Introduction
Stress fractures were first reported in military personnel as "march foot" in the mid-19th century. It was seen in military recruits and was diagnosed with foot pain and swelling.[1] Stress or fatigue fractures occur in normal bone when it is subjected to abnormal forces like military training. Julius Wolff (1836-1902) was a German surgeon who proposed bones will remodel and adapt to the loads being placed on them.[2] A stress fracture occurs when the adaptive ability of the bone is unbalanced. Normal bone is constantly being remodeled by osteoclasts absorbing and osteoblasts laying down new bone. During military training, for instance, the body cannot adapt fast enough, so the bone develops microfractures. If the activity persists, the bone eventually develops a fracture. When an abrupt change in physical activity occurs, there is about a 3-week lag before symptoms begin to manifest. It generally starts with pain after activity, and then the pain will last for progressively longer periods of time.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Stress fractures are due to an abrupt increase in activity or training patterns. There are intrinsic and extrinsic factors contributing to the development of the injury, with the bulk of the information coming from studies on military personnel.[1] Intrinsic factors include poor physical conditioning, female, hormonal disorder, menstrual disorder, poor bone density, reduced muscle mass, genu valgum knees, and a short leg. Extrinsic factors include high-impact sports activities like running, jumping, abrupt increase in physical activity, irregular or angled running surface, poor footwear, running shoe wear older than 6 months, deficient vitamin D and calcium, and smoking.[1] The most common risk factor is an abrupt increase in activity. A longitudinal study of 5000 Finnish male military recruits demonstrated a higher level of high intense activity before entering training helped protect against a stress fracture.[3]
In general, with male and female athletes participating in the same training regime, female athletes have a higher incidence of stress fractures.[4] There is a danger of extrapolating data from military studies to a general running population. Military personnel includes smokers and those less fit; in initial training, running is more limited, with them carrying much heavier loads and having less effective footwear in preventing these injuries. Those at the highest risk for having a stress fracture are female athletes and those with a previous stress fracture.[5]
The most common sites for stress fractures are as follows in decreasing order metatarsals, tibia, tarsals, femur, and fibula, followed by the pelvis. In female athletes, stress fractures of the pelvis and metatarsals are most common. Upper extremity stress fractures are rare but have been reported in gymnasts, weightlifting, and throwing sports. The clavicle, scapula, first rib and proximal humerus/shaft (thrower's shoulder), medial epicondyle (thrower's elbow), olecranon, and radial physis (gymnast's wrist) are the most common sites.[4]
Some stress fractures are more common in specific sports. Runners see tibia and metatarsal with pelvic stress fractures in female runners, and in long-distance running, there is an association with femoral neck and pelvic injuries. Hurdlers see an increased risk of patella fractures. Gymnasts, female soccer players, some American football position players like offensive linemen and linebackers, and Olympic-style weightlifters are at increased risk of spondylolysis. This is a unique stress fracture related to repeated hyperextension of the spine.[6]
Epidemiology
Stress fractures account for about 20% of all sports medicine injuries, and runners who average more than 25 miles a week are considered high risk.[7] Due to the repetitive nature of military training, stress fractures are common among members of the military. From 2009 to 2012, US military members had 5.69 stress fractures per 1000 person-years.[8] In runners, stress fractures account for nearly 16% of all injuries. The most common stress fractures in decreasing order of occurrence are the tibia (23.6%), tarsal navicular (17.6%), metatarsals (16.2%), femur (6.6%), and pelvis (1.6%). [9] These injuries are also more common in women.[7]
In addition to the intrinsic and extrinsic factors already noted, there are additional factors influencing stress fracture development. One factor is a neuromuscular hypothesis.[10] Due to either muscle loss or fatigue, the muscles become increasingly less able to attenuate the forces applied through the bones. This results in higher peak stress and more microdamage. A study done on U.S. Naval Academy plebes showed those who lost the most weight over the shortest period were more likely to develop a stress fracture. The study was interesting because the authors used a dual-energy x-ray absorptiometry scan to measure body composition. Those who lost weight quickly lost, mainly muscle mass and not fat mass.[11] The results of the study support the neuromuscular hypothesis.
Another factor influencing stress fracture development is overtraining, or a more current name would be relative energy deficiency syndrome. This is commonly seen as part of the female athlete triad. The athlete's training volume is too high, and calorie intake is too restricted, resulting in impaired recovery. This leads to disordered menstruation and hormonal imbalances, with estrogen levels falling, leading to osteoporosis with a stress fracture as a result. There is also a similar phenomenon in male endurance athletes who have similar high training volumes and restricted calorie intake. This will result in lower testosterone levels resulting in osteoporosis and stress fracture development.[10]
Vitamin D is another potential factor influencing the development of stress fractures. In a prospective trial of Finnish military recruits, those who sustained a stress fracture had a lower average vitamin D concentration than those who did not. A level 1 randomized trial of female military recruits showed vitamin D supplementation might have prevented a significant percentage of trainees from developing a stress fracture.[10]
Pathophysiology
Wolff's law states that if a force is applied to a normal bone, the bone will respond by remodeling itself to become stronger. Osteocytes are the most common cells in bone and are considered the master orchestrator of both osteoclastic and osteoblastic functions.[7] Osteocytes, through a dense dendritic network, respond to biomechanical stress. These cells secrete mediators that regulate osteoblast and osteoclast activity. It appears cyclic loading can cause the normal dendritic processes of osteocyte signaling to be compromised and thus leads to the impeding the physiological mechanisms of repair.[7] Repetitive loading will stimulate osteoclasts to resorption at a faster rate than osteoblasts can form new bone. The normal cycle of remodeling takes 3 to 4 months.[12]
History and Physical
Patients often present with an insidious onset of pain initially starting after activity. The pain progressively lasts longer and longer after a bout of exercise. If the athlete continues training, the pain will be present upon awakening the morning following a training activity. Additional information should be elicited while taking a history from the patient. Factors like investigating recent increases or changes in training, nutrition, assessing intrinsic and extrinsic risk factors, past medical history, and medications.[12]
Physical exam findings are limited. Patients typically have focal tenderness to palpation and occasional edema at the site of the suspected stress fracture. Clinical diagnostic tests are limited. The use of a tuning fork or low pulsed ultrasound to reproduce the pain has been described but is generally of moderate sensitivity and specificity.[13] The “hop test” (having the patient hop on the affected limb) and fulcrum test (placing tension along a long bone with a suspected stress fracture) are commonly used but have not been well studied.[14] On palpation, spot bony tenderness will be a feature in the majority of patients. Distal to middle third junctions in the tibia or over the 3rd and 4th metatarsal shafts are common areas that can be tender.
Spondylolysis and spondylolisthesis are unique stress fractures and require a high index of suspicion. Spondylosis can be asymptomatic and may be found incidentally on lumbar films. If an athlete reports lower back pain and spondylolysis is suspected, going into lumbar extension will increase the pain.[6] Having the patient flex one leg and extend the lower back (Stork test or single leg hyperextension test) is a common physical exam test for suspected spondylolysis; however, it is neither sensitive nor specific.[15] Spondylolisthesis occurs when the pars defect does not heal, and there is a migration anterior of the vertebral body.
The key to diagnosing a stress fracture is the history of rapidly increasing activity, gradually increasing pain with activity, and a high clinical suspicion.
Evaluation
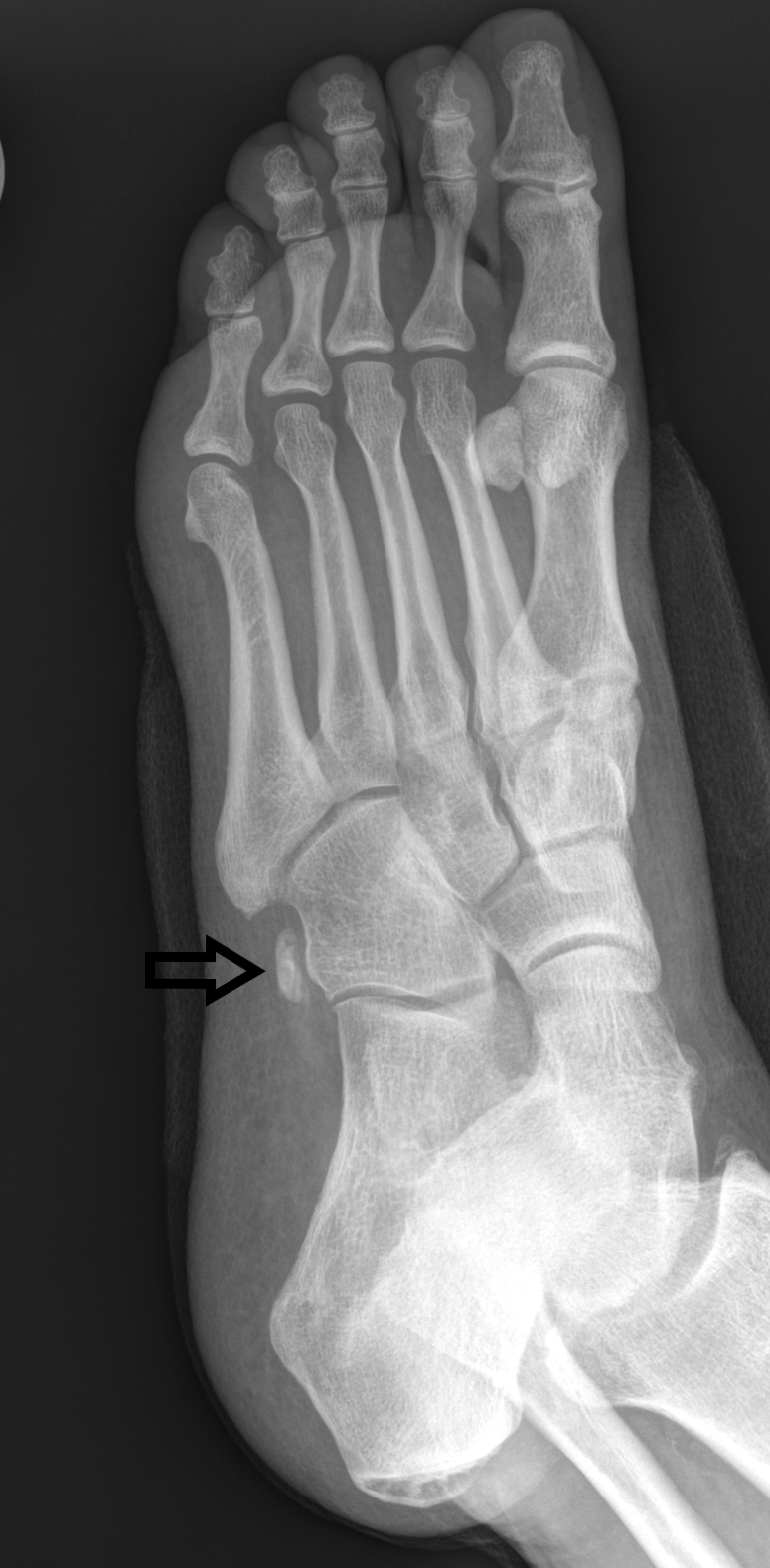
Radiographic imaging of stress fractures starts with plain radiography. It is readily available, inexpensive, and has low radiation exposure. In cortical bone, the changes will be a periosteal and endosteal reaction. If the athlete continues to train despite the pain, a fracture line will develop. In cancellous bone, the finding presents as sclerosis.[4] It takes 2 to 3 weeks before these changes become evident on radiographs, and this will be the biggest drawback.
Plain films in spondylolysis are challenging. To best visualize the par, five views are needed.[15] However, they can miss early changes. If the plain films are negative and the diagnosis is suspected, consider one of three additional modalities. Single-photon emission computerized tomography can help detect early changes. This modality will show stress reactions of the bone before the fracture is seen on plain films. If the results are negative, a fracture is very unlikely. A computed axial tomography scan can help evaluate occult fractures. It also can distinguish between bone healing and a fibrous union. Finally, magnetic resonance imaging can be of value in early detection.[15] It also has the advantage of avoiding additional ionizing radiation.
3 phase bone scans have also been used to image stress fractures. This modality utilizes a radioactive tracer. The tracer is taken up in areas of high bone turnover. It is highly specific, so if the scan is negative, there is no stress fracture. However, its sensitivity is not high. In the right clinical setting, it can be positive for a stress fracture. However, any medical condition causing turnover will also be positive. Conditions like cancer or osteomyelitis can produce similar imaging findings. It is not a helpful modality for follow-up images.[1]
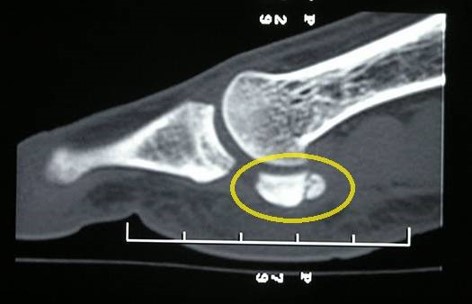
Computer tomography is another modality used to image stress fractures. It has a lower sensitivity than magnetic resonance imaging but is more readily available. It will result in higher radiation exposure than other imaging modalities.[9]
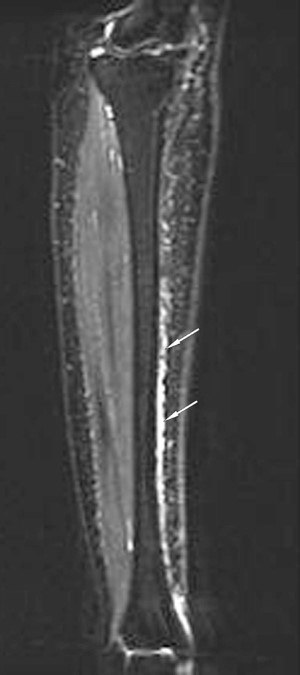
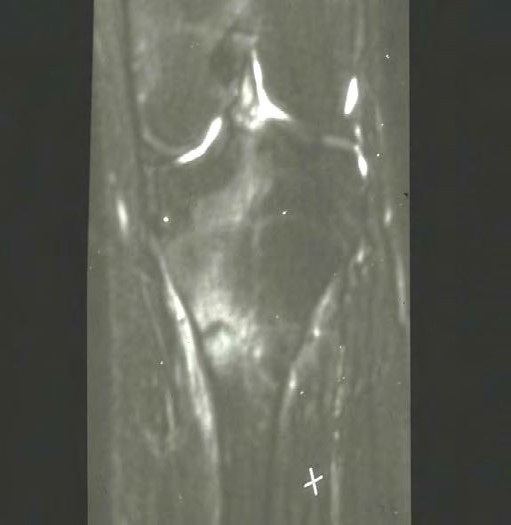
Magnetic resonance imaging has excellent specificity and sensitivity. This modality has a sensitivity of 80 to 100 percent and a specificity of 100 percent. The biggest drawback is the expense of obtaining the images, and they are not readily available. Fredericson developed a classification for stress fractures. Grade 1 findings are periosteal edema only. Grade 2 findings will show mild bone marrow edema on T2 images only. Grade 3 will show moderate bone marrow edema on both T1 and T2 images. Grade 4 findings will show a fracture line.[9]
In general, laboratory studies do not aid in the diagnosis of stress fractures unless they become recurrent and more frequent. Vitamin D levels were evaluated prospectively in a large number of military recruits, and a serum concentration of less than 20 ng/mL (50 nmol/L) is associated with a higher number of stress fractures than levels greater than 20 ng/mL.[7] For recurrent stress fractures, additional testing would include thyroid-stimulating hormone, parathyroid hormone, and a bone mineral density study.
Treatment / Management
Stress fractures are divided into high and low-risk fractures. General principles of management include relative rest/non-weight bearing for a period of 2 to 6 weeks, and then gradual reintroduction of activity.
A low-risk stress fracture can be seen in the posterior tibia, 2nd to 4th metatarsals, femur, inferior and superior pubic rami, sacrum, and fibula.
High-risk stress fractures are seen in areas with challenging blood supply, frequently occurring in areas of maximal tensile load, and are at high risk for non-union.[9][16] They can be difficult to diagnose and require a high index of suspicion. High-risk stress fractures include the femoral neck, anterior tibia, tarsal navicular, talus, sesamoid bones, and 1st and 5th metatarsal bones. With high-risk stress fractures, tension-sided stress fractures are the highest risk because of the tendency to displace secondary summative forces causing distraction caused by an imbalance between gravity and muscular attachments. These stress fractures generally do not respond to conservative management and often result in significant morbidity.[16] Femoral neck stress fractures can be an exception. When a stress fracture is suspected, obtaining magnetic resonance imaging is recommended since plain radiographs are unreliable in this particular injury. Femoral neck stress fractures can be thought of as 2 separate injuries. If the imaging shows tension (superior side) stress fractures, these should be referred for surgical consultation. If the imaging shows a compression (inferior side) stress fracture, and if a fracture line is present, it should be less than 50 percent of the width of the bone, these can be managed non-operatively but need close follow-up. If the pain increases or the fracture increases in size, then a surgical consult should be obtained. If the fracture becomes displaced, it should be treated rapidly as there is a high risk of avascular necrosis to the femoral head.[8]
Patients may also benefit from calcium and vitamin D supplementation, though studies do not demonstrate a clear benefit or quicker healing. In one study of female U.S. Navy recruits, given 2000 milligrams of calcium along with 800 international units of vitamin D had a 20% reduction in stress fractures during training.[17] Some difficulties are generalizing the findings to the population at large or females attending boot camps of the other military branches. However, another study by Tenforde et al. found female athletes using a calcium supplement had a three times higher risk of stress fractures.[5](A1)
Spondylolysis is a unique stress fracture. Few large clinical trials looking at treatment exist, so recovery and return to play can be challenging to manage.[6] Experts recommend treating this conservatively with activity modification, core strengthening, and bracing. Resting from activity can be from 2 weeks to as long as 6 months. The key is waiting for the athlete to become pain-free. Bracing can be helpful, but its use is controversial. The brace should limit lumbar extension and be worn 23 hours a day for up to 6 months.[15] Some limited studies using a rigid lumbosacral orthosis had greater than eighty percent pain-free return to sport.[18] Finally, physical therapy rehabilitation focuses on core strength and spine stabilization, followed by a gradual return to play program with the athlete remaining pain-free. Those refractory cases can be considered for surgical management, and several techniques have been described.[15](A1)
Differential Diagnosis
- Cellulitis
- Osteomyelitis
- Tendonitis
- Tendinopathy
- Exertional compartment syndrome
- Tumors - benign or malignant
- Nerve entrapment
- Arterial entrapment
- Coagulation disorders
- Compartment syndrome
- Neuropathic pain
- Complex regional pain syndrome
Prognosis
Generally, low-risk stress fractures heal, and the athlete can return to the pre-injury activity. Appropriate rehabilitation and gradual return will be necessary.
Complications
Complications are generally few for low-risk stress fractures. Occasionally the patient may experience residual pain. High-risk stress fractures carry considerably higher risks. They are more likely to progress toward non-union and thus require surgical treatment.[10] They are more likely to necessitate a change in the sport for an athlete and may result in greater post-recovery pain.
Postoperative and Rehabilitation Care
High and low-risk stress fractures require the same basic plan for rehabilitation. They both require a period of immobilization to allow the fracture to heal. In general, high-risk injuries will require surgical fixation. The rehabilitation will require two phases of rehab. Phase one would be considered active rest. As the injured site is protected from further injury during a period of immobilization, aerobic fitness can be maintained using no-impact activities like cycling, deep water running, swimming and zero-gravity running. Once the patient has been pain-free for one to two weeks, then phase two can be started. During phase two, rehabilitation beings to strengthen the muscles of the injured extremity, improve proprioception, strengthen the core and pelvic girdle muscles, and return the patient to pre-injury levels of fitness.[9] The goal is to return them to full activity. For those runners who sustain a stress fracture, a gait analysis would be helpful to guide the rehabilitation process. When ready for running, the best plan is to start low and not increase time, intensity, and distance by more than 10 percent per week.
Consultations
For high-risk stress fractures, consultation with orthopedics should be part of the acute treatment plan. For proper preventative measures and non-surgical treatment, consultation with physical medicine and rehabilitation is necessary.
Deterrence and Patient Education
Prevention is the best strategy for stress fractures. Address both intrinsic and extrinsic risk factors. Extrinsic factors are the easiest to address. Optimization of calcium and vitamin D status, proper shoe wear, a gradual increase in training volumes, choosing appropriate running surfaces, and smoking cessation. The intrinsic risk factors are more difficult to modify, but a proper understanding of how they can be additive to the extrinsic risk factors will be important in the overall prevention picture for an athlete. Some of the risk factors like poor physical conditioning, hormonal and menstrual disorder, decreased bone mineral density, and lower body muscle mass can be modified. Other like female sex, genu valgum, and maybe leg length discrepancy cannot be modified but should be taken into account when discussing training volumes.[1]
Enhancing Healthcare Team Outcomes
Treating athletes with stress fractures is a complex problem best managed by an interprofessional team. This is especially true for the diagnosis of the female athlete triad. Treating all stress fractures requires a significant team approach, including parents or significant others, coaches, primary care, gynecology, medical and surgical sports medicine specialists, rehabilitation specialists like a certified athletic trainer, physical therapists, and often a sports nutritionist. Orthopedic and school nurses may be involved with care providing education, and coordinating communication among team members. With the size of this team, open and honest communication and respect for each member's contribution to the care of the athlete will be critical to the return of the patient. The only goal of the team is to return the patient to their activity as quickly and safely as possible. This is only possible when the patient becomes the center of the plan. Patients will have to buy into the process of rehabilitation and return to activity. This will only happen if everyone on the interprofessional healthcare team has the same message. [Level 5]
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
References
McCormick F, Nwachukwu BU, Provencher MT. Stress fractures in runners. Clinics in sports medicine. 2012 Apr:31(2):291-306. doi: 10.1016/j.csm.2011.09.012. Epub [PubMed PMID: 22341018]
Teichtahl AJ, Wluka AE, Wijethilake P, Wang Y, Ghasem-Zadeh A, Cicuttini FM. Wolff's law in action: a mechanism for early knee osteoarthritis. Arthritis research & therapy. 2015 Sep 1:17(1):207. doi: 10.1186/s13075-015-0738-7. Epub 2015 Sep 1 [PubMed PMID: 26324398]
Pihlajamäki H, Parviainen M, Kyröläinen H, Kautiainen H, Kiviranta I. Regular physical exercise before entering military service may protect young adult men from fatigue fractures. BMC musculoskeletal disorders. 2019 Mar 25:20(1):126. doi: 10.1186/s12891-019-2513-4. Epub 2019 Mar 25 [PubMed PMID: 30909910]
Matcuk GR Jr, Mahanty SR, Skalski MR, Patel DB, White EA, Gottsegen CJ. Stress fractures: pathophysiology, clinical presentation, imaging features, and treatment options. Emergency radiology. 2016 Aug:23(4):365-75. doi: 10.1007/s10140-016-1390-5. Epub 2016 Mar 22 [PubMed PMID: 27002328]
Wright AA, Taylor JB, Ford KR, Siska L, Smoliga JM. Risk factors associated with lower extremity stress fractures in runners: a systematic review with meta-analysis. British journal of sports medicine. 2015 Dec:49(23):1517-23. doi: 10.1136/bjsports-2015-094828. Epub 2015 Jul 17 [PubMed PMID: 26582192]
Level 1 (high-level) evidenceGagnet P, Kern K, Andrews K, Elgafy H, Ebraheim N. Spondylolysis and spondylolisthesis: A review of the literature. Journal of orthopaedics. 2018 Jun:15(2):404-407. doi: 10.1016/j.jor.2018.03.008. Epub 2018 Mar 17 [PubMed PMID: 29881164]
Moreira CA, Bilezikian JP. Stress Fractures: Concepts and Therapeutics. The Journal of clinical endocrinology and metabolism. 2017 Feb 1:102(2):525-534. doi: 10.1210/jc.2016-2720. Epub [PubMed PMID: 27732325]
DeFroda SF, Cameron KL, Posner M, Kriz PK, Owens BD. Bone Stress Injuries in the Military: Diagnosis, Management, and Prevention. American journal of orthopedics (Belle Mead, N.J.). 2017 Jul/Aug:46(4):176-183 [PubMed PMID: 28856344]
Kahanov L, Eberman LE, Games KE, Wasik M. Diagnosis, treatment, and rehabilitation of stress fractures in the lower extremity in runners. Open access journal of sports medicine. 2015:6():87-95. doi: 10.2147/OAJSM.S39512. Epub 2015 Mar 27 [PubMed PMID: 25848327]
Miller TL,Best TM, Taking a holistic approach to managing difficult stress fractures. Journal of orthopaedic surgery and research. 2016 Sep 9; [PubMed PMID: 27608681]
Armstrong DW 3rd, Rue JP, Wilckens JH, Frassica FJ. Stress fracture injury in young military men and women. Bone. 2004 Sep:35(3):806-16 [PubMed PMID: 15336620]
Denay KL. Stress Fractures. Current sports medicine reports. 2017 Jan/Feb:16(1):7-8. doi: 10.1249/JSR.0000000000000320. Epub [PubMed PMID: 28067732]
Schneiders AG, Sullivan SJ, Hendrick PA, Hones BD, McMaster AR, Sugden BA, Tomlinson C. The ability of clinical tests to diagnose stress fractures: a systematic review and meta-analysis. The Journal of orthopaedic and sports physical therapy. 2012 Sep:42(9):760-71. doi: 10.2519/jospt.2012.4000. Epub 2012 Jul 19 [PubMed PMID: 22813530]
Level 1 (high-level) evidencePatel DS, Roth M, Kapil N. Stress fractures: diagnosis, treatment, and prevention. American family physician. 2011 Jan 1:83(1):39-46 [PubMed PMID: 21888126]
Lawrence KJ, Elser T, Stromberg R. Lumbar spondylolysis in the adolescent athlete. Physical therapy in sport : official journal of the Association of Chartered Physiotherapists in Sports Medicine. 2016 Jul:20():56-60. doi: 10.1016/j.ptsp.2016.04.003. Epub 2016 Apr 13 [PubMed PMID: 27234265]
McInnis KC, Ramey LN. High-Risk Stress Fractures: Diagnosis and Management. PM & R : the journal of injury, function, and rehabilitation. 2016 Mar:8(3 Suppl):S113-24. doi: 10.1016/j.pmrj.2015.09.019. Epub [PubMed PMID: 26972260]
Lappe J, Cullen D, Haynatzki G, Recker R, Ahlf R, Thompson K. Calcium and vitamin d supplementation decreases incidence of stress fractures in female navy recruits. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2008 May:23(5):741-9. doi: 10.1359/jbmr.080102. Epub [PubMed PMID: 18433305]
Level 1 (high-level) evidenceOverley SC, McAnany SJ, Andelman S, Kim J, Merrill RK, Cho SK, Qureshi SA, Hecht AC. Return to Play in Adolescent Athletes With Symptomatic Spondylolysis Without Listhesis: A Meta-Analysis. Global spine journal. 2018 Apr:8(2):190-197. doi: 10.1177/2192568217734520. Epub 2017 Oct 5 [PubMed PMID: 29662750]
Level 1 (high-level) evidence