():31-8. doi: 10.2147/JPR.S26603. Epub 2012 Feb 16 [PubMed PMID: 22375099]
Harrison C, Epton S, Bojanic S, Green AL, FitzGerald JJ. The Efficacy and Safety of Dorsal Root Ganglion Stimulation as a Treatment for Neuropathic Pain: A Literature Review. Neuromodulation : journal of the International Neuromodulation Society. 2018 Apr:21(3):225-233. doi: 10.1111/ner.12685. Epub 2017 Sep 28 [PubMed PMID: 28960653]
Kanno T, Yaguchi T, Nishizaki T. Noradrenaline stimulates ATP release from DRG neurons by targeting beta(3) adrenoceptors as a factor of neuropathic pain. Journal of cellular physiology. 2010 Aug:224(2):345-51. doi: 10.1002/jcp.22114. Epub [PubMed PMID: 20432431]
Level 3 (low-level) evidenceAmir R, Michaelis M, Devor M. Membrane potential oscillations in dorsal root ganglion neurons: role in normal electrogenesis and neuropathic pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999 Oct 1:19(19):8589-96 [PubMed PMID: 10493758]
Level 3 (low-level) evidenceNakamura SI, Myers RR. Injury to dorsal root ganglia alters innervation of spinal cord dorsal horn lamina involved in nociception. Spine. 2000 Mar 1:25(5):537-42 [PubMed PMID: 10749628]
Level 3 (low-level) evidenceKajander KC, Wakisaka S, Bennett GJ. Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy in the rat. Neuroscience letters. 1992 Apr 27:138(2):225-8 [PubMed PMID: 1319012]
Level 3 (low-level) evidenceMa C, LaMotte RH. Multiple sites for generation of ectopic spontaneous activity in neurons of the chronically compressed dorsal root ganglion. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007 Dec 19:27(51):14059-68 [PubMed PMID: 18094245]
Level 3 (low-level) evidenceDevor M. Ectopic discharge in Abeta afferents as a source of neuropathic pain. Experimental brain research. 2009 Jun:196(1):115-28. doi: 10.1007/s00221-009-1724-6. Epub 2009 Feb 26 [PubMed PMID: 19242687]
Level 3 (low-level) evidenceDeer TR, Grigsby E, Weiner RL, Wilcosky B, Kramer JM. A prospective study of dorsal root ganglion stimulation for the relief of chronic pain. Neuromodulation : journal of the International Neuromodulation Society. 2013 Jan-Feb:16(1):67-71; discussion 71-2. doi: 10.1111/ner.12013. Epub 2012 Dec 14 [PubMed PMID: 23240657]
Level 3 (low-level) evidenceVan Buyten JP, Smet I, Liem L, Russo M, Huygen F. Stimulation of dorsal root ganglia for the management of complex regional pain syndrome: a prospective case series. Pain practice : the official journal of World Institute of Pain. 2015 Mar:15(3):208-16. doi: 10.1111/papr.12170. Epub 2014 Jan 23 [PubMed PMID: 24451048]
Level 2 (mid-level) evidenceMaino P, Koetsier E, Kaelin-Lang A, Gobbi C, Perez R. Efficacious Dorsal Root Ganglion Stimulation for Painful Small Fiber Neuropathy: A Case Report. Pain physician. 2017 Mar:20(3):E459-E463 [PubMed PMID: 28339448]
Level 3 (low-level) evidenceLiem L, Mekhail N. Management of Postherniorrhaphy Chronic Neuropathic Groin Pain: A Role for Dorsal Root Ganglion Stimulation. Pain practice : the official journal of World Institute of Pain. 2016 Sep:16(7):915-23. doi: 10.1111/papr.12424. Epub 2016 Feb 23 [PubMed PMID: 26914499]
Zuidema X, Breel J, Wille F. Paresthesia mapping: a practical workup for successful implantation of the dorsal root ganglion stimulator in refractory groin pain. Neuromodulation : journal of the International Neuromodulation Society. 2014 Oct:17(7):665-9; discussion 669. doi: 10.1111/ner.12113. Epub 2014 Feb 26 [PubMed PMID: 24571400]
Level 3 (low-level) evidenceSchu S, Gulve A, ElDabe S, Baranidharan G, Wolf K, Demmel W, Rasche D, Sharma M, Klase D, Jahnichen G, Wahlstedt A, Nijhuis H, Liem L. Spinal cord stimulation of the dorsal root ganglion for groin pain-a retrospective review. Pain practice : the official journal of World Institute of Pain. 2015 Apr:15(4):293-9. doi: 10.1111/papr.12194. Epub 2014 Apr 1 [PubMed PMID: 24690212]
Level 2 (mid-level) evidenceFeirabend HK, Choufoer H, Ploeger S, Holsheimer J, van Gool JD. Morphometry of human superficial dorsal and dorsolateral column fibres: significance to spinal cord stimulation. Brain : a journal of neurology. 2002 May:125(Pt 5):1137-49 [PubMed PMID: 11960902]
Eldabe S, Burger K, Moser H, Klase D, Schu S, Wahlstedt A, Vanderick B, Francois E, Kramer J, Subbaroyan J. Dorsal Root Ganglion (DRG) Stimulation in the Treatment of Phantom Limb Pain (PLP). Neuromodulation : journal of the International Neuromodulation Society. 2015 Oct:18(7):610-6; discussion 616-7. doi: 10.1111/ner.12338. Epub 2015 Aug 13 [PubMed PMID: 26268453]
Hunter CW, Yang A, Davis T. Selective Radiofrequency Stimulation of the Dorsal Root Ganglion (DRG) as a Method for Predicting Targets for Neuromodulation in Patients With Post Amputation Pain: A Case Series. Neuromodulation : journal of the International Neuromodulation Society. 2017 Oct:20(7):708-718. doi: 10.1111/ner.12595. Epub 2017 Mar 23 [PubMed PMID: 28337820]
Level 2 (mid-level) evidenceLynch PJ, McJunkin T, Eross E, Gooch S, Maloney J. Case report: successful epiradicular peripheral nerve stimulation of the C2 dorsal root ganglion for postherpetic neuralgia. Neuromodulation : journal of the International Neuromodulation Society. 2011 Jan:14(1):58-61; discussion 61. doi: 10.1111/j.1525-1403.2010.00307.x. Epub 2010 Nov 4 [PubMed PMID: 21992163]
Level 3 (low-level) evidenceEldabe S, Espinet A, Wahlstedt A, Kang P, Liem L, Patel NK, Vesper J, Kimber A, Cusack W, Kramer J. Retrospective Case Series on the Treatment of Painful Diabetic Peripheral Neuropathy With Dorsal Root Ganglion Stimulation. Neuromodulation : journal of the International Neuromodulation Society. 2018 Dec:21(8):787-792. doi: 10.1111/ner.12767. Epub 2018 Mar 25 [PubMed PMID: 29575331]
Level 2 (mid-level) evidenceZuidema X, Breel J, Wille F. S3 Dorsal Root Ganglion/Nerve Root Stimulation for Refractory Postsurgical Perineal Pain: Technical Aspects of Anchorless Sacral Transforaminal Lead Placement. Case reports in neurological medicine. 2016:2016():8926578. doi: 10.1155/2016/8926578. Epub 2016 Mar 30 [PubMed PMID: 27123351]
Level 3 (low-level) evidenceMorgalla MH, Fortunato M, Lepski G, Chander BS. Dorsal Root Ganglion Stimulation (DRGS) for the Treatment of Chronic Neuropathic Pain: A Single-Center Study with Long-Term Prospective Results in 62 Cases. Pain physician. 2018 Jul:21(4):E377-E387 [PubMed PMID: 30045604]
Level 3 (low-level) evidenceDeer TR, Mekhail N, Provenzano D, Pope J, Krames E, Leong M, Levy RM, Abejon D, Buchser E, Burton A, Buvanendran A, Candido K, Caraway D, Cousins M, DeJongste M, Diwan S, Eldabe S, Gatzinsky K, Foreman RD, Hayek S, Kim P, Kinfe T, Kloth D, Kumar K, Rizvi S, Lad SP, Liem L, Linderoth B, Mackey S, McDowell G, McRoberts P, Poree L, Prager J, Raso L, Rauck R, Russo M, Simpson B, Slavin K, Staats P, Stanton-Hicks M, Verrills P, Wellington J, Williams K, North R, Neuromodulation Appropriateness Consensus Committee. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation : journal of the International Neuromodulation Society. 2014 Aug:17(6):515-50; discussion 550. doi: 10.1111/ner.12208. Epub [PubMed PMID: 25112889]
Level 3 (low-level) evidence