Introduction
Congenital lung diseases are rare but exceptionally distinct in their presentation, ranging from large masses requiring immediate surgical intervention to small and asymptomatic lesions. The effects of congenital lung malformations can be critical; thus, knowledge of the diagnosis and treatment of these abnormalities is essential. Lung development and anatomy are critical fundamentals in considering these malformations and help in comprehension of the pathophysiology of each disease. Congenital lobar emphysema (CLE), or congenital lobar overinflation, is the excessive inflation of the lung lobes without destroying the alveoli. This condition arises from a "ball valve" effect, causing bronchial blockage and inadequate bronchial cartilage development.[1]
CLE is a rare developmental malformation of the lung with a wide range of presentations that pose a diagnostic and therapeutic dilemma and are associated with high morbidity and mortality. CLE is characterized by respiratory distress due to overexpansion of 1 or more pulmonary lobes of the histologically normal lung without destroying alveolar walls with compression of surrounding lung parenchyma. Air trapping in the lung during the expiratory phase of respiration due to deficient bronchial cartilage causes repeated episodes of respiratory distress. The affected lobe is essentially nonfunctional because of overdistention and air trapping. CLE is frequently recognized in newborns; however, a few cases do not appear until adulthood. This disease is potentially reversible if diagnosed and treated on time.[2]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
In half of patients with CLE, the underlying etiology is idiopathic. In 25% of the patients, CLE is caused by absent bronchial cartilage, hypoplasia, or dysplasia, resulting in bronchial collapse and, thus, air trapping during expiration. Other CLE etiologies include:
- Parenchymal diseases
- External bronchial obstruction
- Internal bronchial obstruction [2]
CLE could be caused by parenchymal disease, including the theory of polyalveolar lobes in which experts have proposed that a 3- to 5-fold increase in the number of alveoli develops in the affected lung lobes compared to normal lobes. The mechanism of underlying air trapping in polyalveolar lobes is not known.[3] CLE could also be due to internal bronchial disease. The bronchial disease can be stenosis, atresia, or bronchomalacia.[4][5] Diseases from structures adjoining lungs or bronchi (eg, vessels and mediastinal masses) may also cause CLE.[6]
Epidemiology
The incidence of CLE among live births is 1 out of 20,000 to 30,000.[1] The occurrence is more in men than women, with a ratio of 3 to 1. CLE is more common in infants, with 33% of the cases being symptomatic at birth, and is extremely rare among adults. Due to the severity of symptoms, most are diagnosed within the first 6 months of birth. The left upper lobe is most commonly involved (43%), followed by the right middle lobe (32%) and right upper lobe (21%). The involvement of the lower lobe is rare and only seen in 2% of cases.[2] A quarter of the cases are caused by deformity of bronchial cartilages, and another quarter are due to obstruction of bronchi. CLE is also associated with anomalies of other systems, especially cardiac, in up to 20% of the cases.[7]
Reproductive outcomes vary significantly among different racial and ethnic groups, with Black individuals experiencing a greater incidence of preterm births, low birth weights, and infant mortality compared to White individuals. Studies have shown that the prevalence of certain birth defects differs among these groups. While the overall risk of congenital anomalies is similar between White and Asian individuals, Asians have a higher likelihood of developing craniofacial and musculoskeletal anomalies, according to research results by Egbe A et al (2015).[8][9]
Pathophysiology
CLE is a rare cystic lung malformation characterized by the overinflation of a pulmonary lobe. This hyperinflation of alveoli causes a mediastinal shift to the opposite side, compressing the contralateral lung and leading to the collapse of the remaining lung. Although 90% of cases present before 6 months of age, CLE can manifest as late as 5 years old, with boys more commonly affected than girls. The primary symptoms include respiratory distress, dyspnea, recurrent respiratory tract infections, and cyanosis. Literature reviews indicate that the left upper lobe is most frequently impacted, followed by the middle and right upper lobes, with the lower lobes rarely affected.[10]
Histopathology
Congenital cystic pulmonary lesions are represented by the following entities: congenital pulmonary airway malformation (CPAM), formerly congenital cystic adenomatoid malformation, extralobular and intralobular sequestration, CLE (overexpansion), and bronchogenic cyst. The developmental model of CPAM histogenesis by Stocker proposed perturbations designated as CPAM type 0 to type 4 without known or specific pathogenetic mechanisms along the airway from the bronchus to the alveolus.[11] Additionally, mutational events may occur either at the bodily level in KRAS (CPAM types 1 and possibly 3) or germline variants in congenital acinar dysplasia, formerly CPAM type 0, and pleuropulmonary blastoma, type I, formerly CPAM type 4. The potential for overt malignant progression exists in the case of pleuropulmonary blastoma type I and CPAM type 1 in some cases to well-differentiated mucinous adenocarcinoma. On the other hand, CPAM type 2 is an acquired lesion resulting from an interruption in lung development secondary to bronchial atresia. The latter is also regarded as the etiology of extralobular and intralobular sequestration, whose pathologic features are similar, if not identical, to CPAM type 2. These observations have provided important insights into the pathogenetic mechanisms in the development of the CPAMs since the Stocker classification.[12]
Histologically, no tissue destruction was observed, such as in acquired emphysema. Additionally, no destruction of alveolar walls and septum occurs, and normal acinus structure is present. However, no acinar maturation with age and overinflated alveoli are observed. The primary forms of CLE are hypoalveolar and polyalveolar. In many CLE cases, normal numbers of radial alveoli are present. Nonetheless, no remarkable progression with age is noted compared to normal individuals, inferring that acinar development in the concerned lung ends in the postpartum period. In the hypoalveolar form, fewer than the expected number of alveoli are present, while the polyalveolar form has more alveoli than expected.[3] The airways and arteries are normal in number, size, and structure.
History and Physical
Persistent wheezing in infants frequently requires a thorough etiological investigation for accurate diagnosis. Early onset and the lack of a symptom-free period between wheezing episodes can suggest the presence of malformations. One of them is CLE, a prevalent bronchopulmonary malformation marked by the overinflation of a lung lobe without parenchymal destruction. CLE presents as respiratory distress due to lung lobe overexpansion, compressing adjacent lobes, leading to air entrapment during expiration and recurrent respiratory distress, typically identified during the neonatal stage or later in childhood.[13]
Almost 50% of the patients are symptomatic at birth, and the other 50% become symptomatic within the first 6 months of life. The patient usually presents with difficulty in feeding and breathing, wheezing, retractions, and cyanosis. A history of chronic cough and recurrent respiratory infections is commonly noted. Due to overlapping symptoms, some patients are misdiagnosed with pneumonia or pneumothorax instead of CLE.[4] Lobes affected with CLE are overinflated; thus, ventilation and perfusion are impaired. Due to progressive overinflation, adjacent organs are compressed, leading to further impairment of ventilation or perfusion and, ultimately, respiratory failure. On physical examination, wheezing and rhonchi can frequently be auscultated. The affected lobe is hyperresonant on percussion, and breath sounds are diminished in the area. Congenital cardiac defects are typically associated with CLE.[14]
Evaluation
Clinicians should have a high clinical suspicion for CLE to be able to diagnose patients presenting with symptoms in a timely fashion. Patients with CLE develop air-trapping and hyperinflation in the affected lobes, which are also poorly perfused. Pediatric and adult radiologists need to be familiar with the radiological features of CLE to maximize diagnostic accuracy. Additionally, patients with CLE can be diagnosed prenatally. Affected fetal lungs with CLE can show hyperechogenicity without abnormal blood flow and may also be accompanied by polyhydramnios or mediastinal shift. Magnetic resonance imaging (MRI) is also safe prenatally to confirm the diagnosis.[15][16]
A chest radiograph is typically the initial imaging study performed, which may reveal a distended, hyperlucent affected lobe with a fine vascular network, with this distension pushing the mediastinum and compressing the ipsilateral lobe (see Image. Congenital Lobar Emphysema). A chest computed tomography (CT) scan is preferred for a more specific diagnosis and to determine the topography, which helps evaluate the affected lobe and surrounding structures. CT scan of the chest with intravenous contrast can help evaluate the vascular anomalies related to CLE. The left upper lobe is most commonly affected (43%), followed by the right middle lobe (32%) and the right upper lobe (21%), with the lower lobes being rarely affected (<1%). Bilateral CLE has been documented but is uncommon.[17][18]
MRI with 3-dimensional and vascular sequences is an alternative diagnostic tool that does not require contrast injection and helps identify malformations with a vascular component. In atypical cases, bronchoscopy may be performed to rule out an intrabronchial foreign body, mucous plug, or bronchial anomaly contributing to emphysema. Lung scintigraphy is valuable for visualizing ventilation and perfusion disorders in the affected area and distinguishing CLE from compensatory emphysema.[19][20] However, the definitive diagnosis is typically confirmed through pathological examination following surgical excision, the standard CLE treatment.
Treatment / Management
The clinical severity of patients with CLE defines the treatment of choice. Apart from a few patients who are asymptomatic, almost half show symptoms within the first month of birth. Patients with mild or moderate symptoms could be treated conservatively. Lobectomy is the gold standard for treatment in cases with severe symptoms; however, the conservative treatment seems to increase due to antenatal diagnosis and intrauterine regression.[21][22](B2)
In those undergoing lobectomy, all procedures are performed under general anesthesia using a single-lumen endotracheal tube, with patients positioned laterally. For those undergoing open thoracotomy, a muscle-sparing lateral thoracotomy should be employed. The distended lobe usually protrudes from the thoracic cavity once the chest is opened. The pulmonary artery branches are ligated first, followed by the vein, and finally, the bronchus.
For patients having a thoracoscopic lobectomy, CO2 is insufflated at a pressure of 4 mm Hg, with a maximum of 6 mm Hg, to deflate the lung. Port positions are customized for each patient; an extra port is occasionally used for retraction. During lower lobectomy, the ports are positioned as low as possible. The affected lobe is resected and removed through a mini-thoracotomy. At the end of the procedure, the intrathoracic CO2 is evacuated, and a small chest tube is inserted.[23][24][25](B3)
Differential Diagnosis
Differential diagnoses that should also be considered when evaluating CLE include:
- Congenital cystic adenomatoid malformations: CLE and congenital cystic adenomatoid malformation type III have similar ultrasound findings, showing a highly echogenic mass. Congenital lobar emphysema is devoid of cystic lesions compared to cystic adenomatoid malformation.[26]
- Pneumonia: Patients with pneumonia present with fever, chest retractions, and cyanosis. The affected lung on the chest radiograph may present with collapse consolidation, which usually improves with antibiotics; unlike CLE, the affected lung in pneumonia is not hyperinflated.[21]
- Tension pneumothorax: Like CLE, patients with tension pneumothorax also present with respiratory distress; the physical examination will reveal hyperresonance and decreased breath sounds on the affected lung, and the chest radiograph will show that the mediastinum deviates to the contralateral lung. Tension pneumothorax is a medical emergency, and patients have hypotension, and it needs prompt decompression. The chest radiograph of the affected lung in tension pneumothorax shows depression of the hemidiaphragm, which is not seen in CLE.[27]
- Congenital diaphragmatic hernia: CLE and congenital diaphragmatic hernia present with hyperlucent lung on chest radiograph and can be differentiated from CLE because of the gas-filled bowel loops in the chest.[28]
Prognosis
The prognosis following surgical resection is excellent in patients with CLE. In a single institution case series, between January 2015 and January 2019, 37 neonates and infants with congenital lobar overinflation were treated, with an average age of 111.43 plus or minus 65.19 days, and 22 were boys. All cases exhibited respiratory distress, and 19 also presented with cyanosis. Fifteen cases had recurrent pneumonia and fever. Clinical findings included diminished breath sounds on the affected side in 30 cases and wheezes in 22 cases. Emphysema was observed in all cases via chest radiograph, and confirmatory chest CT scans were performed. The left upper lobe was affected in 23 cases, the right middle lobe in 7 cases, and the right upper lobe in 7 cases.
Lobectomy was performed on 31 patients, with a mean age of 147.58 plus or minus 81.49 days at the time of surgery, and 19 of these patients were male. Postoperative complications occurred in 5 cases, with 2 requiring postoperative ventilation. No morbidity or mortality was reported. Follow-up periods ranged from 3 months to 1 year, and all patients were doing well except for one who was lost to follow-up after 3 months.[7] In mild and moderate cases, nonsurgical conservative management has good results. In patients with severe symptoms, lobectomy has an excellent prognosis, and mortality with surgery is very low.[29]
Complications
CLE can have the following complications:
- Cyanosis
- Respiratory failure
- Surgical complications due to lobectomy
- Infantile death
Consultations
Consultation with the following specialties may be needed for appropriate diagnosis and treatment:
- Neonatologists
- Radiologists
- Pediatricians
- Pediatric pulmonologists
- Pediatric surgeons
- Thoracic surgeons
Deterrence and Patient Education
Patients with CLE can present differently. The usual presentation is respiratory distress, wheezing, cyanosis, and difficulty feeding. The newly diagnosed patient's parents should be given emotional support and detailed information about the condition, its treatment, prognosis, and effects on everyday life.
Asymptomatic patient: If the patient is asymptomatic, the parents should be guided about conservative management. Regular follow-up and observation should be performed, and patients or their caregivers should be advised of the steps to take if the patient becomes symptomatic.
Symptomatic patient: If the patient has severe symptoms, parents should be guided about the possibility of surgery (lobectomy), its prognosis, and lifestyle changes accordingly. The parents should be provided with educational material about the disease. Flow charts, diagrams, and videos should explain every aspect of the disease, treatment, and lifestyle changes until the parents fully understand everything and are satisfied with the care provision.
Pearls and Other Issues
Essential points to remember when managing CLE include:
- CLE is a rare developmental malformation of the lungs.
- The primary etiology is the deficient development of bronchial cartilages, leading to overinflation of the affected lobe.
- CLE usually presents with respiratory distress, wheezing, rhonchi, cyanosis, and difficulty in feeding.
- Chest radiographs and CT scans are used for diagnosis.
- The best treatment in severe cases is lobectomy.
- CLE usually has a good prognosis, even in patients with severe symptoms after lobectomy.
Enhancing Healthcare Team Outcomes
Healthcare professionals play a crucial role in ensuring that parents of patients with CLE understand the disease and the available treatments. The diagnosis of CLE in a newborn is often distressing and can cause significant anxiety; thus, clinicians must also provide emotional support to the parents. An effective therapeutic approach to CLE involves an interprofessional team comprising a primary care physician, pediatrician, physician assistant, nurse practitioner, radiologist, pediatric pulmonologist, pediatric surgeon, and thoracic surgeon. This team collaborates to establish a definitive diagnosis and deliver appropriate treatment. Conservative treatment and close monitoring by primary care clinicians or pediatricians are recommended for patients with mild or moderate symptoms. However, those with severe symptoms, such as respiratory failure and cyanosis, should be referred to a thoracic surgeon for lobectomy. Interprofessional communication and coordinated care among these healthcare providers are essential to enhance patient-centered care, improve outcomes, ensure patient safety, and optimize team performance in managing CLE.
Media
(Click Image to Enlarge)
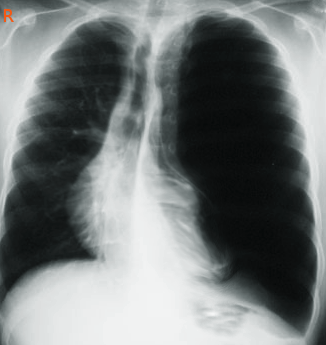
Congenital Lobar Emphysema. A chest radiograph in a patient reveals characteristic findings of congenital lobar emphysema, including a distended, hyperlucent affected lobe with a fine vascular network. This distension pushes the mediastinum and compresses the ipsilateral lobe.
Contributed by S Bhimji, MD
References
Luo J, Liu P. Congenital Lobar Emphysema. Radiology. 2024 Jun:311(3):e233554. doi: 10.1148/radiol.233554. Epub [PubMed PMID: 38916506]
Demir OF, Hangul M, Kose M. Congenital lobar emphysema: diagnosis and treatment options. International journal of chronic obstructive pulmonary disease. 2019:14():921-928. doi: 10.2147/COPD.S170581. Epub 2019 May 1 [PubMed PMID: 31118601]
Hislop A, Reid L. New pathological findings in emphysema of childhood. 1. Polyalveolar lobe with emphysema. Thorax. 1970 Nov:25(6):682-90 [PubMed PMID: 5494677]
Michelson E. Clinical spectrum of infantile lobar emphysema. The Annals of thoracic surgery. 1977 Aug:24(2):182-96 [PubMed PMID: 327959]
Level 3 (low-level) evidenceWarner JO, Rubin S, Heard BE. Congenital lobar emphysema: a case with bronchial atresia and abnormal bronchial cartilages. British journal of diseases of the chest. 1982 Apr:76(2):177-84 [PubMed PMID: 7093137]
Level 3 (low-level) evidenceCochran ST, Gyepes MT, Smith LE. Obstruction of the airways by the heart and pulmonary vessels in infants. Pediatric radiology. 1977 Sep 1:6(2):81-7 [PubMed PMID: 142958]
Abdel-Bary M, Abdel-Naser M, Okasha A, Zaki M, Abdel-Baseer K. Clinical and surgical aspects of congenital lobar over-inflation: a single center retrospective study. Journal of cardiothoracic surgery. 2020 May 19:15(1):102. doi: 10.1186/s13019-020-01145-8. Epub 2020 May 19 [PubMed PMID: 32429981]
Level 2 (mid-level) evidenceLashkarbolouk N, Mazandarani M, Azari AA, Ghorbani S, Shahkar L. The ten-year evaluation of clinical characteristics in congenital lung anomaly in pediatrics; a retrospective study in North of Iran. BMC pediatrics. 2024 Jul 6:24(1):435. doi: 10.1186/s12887-024-04911-y. Epub 2024 Jul 6 [PubMed PMID: 38971736]
Level 2 (mid-level) evidenceEgbe A, Lee S, Ho D, Uppu S. Effect of Race on the Prevalence of Congenital Malformations among Newborns in the United States. Ethnicity & disease. 2015 Spring:25(2):226-31 [PubMed PMID: 26118153]
Suryawanshi K, Nikumbh D, Singhavi S, Damle R, Dravid N. Congenital Lobar Emphysema with Pulmonary Extramedullary Hematopoiesis. Turk patoloji dergisi. 2017:33(1):74-76. doi: 10.5146/tjpath.2014.01291. Epub [PubMed PMID: 25652558]
Stocker JT. Cystic lung disease in infants and children. Fetal and pediatric pathology. 2009:28(4):155-84 [PubMed PMID: 19842869]
Dehner LP, Schultz KAP, Hill DA. Congenital Pulmonary Airway Malformations With a Reconsideration and Current Perspective on the Stocker Classification. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. 2023 May-Jun:26(3):241-249. doi: 10.1177/10935266221146823. Epub 2023 Feb 21 [PubMed PMID: 37334833]
Level 3 (low-level) evidenceBenbouziane N, Larda L, Pongo C, Alaoui-Inboui FZ, Slaoui B. Congenital Lobar Emphysema in Children: Case Series. Cureus. 2023 Nov:15(11):e49416. doi: 10.7759/cureus.49416. Epub 2023 Nov 26 [PubMed PMID: 38149169]
Level 2 (mid-level) evidenceMoideen I, Nair SG, Cherian A, Rao SG. Congenital lobar emphysema associated with congenital heart disease. Journal of cardiothoracic and vascular anesthesia. 2006 Apr:20(2):239-41 [PubMed PMID: 16616669]
Level 3 (low-level) evidenceOlutoye OO, Coleman BG, Hubbard AM, Adzick NS. Prenatal diagnosis and management of congenital lobar emphysema. Journal of pediatric surgery. 2000 May:35(5):792-5 [PubMed PMID: 10813352]
Level 3 (low-level) evidenceOliver ER, DeBari SE, Horii SC, Pogoriler JE, Victoria T, Khalek N, Howell LJ, Adzick NS, Coleman BG. Congenital Lobar Overinflation: A Rare Enigmatic Lung Lesion on Prenatal Ultrasound and Magnetic Resonance Imaging. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2019 May:38(5):1229-1239. doi: 10.1002/jum.14801. Epub 2018 Sep 12 [PubMed PMID: 30208226]
Sasieta HC, Nichols FC, Kuzo RS, Boland JM, Utz JP. Congenital Lobar Emphysema in an Adult. American journal of respiratory and critical care medicine. 2016 Aug 1:194(3):377-8. doi: 10.1164/rccm.201602-0289IM. Epub [PubMed PMID: 27275927]
Berrocal T, Madrid C, Novo S, Gutiérrez J, Arjonilla A, Gómez-León N. Congenital anomalies of the tracheobronchial tree, lung, and mediastinum: embryology, radiology, and pathology. Radiographics : a review publication of the Radiological Society of North America, Inc. 2004 Jan-Feb:24(1):e17 [PubMed PMID: 14610245]
von Ranke FM, Freitas HMP, Dinoá V, Miraldi F, Marchiori E. Congenital lobar emphysema. Radiologia brasileira. 2018 May-Jun:51(3):205-206. doi: 10.1590/0100-3984.2016.0224. Epub [PubMed PMID: 29991847]
Sharma PK, Khare C, Chanchlani R. Congenital Lobar Emphysema: Gross Appearances of the Lung. Journal of pediatric surgery. 2024 May:59(5):1015. doi: 10.1016/j.jpedsurg.2024.01.003. Epub 2024 Jan 11 [PubMed PMID: 38290914]
Ulku R, Onat S, Ozçelik C. Congenital lobar emphysema: differential diagnosis and therapeutic approach. Pediatrics international : official journal of the Japan Pediatric Society. 2008 Oct:50(5):658-61. doi: 10.1111/j.1442-200X.2008.02630.x. Epub [PubMed PMID: 19261115]
Level 2 (mid-level) evidenceCeran S, Altuntas B, Sunam GS, Bulut I. Congenital lobar emphysema: is surgery routinely necessary? African journal of paediatric surgery : AJPS. 2010 Jan-Apr:7(1):36-7. doi: 10.4103/0189-6725.59360. Epub [PubMed PMID: 20098010]
Level 3 (low-level) evidenceBawazir OA. Congenital lobar emphysema: Thoracotomy versus minimally invasive surgery. Annals of thoracic medicine. 2020 Jan-Mar:15(1):21-25. doi: 10.4103/atm.ATM_203_19. Epub 2020 Jan 2 [PubMed PMID: 32002043]
Hegde BN, Tsao K, Hirose S. Management of Congenital Lung Malformations. Clinics in perinatology. 2022 Dec:49(4):907-926. doi: 10.1016/j.clp.2022.08.003. Epub [PubMed PMID: 36328607]
Kunisaki SM. Narrative review of congenital lung lesions. Translational pediatrics. 2021 May:10(5):1418-1431. doi: 10.21037/tp-20-133. Epub [PubMed PMID: 34189102]
Level 3 (low-level) evidenceHong C, Deng H, Li M, Zhou WP, Tang J, Xia B, Yu G, Zhang L. Gene expression profiling reveals differential patterns between microcystic congenital cystic adenomatoid malformation and congenital lobar emphysema. Early human development. 2019 Jan:128():77-80. doi: 10.1016/j.earlhumdev.2018.12.014. Epub 2018 Dec 22 [PubMed PMID: 30583279]
Reed A, Lucas SF, Nowacka A, Eze C. Iatrogenic pneumothorax in a 4-week-old girl: new diagnosis of congenital lobar emphysema. BMJ case reports. 2020 Feb 20:13(2):. doi: 10.1136/bcr-2019-233302. Epub 2020 Feb 20 [PubMed PMID: 32086328]
Level 3 (low-level) evidenceDillman JR, Sanchez R, Ladino-Torres MF, Yarram SG, Strouse PJ, Lucaya J. Expanding upon the unilateral hyperlucent hemithorax in children. Radiographics : a review publication of the Radiological Society of North America, Inc. 2011 May-Jun:31(3):723-41. doi: 10.1148/rg.313105132. Epub [PubMed PMID: 21571653]
Keith HH. Congenital lobar emphysema. Pediatric annals. 1977 Jul:6(7):34, 36-41 [PubMed PMID: 882286]