Introduction
Echocardiography is the use of ultrasound to evaluate the structural components of the heart in a minimally invasive strategy. Although, prior to the invention of today's routinely used 2-dimensional echocardiography, there was motion-based (M-mode) echocardiography. In 1953, Inge Edler, regarded as the father of echocardiography, first described M-mode technology, which began the era of diagnostic noninvasive echocardiography.[1] M-Mode echocardiography was the combination of amplitude-based (A-mode) ultrasonography with Brightness-based (B-mode) techniques, which allowed the addition of a "time" dimension when the B-mode was swept across the oscilloscope; however this was not a picture, per se, but how structures evolved through the cardiac cycle.[2] This was the primary technology until two-dimensional (2D) ultrasonography was developed over the next decades. In 1973, S.L. Johnson developed 2D ultrasonography and doppler technology, which ultimately allowed physicians to detect blood flow in vessels, and, in 1979, Holen and Hatle found that by using the Bernoulli equation they could detect pressure gradients.[1] The combination of all these technologies is the echocardiography that is commonly used every day in today's medical profession.
The most commonly used technique among these is transthoracic echocardiography (TTE). This allows the clinician to obtain real-time sizes, structure, and function of the heart during the cardiac cycle. Another useful and important use of these methods is stress echocardiography. Stress echocardiography is the combination of standard transthoracic echocardiography and either pharmacological or physical stress to the cardiac structures to assess wall motion abnormalities. Physical stresses may include running on a treadmill, and pharmacological stress, including medications.[3] When higher resolution imaging of cardiac structures, including valves, is required, transesophageal echocardiography (TEE) is considered. TEE is more invasive than standard TTE, as it requires the insertion of a probe into the patient's esophagus to obtain images not hindered by the patient's chest wall, including; muscle, tissue, and bone. When more accurate and even higher-resolution imaging is needed, during intracardiac procedures, intracardiac echocardiography (ICE) is an option that can be considered.
Echocardiography is a low cost, at times minimally invasive, and readily available test that can provide information that can change the treatment course, and in some cases, provide real-time life-saving information. Many of the clinical uses of echocardiography are multidisciplinary in practice, and the overlap between the different utilities of echocardiogram is large. The addition of contrast to echocardiography, or the addition of strain to TTE are all examples of combinations of these utilities. The utilization of echocardiography is vast and can be applied in a variety of ways and a wide range of situations, and these forms will be discussed in detail.
Anatomy and Physiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Anatomy and Physiology
Echocardiography is one of the most commonly used, non-invasive method for looking at cardiac anatomy. Echocardiography is used to provide thin cross-sections of cardiac structures, this includes; left and right atrium, left and right ventricles, valves, and associated valvular structures.[4] The integration of doppler to echocardiography allows clinicians to assess cardiovascular hemodynamics, including blood flow across valves to diagnose valvular stenosis and/or regurgitation.[5] An important aspect of anatomy and physiology that is evaluated on echocardiography is the function of cardiac muscle during systole and diastole. The assessment of cardiac muscle during contractions is the bases of stress testing.[3] Those patients whose stress echocardiogram is positive for wall motion abnormalities would be considered for more invasive procedures, including cardiac catheterization.
There are four standard views of the heart chambers that are evaluated when using TTE; these include parasternal long axis, parasternal short axis, apical four-chamber, and subxiphoid. The parasternal long-axis view is obtained by placing the probe left of the sternum at the 4th intercostal space perpendicular to the chest wall. As the heart is rotated left in the chest, the initial chamber visualized is the right ventricular; however, views of the left ventricle left atrium, and aortic outflow tract is also obtained, as well as their associated valves, which include the mitral valve, tricuspid, and aortic valve.[6]
The parasternal short axis view is obtained by positioning the probe 90 degrees from the long axis view. The short axis will obtain a cross-section of the left ventricle, which is useful in assessing left ventricular wall function, as well as it's associated papillary muscles; additionally, a cross-section of the right ventricle can also be visualized.[6] The apical four-chamber view is obtained by positioning the probe by the left nipple in males, and beneath the left breast in females, the probe is directed cephalad towards the heart and right shoulder. Using this view, one is able to better compare the ventricles and to look for evidence of right heart strain that one would have from a large pulmonary embolism.[7] The subxiphoid view will obtain views of all heart chambers, the left lobe of the liver, and the inferior vena cava (IVC) as it enters the right atrium. Compressibility of the IVC has been used to assess the fluid status of patients, but in fact, only gives information about preload. If easily compressible (greater than 50% collapse), the patient may have low preload, and if not easily compressible, the patient has a high preload. IVC compressibility has been studied with variable recommendations; therefore, it should be used in conjunction with other modes of volume assessment, not as a lone marker.
Indications
The indications for the use of transthoracic echocardiography is vast, and the basis of using this test is known or suspected cardiac disease. The Journal of the American Society of Echocardiography has published appropriate use criteria in 2019, which covers the criteria in its entirety.[8]
The use of echocardiography in asymptomatic patients includes screening patients who have first degree relatives with hypertrophic cardiomyopathy (HCM), aortic aneurysms or dissections, and those athletes that have electrocardiographic (ECG) changes. These patients may not have any immediate complaints, but their strong family history should give the clinician a high pretest probability to order an Echocardiogram. For example, patients with HCM may have a family history of sudden cardiac death in 1st-degree relatives at a young age.
Second, the initial evaluation of patients with clinical signs of or symptoms of heart disease. This includes palpitations, syncope, pre-syncope, newly diagnosed bundle branch blocks, arrhythmias, ACS, exertional shortness of breath, and evaluation of pulmonary hypertension.
Third, follow-up on previously diagnosed heart failure or valvular disease. In regards to the former, a re-evaluation of the patient's ejection fraction with echocardiography, especially in those with acute heart failure exacerbations, would provide important information to better treat the patient.
Finally, the evaluation of patent foramen ovale in those with cerebrovascular accidents (CVA).[8] One of the common causes of CVA is cardioembolic disease. Echocardiography with "bubble study" is a way to evaluate for a patent foramen ovale (PFO). Agitated saline is injected into the heart chambers, and flow across the atrium is evaluated for a defect. This defect may require surgical closure.
Bedside echocardiography has many indications in addition to the above listed. Since the early 1990s, bedside echocardiography use by intensivists and emergency medicine physicians has been increasing. Bedside echocardiography is routinely employed in critically ill patients who may or may not have previously diagnosed cardiac disease, but now require vascular support. Bedside echocardiography in the undifferentiated shortness breath can help elucidate cardiac failure vs. pericardial effusion vs. non-cardiac etiology. Some of the most common indications for bedside echocardiography include suspicion for pulmonary embolism, hypotension or respiratory failure of unknown etiology, complications after cardiothoracic surgery, identifications of pericardial effusion, and evaluation of right ventricular dysfunction.[9]
The indications for the use of stress echocardiography is the suspicion for coronary artery disease. This includes patients with stable angina with intermediate probability for coronary artery disease, with a pre-test probability of 15% to 85%.[10] This includes Suspected CAD with negative cardiac troponin and/or non-diagnostic ECG. If a patient has unstable angina and/or increasing troponin levels, the decision may be made to proceed with Percutaneous Coronary Intervention instead of stress testing.
Transesophageal Echocardiography (TEE) is used because it provides a superior imaging quality of the posterior heart chambers and structures when compared to TTE, can be used during coronary artery bypass graft surgery, aids in placement of transcatheter devices, and may be performed at the bedside in clinically ill patients.[11] This includes assessing valvular structures when the diagnosis of Infective endocarditis is suspected, or pre-operative evaluations of thrombus prior to cardioversion. TTE is a good initiate test of choice to evaluate cardiac structures for vegetation seen in infective endocarditis; however, if clinical suspicion is high, a TEE must be obtained to properly evaluate the valves and associated valvular structures.
Intracardiac echocardiography (ICE) is used during transcatheter cardiac interventions. This includes closure of atrial septal defects, catheter-directed biopsy, thrombectomies, and during electrophysiological procedures, which include transeptal puncture.[12] As ICE obtains images from within the heart, higher resolution images are obtained to help guide catheter-directed procedures. Due to advances in TEE, there has been limited use for ICE; however, as there has been an increase in transvenous catheter procedures, ICE is being considered further.[12]
Intravascular ultrasound (IVUS) may be performed during cardiac catheterization to directly visualize atherosclerosis within the vessel walls; this is utilized when the extent and severity of stenosis are indeterminant during PCI.[13] Clinical trials have found improved luminal gains, decreased re-stenosis, stent thrombosis, myocardial infarction (MI), and the need for revascularization when compared to angiography alone. To overcome the limitations of both angiography and IVUS, the use of both during the same intervention can provide more accurate reconstructions of plaques size and volume.[14]
Strain rate (STR) Echocardiography, also known as deformation echocardiography, is indicated when there is high suspicion for wall motion abnormalities. However, TTE is initially used to evaluate wall abnormalities; it is based on visual estimates, which may be subjective because it is operator dependant.[15] If TTE imaging is not clear or evident enough, ST and STR echocardiography may be utilized as an adjunct for a more accurate and objective evaluation of wall motion abnormalities.
Three-dimensional (3D) echocardiography is used when a clearer image of cardiac structures, and how to relate to each other is required. This is used most often used with presurgical planning for interventional procedures, as it can prove improved views of valvular structures.[16] 3D echocardiography has also found to add prognostic value to wall motion abnormalities in those with ischemia and assessing viability in patients with myocardial infarction.[17]
Contrast echocardiography has been studied extensively in the assessment of myocardial perfusion and has been found to be of independent prognostic and diagnostic benefit.[18] The injection of agitated saline uses air microbubbles as contrast, which are used to detect right-to-left shunts in patients who are found to have a CVA or for evaluation of pulmonary arteriovenous malformations. Further clinical indications include the assessment of wall motion abnormalities at rest, apical left ventricular thrombus, rupture, and aneurysm, apical hypertrophy, cardiac masses, and during perfusion imaging.[18]
Contraindications
Standard echocardiography does not have absolute contraindications, other than a patient's refusal. It is important to know that patient body habitus may limit the image clarity and amount of useful data that can be obtained.
There are, however, contraindications to stress and transesophageal echocardiography. Some of the common contraindications to stress echocardiography include acute myocardial infarction within two days, severe symptomatic aortic stenosis, heart failure exacerbation, acute pericarditis, uncontrolled arrhythmias, hypertension (more than 200/110 mmHg), and unstable angina not stabilized medically.[10] Contraindications to transesophageal echocardiography include esophageal disease with strictures, tumors, acute gastrointestinal bleeding, and diverticula.[19] Further relative contraindications include hiatal hernias, facial or airway trauma, or chest radiation. Procedures with relative contraindications can be done when a full list of risks and benefits have been thoroughly discussed and agreed upon with the patient. Invasive imaging techniques have the same contraindications as the procedures needed to perform them, including cardiac catheterization.
Equipment
The equipment needed for transthoracic echocardiography includes an ultrasound machine with Doppler capabilities, a cardiac ultrasound probe, and an ultrasound gel. When undergoing stress echocardiography, ECG monitoring is required, and medications needed to increase heart rate, including, but not limited to, dobutamine, atropine, adenosine, and regadenoson. Finally, those undergoing transesophageal echocardiography will need possible tracheal intubation and sedation, with potential paralytic agents prior to the procedure.[11] These procedures are usually performed in an operating room, endoscopy suite, or catheterization laboratory under close monitoring by a cardiologist and/or anesthesiologist.
Personnel
When performing a transthoracic echocardiogram, a trained cardiovascular technician/sonographer is necessary. In the United States, an individual needs at least an associate's degree to become a cardiovascular sonographer.[20] After the adequate images have been obtained, a trained cardiologist is needed to read the echocardiography images. During transesophageal echocardiography, an anesthesiologist or CRNA is necessary to perform sedation and endotracheal intubation prior to the procedure if necessary, as well as a cardiologist must be present to oversee the procedure and guarantee adequate images are obtained.
When obtaining invasive imaging, an interventional cardiologist must be present because vessel access must be obtained, as well as a catheter must be advanced into the heart. However, within the emergency department and intensive care units, intensivists and emergency medicine physicians who are credentialed in bedside echocardiography are able to utilize echocardiography and interpret the results in the acute setting and administer appropriate measures.
Preparation
Transthoracic echocardiography needs minimal preparations; however, this includes removing clothing over the chest and potentially cardiac monitoring stickers, and the patient should lay in a decubitus position on their left side. It is important to keep patients modest and covered when possible. Preparations for transesophageal echocardiography are more intensive, as the patient would need to be sedated, and for more difficult studies, the patient may need to be intubated. When performing invasive imaging, the femoral and radial vessel insertion sites, including the groin or wrist, would need to be cleaned, prepped, and draped in a sterile technique. Prior to any procedure, a time out is completed, where the name, date of birth, and procedure being performed is read aloud and agreed upon by all involved.
Technique or Treatment
When performing transthoracic echocardiography, the patient should be in a left decubitus position. This allows the heart to fall closer to the anterior thoracic wall, making sonography easier. The probe is placed on the skin at multiple positions, including parasternal long axis, parasternal short axis, apical four-chamber, and subxiphoid. The probe is positioned in the intercostal spaces to avoid the scattering effects of bone.
While performing a TEE, the patient is in the supine position, with the head of the bed in a slight angle while and the probe is inserted into the mouth and advanced into the esophagus. A TEE bite block is inserted into the mouth, to allow easier transit of the probe. While performing any invasive imaging techniques, access is obtained at the femoral or radial vessels.
Complications
There is limited risk associated with TTE. There are, however, risks of esophageal perforation and bleeding with TEE. In those undergoing stress echocardiography, there is a risk of cardiac arrhythmias when administering medications to increase a patient's heart rate. Intracardiac echocardiography has an additional risk of bleeding as venous access must be obtained to insert the catheter.[21] Furthermore, there is a risk of puncture to the cardiac structures while the catheter is in the heart anatomy, including wall rupture, hematoma, effusion, or tamponade.
Clinical Significance
Transthoracic Echocardiography (TTE): When a patient presents clinically with dyspnea or chest pain, and there is a high pre-test probability that the shortness of breath is of cardiac origin, TTE is recommended. It is useful in identifying the cause, location, and severity of myocardial ischemia, atrial sizes, ejections fraction (EF), left ventricular hypertrophy, and presence of valvular disease.[22] An EF is a common piece of information that is available from a TTE, especially in those with systolic congestive heart failure (CHF), because it dictates the course of treatment. An important assessment of cardiac structure by TTE is the size and thickness of the cardiac structures. Left and right atrial size will help aid in the diagnosis of diastolic dysfunction, and right and left ventricular hypertrophy will aid in the diagnosis of heart failure. This is especially important when the diagnosis of hypertrophic cardiomyopathy (HCM) is suspected. Asymmetric hypertrophy of left and right ventricles is the bases of the diagnosis for HCM. Patients who are found to have preserved ejection fraction (>50%) CHF are treated mainly with diuretics (furosemide, bumetanide) for symptom management.[23] On the contrary, those who are found to have reduced EF (<50%) CHF may be treated with diuretics, with the addition of beta-blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and aldosterone antagonist which have shown to have a mortality benefit. This benefit is not seen in those with preserved EF heart failure. The evaluation of the mitral, tricuspid, aortic, and pulmonary valves is another important indication of TTE. Patients who have a new-onset heart murmur, clinical suspicion for infective endocarditis, dyspnea, and near-syncope may have structural valve pathology including tricuspid, mitral, aortic, and pulmonary stenosis and regurgitation. TEE is more sensitive for the diagnosis of valvular pathology, although TTE is generally performed first, as it is less invasive.[22] Another aspect of TTE is assessing heart chamber pressures, and the most useful of these is right pulmonary artery pressure, which aids in the diagnosis of pulmonary artery hypertension. In the acute setting, bedside TTE can assess pericardial effusions, and aid in the diagnosis of cardiac tamponade, this may require immediate interventions, including pericardiocentesis.
Bedside Echocardiography: The use of bedside echocardiography is widespread and allows a physician to obtain immediate information in the acute setting. A common use is an assessment of cardiovascular response while hydrating a hypovolemic patient, however, as mentioned above, this gives you information about pre-load, and further clinical assessment must be taken into account.[24] The use of bedside TTE for the assessment of pulmonary embolism has shown to have a specificity of 83% and sensitivity of 53%.[25] The most common "sign" used was the demonstration of right heart strain due to a pulmonary embolism. Due to its low sensitivity but high specificity, it may be an adequate rule-in test during critical situations at the bedside.
Transesophageal Echocardiography (TEE): When a patient has a high index of suspicion for infective endocarditis, it is important to have a much clearer image of the valvular structure to identify valvular prolapse, rupture, or infective vegetation on the valves[11] The ability to recognize vegetation will drive the time course of antibiotic treatment, TTE is not always sensitive enough to identity vegetation. Patients who undergo cardioversion for atrial fibrillation or flutter, who have not been anticoagulated previously, will need to undergo TEE to assess for thrombus first. TEE is able to assess the left atrial appendage (LAA) for the presence of thrombus, which is the most commonplace for the formation of clots. If a patient were to undergo cardioversion with a thrombus present, it could dislodge and cause a cerebrovascular accident. If a thrombus is not identified on echocardiography, the cardioversion may proceed.[11] Finally, when patients are undergoing intracardiac transcatheter valve procedures, septal closure device placement, or catheter ablation, TTE is generally used as it provides clearer imaging to assist in the procedures in real-time.
Stress Echocardiography: When appropriate, stress echocardiography is a great initial modality to assess for cardiac ischemia. First, the patient's heart function is assessed at rest to determine a baseline, next the heart is stressed using either running on a treadmill or pharmacological stressors, including dobutamine, adenosine, or dipyridamole.[22] Next, using ECG and echocardiogram, cardiac rhythm and images are obtained after the patient has achieved appropriate exercise capacity or 85% of age-predicted maximum heart rate.[22] If ECG changes and new or worsening wall motion abnormalities are seen on echocardiography, the patient will likely need to undergo cardiac catheterization and potentially percutaneous coronary intervention (PCI) with coronary stent placement if there is stenosis identified. If patients have significant chest pain or are unable to reach appropriate exercise capacity prior to completion, stress testing may be halted, and the patient would be referred for cardiac catheterization with possible PCI.
Intracardiac Echocardiography (ICE): The use of ICE is less frequent given its invasive technique, cost, steep learning curve, and due to the advancements in TTE and TEE.[12] The advantages of ICE include higher resolution imaging and avoids the need for sedation or endotracheal intubation.[26] As atrial septal defect closures are becoming more common to perform via transvenous route, and because TTE and TEE imaging may be limited, ICE provides better visualization of intra-cardiac structures for a safer and more accurate transeptal catheterization needed for repair.[27] Furthermore, the use of ICE during catheter ablations is helpful as it improves tissue contact while using the circular mapping catheter. ICE may lower procedural time and improve the optimal size of lesion formation during ablation, and thus, leading to improved success rates. However, the use of ICE during left-atrial appendage (LAA) closure was evaluated in the ICE-CHIP trial, and the study found the use of ICE form the right atrium, was inadequate at identifying a thrombus in the LAA.[26] Further advances in ICE are needed for LAA visualization, and at this time, TEE is more sensitive at identifying a thrombus in the LAA when compared to ICE.
Intravascular Ultrasound (IVUS): The mainstay of treatment for coronary artery disease is cardiac catheterization when appropriate, and most commonly, it is performed under the guidance of angiography. Contrast is introduced into the cardiac vasculature while radiography is performed, allowing the visualization of coronary anatomy. This is also the technique interventional cardiologists utilize to guide their wire and assess stenosis. When stenosis is <40%, no intervention is generally required, but with a greater than 70% stenosis, a percutaneous coronary intervention (PCI) is performed.[28] However, if lesions are in the indeterminate range, 40% to 70% stenosis, further imaging, such as IVUS, is required to better assess the lesion and guide intervention.[13] The blood flow and pressure is measured proximal and distal to the lesion, and the flow and pressure differential is calculated across the area of stenosis. If the lesion is causing significant flow defects, coronary stenting is likely to be performed. This utilization is called Fractional flow reserve (FFR), and Instantaneous wave-free Ratio (IFR), which is discussed in much greater detail in it's associated StatPearls.[28]
Strain rate (STR) Echocardiography: TTE is able to detect wall motion abnormalities in those with ischemic or non-ischemic cardiomyopathy; however, when these abnormalities are sub-clinical, STR echocardiography is a more sensitive utility. Early detection in asymptomatic patients is an important use of STR echocardiography, especially in those with diabetes, amyloidosis, and muscular dystrophies.[15] Furthermore, it has been shown that STR imaging is sensitive enough to detect early cardiac injury secondary to doxorubicin-based chemotherapy.[15] Dandel et al. described the utility of differentiating between an athlete's physiological hypertrophy and asymptomatic HCM. This utility is important as HCM is known to cause sudden cardiac death in young athletes. A study from Belgium also described that the use of STR echocardiography was the superior method in identifying cardiac tissue changes and fibrosis in patients with HCM.[29] Another important utility is the assessment of cardiac viability. The augmentation of STR imaging with dobutamine has been shown to improve the diagnostic and prognostic evaluation of ischemia and post-myocardial infarction scar tissue formation.[15] It is important to understand that STR imaging can be applied to other forms of echocardiography; this includes TEE, TTE, and 3D echocardiography. It is an adjunct to improve visualization of wall motion defects.
3-dimensional (3D) Echocardiography: The use of traditional 2D echocardiography is limited due to foreshortened views of the ventricles and atria, and this can underestimate volumes.[30] 3D imaging allows the user to select images that are not foreshortened, which eliminates the geometric assumptions performed when assessing ventricular and atrial volumes. 3D echocardiography can further improve the visualization of shapes and spatial relationships between cardiac structures, as well as improve the visualization and function of valves and valvular structure.[30] Qiangjun et al. describe a case of hepatic metastasis to the tricuspid and pulmonic valve, in which 2D echocardiography only visualized severe tricuspid and pulmonary regurgitation. However, 3D echocardiography was able to detect fixed, retracted, and thickened valves during the cardiac cycle; this utility provided significant data to the practitioners to allow for the diagnosis of metastatic disease.[31] The left atrial appendage is the most common place where thrombi form in patients with atrial fibrillation, and in those with a contraindication to anticoagulation, a left atrial appendage closure device such as a Watchman device may be placed. Interventional cardiologists are able to use 3D echocardiography to simulate the procedure morphology prior to placing the closure device.[30] 3D echocardiography is an invaluable tool that is being used more frequently with all forms of echocardiography to improve clinical assessments.
Contrast Echocardiography: When a patient has an embolic cerebrovascular accident, the most common origin is cardioembolic. Contrast-enhanced echocardiography is performed to assess for the presence of a patent foramen ovale, which may require surgical closure.[18] Agitated saline is used as a contrast media and injected into the right atrium, which is routinely called a "bubble study," TTE is performed to detect the passing of the bubbles from the right atrium to the left atrium. When a patient is to undergo cardioversion for arrhythmia and has not been anticoagulated for at least three weeks, a TEE is often performed to identify possible left atrial appendage thrombus and prevent dislodgement during cardioversion. Echocardiography contrast may obscure the visualization of a thrombus; on the other hand, hypoechogenic thrombi may be obscured during standard TEE.[32] Two studies involved using contrast-enhanced and non-contrast-enhanced TEE in the detection of cardiac thrombi found that contrast-enhanced imaging was able to better delineate thrombi and was able to further identify the presence of thrombi more frequently than non-contrast-enhanced imaging.[33][34] If non-contrast imaging is inconclusive, then the use of contrast-enhanced imaging may be warranted.
Enhancing Healthcare Team Outcomes
The decision to order an echocardiogram requires an interprofessional team approach. The assessment of fluid status is a physical exam finding that can be performed not only by physicians but also by the nursing staff, and this can be used to help aid in the decision to order an echocardiogram. Interprofessional communication is important when treating any patient, including those with a cardiac history, and it is vital for all staff to continuously assess a patient's fluid status and need for further imaging assessments. Adequate communication about the specific indication for echocardiography for each specific patient, will help ensure optimal views and help improve the clinical utility of this test.
Media
(Click Video to Play)
Transesophageal Echocardiogram. Transesophageal echocardiogram, before and after patent foramen ovale closure, revealing delayed intracardiac shunting and hypoxemia after massive pulmonary embolism.
Weig T, Dolch M, Frey L, et al. Delayed intracardial shunting and hypoxemia after massive pulmonary embolism in a patient with a biventricular assist device. J Cardiothorac Surg. 2011;6:133.
doi: 10.1186/1749-8090-6-133.
(Click Image to Enlarge)
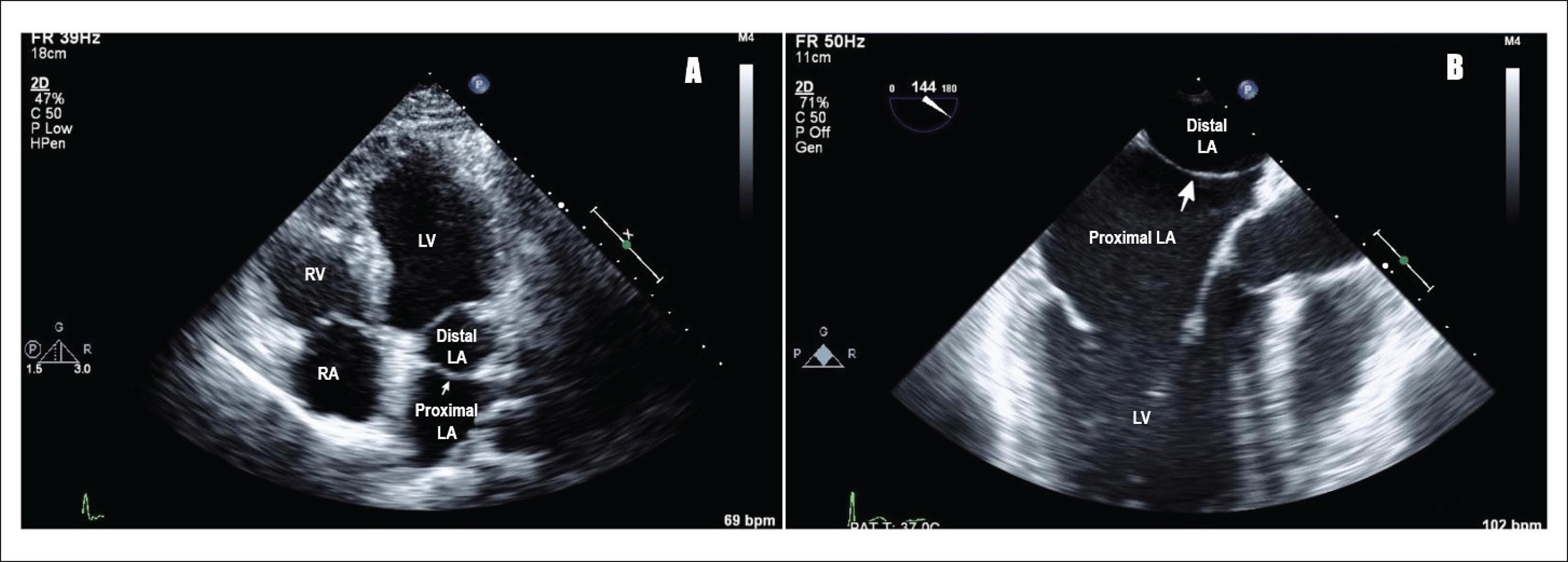
Cor Triatriatum. A) Transthoracic echocardiogram showing cor triatriatum: proximal and distal left atrium separated by a membrane (pointing white arrow), LA (left atrium), LV (left ventricle), RV (right ventricle), RA (right atrium). B) Transesophageal echocardiogram showing cor triatriatum: proximal and distal left atrium separated by a membrane (pointing white arrow), LA (left atrium), and LV (left ventricle).
Raheja H, Namana V, Moskovits N, Hollander G, Shani J. Cor triatriatum sinistrum. Arq. Bras. Cardiol. 2018;110(1). doi: 10.5935/abc.20170138. CC license 4.0.
References
Singh S, Goyal A. The origin of echocardiography: a tribute to Inge Edler. Texas Heart Institute journal. 2007:34(4):431-8 [PubMed PMID: 18172524]
Feigenbaum H. Role of M-mode technique in today's echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2010 Mar:23(3):240-57; 335-7. doi: 10.1016/j.echo.2010.01.015. Epub [PubMed PMID: 20206828]
Kossaify A, Bassil E, Kossaify M. Stress Echocardiography: Concept and Criteria, Structure and Steps, Obstacles and Outcomes, Focused Update and Review. Cardiology research. 2020 Apr:11(2):89-96. doi: 10.14740/cr851. Epub 2020 Mar 10 [PubMed PMID: 32256915]
Faletra FF, Ho SY, Leo LA, Paiocchi VL, Mankad S, Vannan M, Moccetti T. Which Cardiac Structure Lies Nearby? Revisiting Two-Dimensional Cross-Sectional Anatomy. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2018 Sep:31(9):967-975. doi: 10.1016/j.echo.2018.04.014. Epub 2018 Jun 27 [PubMed PMID: 29958761]
Level 2 (mid-level) evidencePearlman AS. Celebrating (More Than) 50 Years of Doppler Echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2018 Dec:31(12):1307. doi: 10.1016/j.echo.2018.08.002. Epub 2018 Sep 18 [PubMed PMID: 30241928]
Rylski B, Branchetti E, Bavaria JE, Vallabhajosyula P, Szeto WY, Milewski RK, Desai ND. Modeling of predissection aortic size in acute type A dissection: More than 90% fail to meet the guidelines for elective ascending replacement. The Journal of thoracic and cardiovascular surgery. 2014 Sep:148(3):944-8.e1. doi: 10.1016/j.jtcvs.2014.05.050. Epub 2014 May 22 [PubMed PMID: 24998700]
Level 2 (mid-level) evidenceLang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European heart journal. Cardiovascular Imaging. 2015 Mar:16(3):233-70. doi: 10.1093/ehjci/jev014. Epub [PubMed PMID: 25712077]
Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P, Rating Panel Members, Appropriate Use Criteria Task Force. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2019 Appropriate Use Criteria for Multimodality Imaging in the Assessment of Cardiac Structure and Function in Nonvalvular Heart Disease : A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and the Society of Thoracic Surgeons. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2019 Aug:26(4):1392-1413. doi: 10.1007/s12350-019-01751-7. Epub [PubMed PMID: 31250324]
Casaroto E, Mohovic T, Pinto LM, Lara TR. Bedside echocardiography in critically ill patients. Einstein (Sao Paulo, Brazil). 2015 Oct-Dec:13(4):644-6. doi: 10.1590/S1679-45082015MD3271. Epub [PubMed PMID: 26761560]
Steeds RP, Wheeler R, Bhattacharyya S, Reiken J, Nihoyannopoulos P, Senior R, Monaghan MJ, Sharma V. Stress echocardiography in coronary artery disease: a practical guideline from the British Society of Echocardiography. Echo research and practice. 2019 Jun 1:6(2):G17-G33. doi: 10.1530/ERP-18-0068. Epub [PubMed PMID: 30921767]
Hahn RT, Abraham T, Adams MS, Bruce CJ, Glas KE, Lang RM, Reeves ST, Shanewise JS, Siu SC, Stewart W, Picard MH, American Society of Echocardiography, Society of Cardiovascular Anesthesiologists. Guidelines for performing a comprehensive transesophageal echocardiographic examination: recommendations from the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. Anesthesia and analgesia. 2014 Jan:118(1):21-68. doi: 10.1213/ANE.0000000000000016. Epub [PubMed PMID: 24356157]
Alkhouli M, Hijazi ZM, Holmes DR Jr, Rihal CS, Wiegers SE. Intracardiac Echocardiography in Structural Heart Disease Interventions. JACC. Cardiovascular interventions. 2018 Nov 12:11(21):2133-2147. doi: 10.1016/j.jcin.2018.06.056. Epub [PubMed PMID: 30409271]
Otake H, Kubo T, Shinke T, Hibi K, Tanaka S, Ishida M, Kataoka T, Takaya T, Iwasaki M, Sonoda S, Ioji T, Akasaka T. OPtical frequency domain imaging vs. INtravascular ultrasound in percutaneous coronary InterventiON in patients with Acute Coronary Syndrome: Study protocol for a randomized controlled trial. Journal of cardiology. 2020 Sep:76(3):317-321. doi: 10.1016/j.jjcc.2020.03.010. Epub 2020 Apr 24 [PubMed PMID: 32340781]
Level 1 (high-level) evidenceShi C, Luo X, Guo J, Najdovski Z, Fukuda T, Ren H. Three-Dimensional Intravascular Reconstruction Techniques Based on Intravascular Ultrasound: A Technical Review. IEEE journal of biomedical and health informatics. 2018 May:22(3):806-817. doi: 10.1109/JBHI.2017.2703903. Epub 2017 May 12 [PubMed PMID: 28504955]
Dandel M, Lehmkuhl H, Knosalla C, Suramelashvili N, Hetzer R. Strain and strain rate imaging by echocardiography - basic concepts and clinical applicability. Current cardiology reviews. 2009 May:5(2):133-48. doi: 10.2174/157340309788166642. Epub [PubMed PMID: 20436854]
Levine RA, Handschumacher MD, Sanfilippo AJ, Hagege AA, Harrigan P, Marshall JE, Weyman AE. Three-dimensional echocardiographic reconstruction of the mitral valve, with implications for the diagnosis of mitral valve prolapse. Circulation. 1989 Sep:80(3):589-98 [PubMed PMID: 2766511]
Corsi C, Lang RM, Veronesi F, Weinert L, Caiani EG, MacEneaney P, Lamberti C, Mor-Avi V. Volumetric quantification of global and regional left ventricular function from real-time three-dimensional echocardiographic images. Circulation. 2005 Aug 23:112(8):1161-70 [PubMed PMID: 16103242]
Level 1 (high-level) evidenceEskandari M, Monaghan M. Contrast echocardiography in daily clinical practice. Herz. 2017 May:42(3):271-278. doi: 10.1007/s00059-017-4533-x. Epub [PubMed PMID: 28160033]
O'Rourke MC, Goldstein S, Mendenhall BR. Transesophageal Echocardiogram. StatPearls. 2023 Jan:(): [PubMed PMID: 28723055]
Nicastro I, Barletta V, Conte L, Fabiani I, Morgantini A, Lastrucci G, Bello VD. Professional Education, Training and Role of the Cardiac Sonographer in Different Countries. Journal of cardiovascular echography. 2013 Jan-Mar:23(1):18-23. doi: 10.4103/2211-4122.117981. Epub [PubMed PMID: 28465879]
Basman C, Parmar YJ, Kronzon I. Intracardiac Echocardiography for Structural Heart and Electrophysiological Interventions. Current cardiology reports. 2017 Sep 6:19(10):102. doi: 10.1007/s11886-017-0902-6. Epub 2017 Sep 6 [PubMed PMID: 28879526]
Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG, American Society of Echocardiography. American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2007 Sep:20(9):1021-41 [PubMed PMID: 17765820]
Xie F, Zheng C, Yuh-Jer Shen A, Chen W. Extracting and analyzing ejection fraction values from electronic echocardiography reports in a large health maintenance organization. Health informatics journal. 2017 Dec:23(4):319-328. doi: 10.1177/1460458216651917. Epub 2016 Jun 7 [PubMed PMID: 27271114]
Boyd JH, Sirounis D, Maizel J, Slama M. Echocardiography as a guide for fluid management. Critical care (London, England). 2016 Sep 4:20(1):274. doi: 10.1186/s13054-016-1407-1. Epub 2016 Sep 4 [PubMed PMID: 27592289]
Fields JM, Davis J, Girson L, Au A, Potts J, Morgan CJ, Vetter I, Riesenberg LA. Transthoracic Echocardiography for Diagnosing Pulmonary Embolism: A Systematic Review and Meta-Analysis. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2017 Jul:30(7):714-723.e4. doi: 10.1016/j.echo.2017.03.004. Epub 2017 May 9 [PubMed PMID: 28495379]
Level 1 (high-level) evidenceSaksena S, Sra J, Jordaens L, Kusumoto F, Knight B, Natale A, Kocheril A, Nanda NC, Nagarakanti R, Simon AM, Viggiano MA, Lokhandwala T, Chandler ML, ICE-CHIP Investigator Study Group. A prospective comparison of cardiac imaging using intracardiac echocardiography with transesophageal echocardiography in patients with atrial fibrillation: the intracardiac echocardiography guided cardioversion helps interventional procedures study. Circulation. Arrhythmia and electrophysiology. 2010 Dec:3(6):571-7. doi: 10.1161/CIRCEP.110.936161. Epub 2010 Sep 18 [PubMed PMID: 20852299]
Level 1 (high-level) evidenceEnriquez A, Saenz LC, Rosso R, Silvestry FE, Callans D, Marchlinski FE, Garcia F. Use of Intracardiac Echocardiography in Interventional Cardiology: Working With the Anatomy Rather Than Fighting It. Circulation. 2018 May 22:137(21):2278-2294. doi: 10.1161/CIRCULATIONAHA.117.031343. Epub [PubMed PMID: 29784681]
Soos MP, Gonzalez-Morales D, McComb D. Instantaneous Wave-Free Ratio. StatPearls. 2023 Jan:(): [PubMed PMID: 31082183]
Pagourelias ED, Mirea O, Duchenne J, Unlu S, Van Cleemput J, Papadopoulos CE, Bogaert J, Vassilikos VP, Voigt JU. Speckle tracking deformation imaging to detect regional fibrosis in hypertrophic cardiomyopathy: a comparison between 2D and 3D echo modalities. European heart journal. Cardiovascular Imaging. 2020 Oct 20:21(11):1262-1272. doi: 10.1093/ehjci/jeaa057. Epub [PubMed PMID: 32294170]
Tanabe K. Three-Dimensional Echocardiography - Role in Clinical Practice and Future Directions. Circulation journal : official journal of the Japanese Circulation Society. 2020 Jun 25:84(7):1047-1054. doi: 10.1253/circj.CJ-20-0239. Epub 2020 May 12 [PubMed PMID: 32404540]
Level 3 (low-level) evidenceCai Q, Beckles DL, Ahmad M. Three-dimensional echocardiography assessment of carcinoid valvular heart disease: Images of each and all. Echocardiography (Mount Kisco, N.Y.). 2020 May:37(5):791-793. doi: 10.1111/echo.14679. Epub 2020 May 9 [PubMed PMID: 32386253]
Kato H, Nakanishi M, Maekawa N, Ohnishi T, Yamamoto M. Evaluation of left atrial appendage stasis in patients with atrial fibrillation using transesophageal echocardiography with an intravenous albumin-contrast agent. The American journal of cardiology. 1996 Aug 1:78(3):365-9 [PubMed PMID: 8759824]
von der Recke G, Schmidt H, Illien S, Lüderitz B, Omran H. Use of transesophageal contrast echocardiography for excluding left atrial appendage thrombi in patients with atrial fibrillation before cardioversion. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2002 Oct:15(10 Pt 2):1256-61 [PubMed PMID: 12411914]
Bernier M, Abdelmoneim SS, Stuart Moir W, Eifert Rain SS, Chandrasekaran K, Ammash NM, Mulvagh SL. CUTE-CV: a prospective study of enhanced left atrial appendage visualization with microbubble contrast agent use during transesophageal echocardiography guided cardioversion. Echocardiography (Mount Kisco, N.Y.). 2013 Oct:30(9):1091-7. doi: 10.1111/echo.12240. Epub 2013 May 11 [PubMed PMID: 23662846]