Introduction
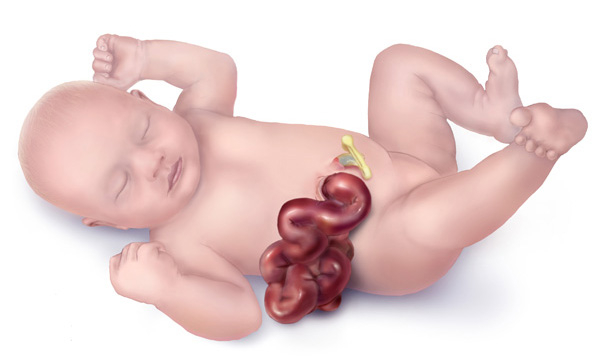
Gastroschisis is a paraumbilical, full-thickness abdominal wall defect associated with protrusion of the bowel through the defect. It is rarely associated with genetic conditions. A membrane does not cover the bowel exposed in utero and, as a result, may be matted, dilated, and covered with a fibrinous inflammatory rind. Infants have a high proportion of intrauterine growth restriction. Diagnosis is often made on the 20-week ultrasound with free-floating bowel loops in the uterine cavity. Maternal serum alpha-fetoprotein (AFP) is elevated in pregnancies with gastroschisis. Compared with other abdominal wall defects diagnosed prenatally, such as omphalocele, only 10 percent of cases with gastroschisis are associated with malformations outside of the gastrointestinal tract. Additional gastrointestinal problems occur in up to a quarter of cases. Infants can be classified and simple or complex, which can help stratify outcomes and care for infants born with gastroschisis. Complexity is based on the absence or presence of intestinal atresia, stenosis, bowel perforation, necrosis, malrotation, or volvulus. Infants may benefit from delivery in a facility with resources such as high-risk obstetrics, neonatology, and neonatal intensive care unit, and a pediatric surgeon—a trial of labor rather than scheduled cesarean birth for most patients. Spontaneous delivery usually occurs between 37 to 38 weeks gestation. A trial of spontaneous vaginal delivery is supported. The exposed infant bowel is protected following birth, an orogastric tube is placed, as are peripheral IVs. The airway is stabilized. Gastroschisis closure can be performed operatively or through slow bowel reductions utilizing a spring-loaded silo to contain the bowel. While a small percentage of infants have intestinal atresia, bowel loss, and prolonged hospitalizations, the overall survival is greater than 90%.[1]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The cause of gastroschisis is incompletely understood. Gastroschisis is caused by a failure of the formation and development of the ventral body wall during embryogenesis, resulting in herniation of the bowel. There several implicated causative factors that may contribute to the development of gastroschisis, including tobacco, specific environmental exposures (nitrosamines, for example, atrazine), cyclooxygenase inhibitor use (aspirin and ibuprofen), and decongestants (pseudoephedrine and phenylpropanolamine).[2][3][4]
Epidemiology
Gastroschisis occurs in 1 in 4000 live births.[5] The incidence of gastroschisis is increasing worldwide.[6] The incidence of gastroschisis between male infants is similar to that of females. There is a higher incidence in Hispanic, singleton pregnancies, and younger women less than 20 years of age.
Pathophysiology
During the 4th week of gestation, the abdominal wall forms through the craniocaudal and mediolateral directions. The liver and midgut herniate through the umbilical cord during the sixth week of gestation. The midgut has completed rotation and returns to the abdominal cavity at the 10th week of gestation.
History and Physical
The characteristic clinical finding in newborns born with gastroschisis is a full-thickness paraumbilical abdominal wall defect (to the right of the umbilicus) with the associated evisceration of the bowel. The abdominal wall defect tends to be approximately 4 cm in size. Unlike omphalocele, there is no membrane covering the bowel. The bowel is often thick and matted. The exposure to the amniotic fluid and the amount of time the bowel has been in contact with the amniotic fluid influence the amount of bowel wall thickening and rind. Within the matted bowel, potential bowel atresias or in-utero ruptures can be visualized. Additional abdominal contents can also herniate through this defect, including the stomach, liver, and bladder. Rarely, the bowel can be exposed and present on a small stalk with a tiny gastroschisis defect. This finding is often concerning, as this finding may be associated with a significant loss of bowel length (vanishing gastroschisis).
Evaluation
Gastroschisis is often diagnosed on a prenatal ultrasound performed at approximately 20 weeks gestation.[7][8][9] In the presence of gastroschisis, serum alpha-fetoprotein (AFP) level is elevated.[7][9] On ultrasound, a defect to the right of the umbilical cord and free-floating bowel loops are visualized. Bowel exposed to amniotic fluid may become thickened and dilated. Late diagnosed gastroschisis, such as at the time of delivery, can result in changes in neonatal management. Late diagnosed gastroschisis often has fewer changes from the amniotic fluid. However, there may also be bowel loss if the defect that the bowel herniated through was small and compromised the intestine as the abdominal wall defect attempted to close.
Infants the gastroschisis may have intrauterine growth restriction (IUGR). Pregnancy may become complicated by fetal demise and spontaneous preterm birth.[10][11] Approximately 10 percent of cases are associated with malformations outside of the gastrointestinal tract. In up to 25 percent of cases, additional gastrointestinal problems (e.g., intestinal atresia, stenosis, perforation, necrosis, malrotation, volvulus) are present. The karyotype is abnormal in 1 percent of cases, usually in the setting of associated abnormalities.
Treatment / Management
Pregnancy Management
Infants with gastroschisis should be monitoring for fetal growth. Growth restriction can be seen in up to 60% of cases. If there is a concern or risk of intrauterine demise, then antenatal testing can be considered. The prevalence of chromosomal anomalies in infants with isolated gastroschisis is that of the baseline population risk. However, in the setting of extraintestinal structural abnormalities, then the risk of chromosomal anomalies increases, and amniocentesis for parental decision-making and management of the newborn may be warranted.[12] Once gastroschisis is diagnosed, fetal growth and amniotic fluid volume are charted via ultrasound at 3 to 4-week intervals starting at 24 weeks gestation.[13] Oligohydramnios may be related to fetal growth restriction and is a risk for cord compression, while polyhydramnios may be predictive of bowel atresia.[14] Growth restriction in fetuses in the setting of abdominal wall defects may predict increased adverse neonatal outcomes.[15][16](A1)
Delivery
Infants with gastroschisis have no contraindication to vaginal delivery based on gastroschisis alone. The timing of delivery is based on gestation age (lung maturity), ultrasound findings (fetal growth profile, bowel appearance), and fetal testing results. Care for the infant is usually managed by a group of specialists, including a maternal-fetal medicine specialist, neonatologist, and pediatric surgery, to discuss patient-specific factors before delivery. The mean gestational age for spontaneous delivery in women carrying infants with gastroschisis is the 36th week of gestation.[17][18] A survey of practice patterns demonstrated forty percent of maternal-fetal medicine specialists deliver infants at 37 weeks, 30 percent deliver at 39 weeks if gastroschisis is stable. The remainder delivers infants before 37 weeks.[13] Overall, many maternal-medicine specialists allow a trial of vaginal delivery. When there is marked liver herniation, a cesarean section should be considered. One factor that some specialists utilize to determine continued gestation is if the bowel does not dilate greater than 25 mm after 37 weeks gestation. (A1)
Management of the Neonate
Management of the neonate begins with delivery room care. Evaporative fluid losses through the exposed bowel are 2.5 times the amount of that of a healthy newborn.[19][20][21] However, over resuscitation has detrimental effects, including bowel and total body edema, an increase in time to abdominal wall closure, and an increased risk of abdominal compartment syndrome.[21][6] Initial management includes protecting the exposed bowel by placing the lower half of the infant into a bowel bag. This maneuver allows the protection of the bowel as well as visualization of the blood flow and perfusion of the bowel. An orogastric tube should be inserted to decompress the stomach. Placement of peripheral intravenous access to provide antibiotics and maintenance fluid is performed. Intravenous fluids are administered two to three-fold due to the exposed bowel fluid losses. The airway should be evaluated and maintained. (A1)
Surgical Management of Gastroschisis
The primary goal of gastroschisis repair to return the exposed bowel and new organs to the abdominal cavity while minimizing intestinal injury or increased intra-abdominal pressure. Two treatment options are present for gastroschisis. The first is primary repair, and the second is delayed closure (usually utilizing a temporary silo and performing serial reduction of bowel contents). Primary closure near birth is performed either operatively or following successful bowel reduction back into the abdominal cavity and performing sutureless gastroschisis repair. A randomized control trial evaluated primary closure versus delayed primary closure following silo reduction in which there were no differences seen between two groups in length of stay, time to enteral feeds, or ventilator times.[22](A1)
The umbilicus is now preserved during the closure as it leads to excellent cosmetic results. A prospective randomized study comparing sutureless closure to sutured repair noted that the time to full feeds and length of stay was significantly longer in the sutureless group, despite no additional complications.[23] However, a multi-institutional review demonstrated that sutureless abdominal wall closure of neonates with gastroschisis was associated with less general anesthetics, antibiotic use, surgical site/deep space infections, and decreased ventilator time.[24](A1)
The bowel is inspected at the time of reduction for obstructing bands, perforation, or atresia. However, bowel anastomosis in the setting of possible atresia is usually not commenced in the setting of edematous bowel. At the initial assessment of the gastroschisis, obvious bowel atresia can be turned into an end ostomy to allow an earlier resumption of feeding while waiting for the intestines to normalize. Care is exercised during the reduction of bowel contents. Perfusion of the bowel is monitored frequently as the mesentery can be compromised based on the pressure of the silo, the bowel at the fascia as it is being reduced, as well as once the bowel is reduced into the abdominal cavity. Abdominal compartment syndrome with pressures higher than 10 to 15 mmHg is often associated with decreased renal and intestinal perfusion, whereas above 20 mmHg, they correlate with organ dysfunction and complications.[25][26](B2)
Differential Diagnosis
The differential diagnosis of gastroschisis in a newborn is those of newborn abdominal wall and umbilical defects.[27] Omphalocele is the first differential diagnosis. The membranous sac covering the bowel contents and the often intact umbilical cord assist in differentiating the two diagnoses. The membrane covering the omphalocele may have ruptured during in utero development or at birth. However, the location of the liver and cord vessel insertion site may assist in differentiating omphalocele (cord inserts into the apex of the omphalocele membrane) vs. gastroschisis (cod inserts adjacent to the abdominal wall defect with the defect usually to the right of the umbilicus). Additional diagnosis to consider: umbilical hernia (cord inserts into the hernia sac), pentalogy of Cantrell (lower sternal defect, anterior diaphragm defect, pericardial defect, omphalocele, congenital heart anomalies), bladder exstrophy (low umbilical cord insertion with non-visualization of the bladder), cloacal exstrophy (cord insertion is low with exposed bowel and bladder and omphalocele), amniotic band sequence (constriction of rings and limbs - also called limb body wall complex).[28][29]
Prognosis
Compared with infants born with other abdominal wall defects, infants born with gastroschisis have the most favorable prognosis, with excellent long-term outcomes.[30] The overall survival rate for live-born infants is 98 percent in neonates born in North America.[31]
Categorizing infants born with gastroschisis into “simple” or “complex” based on the absence or presence of intestinal atresia, stenosis, bowel perforation, necrosis, malrotation, or volvulus aids in stratifying infant outcome further.[32] Up to 75 percent of gastroschisis cases are simple, and 25 percent are complex. Infants born with complex gastroschisis have more gastrointestinal, respiratory, and infectious complications in the neonatal period.[33] A systematic review and meta-analysis compared simple versus complex gastroschisis outcomes. Infants born with complex gastroschisis were associated with having a higher risk of in-hospital mortality, short bowel syndrome, bowel obstruction, necrotizing enterocolitis, parenteral nutrition, and tube feedings on discharge.[33] Infants with complex gastroschisis also were likely to have a 2-month longer hospitalization.[34]
Most infants with gastroschisis have an intestinal rotational anomaly, which is not repaired. The incidence of volvulus between infants born with omphalocele versus gastroschisis is higher for infants born with omphalocele (4.4% vs. 1.0%).[35] Intestinal adhesions and adhesive bowel obstruction are at increased risk after gastroschisis.[36] Cryptorchidism (undescended testicles) is associated with 15% to 30% of gastroschisis cases, with observational management for the first year of life, at which time an orchiopexy can be performed.[37]
Long-term outcomes are not well defined but are overall favorable. The cosmetic appearance of the abdomen and umbilicus in infants where the umbilicus is sacrificed reports psychosocial stress in the absence of an umbilicus.[37] An umbilicoplasty or umbilical reconstruction surgery can be considered in this patient group. Neurodevelopmental delay, learning issues, and overall health-related quality of life have not been well defined but are reported in preliminary studies to be within a normal range.[36][38]
Complications
Complications
Preterm delivery is more common in infants with gastroschisis at 28% versus infants without 6%. Complications can occur in infants with gastroschisis based on the need for total parenteral nutrition (TPN) and resultant line sepsis, bowel configuration leading to necrotizing enterocolitis (NEC), and abdominal wound infections from the gastroschisis closure.
Risk Stratification
Patients can be defined as “simple” versus “complex” based on the presence of intestinal complications (atresia, ischemia, perforation, or development of necrotizing enterocolitis). Patients with complex defects have a higher mortality rate, require multiple operative interventions, and have a prolonged hospitalization, increased rates of sepsis, and higher rates of prolonged cholestasis and need for intestinal transplantation due to intestinal failure.[32][39][40][41]
Management of Intestinal Atresia in the Setting of Gastroschisis
Up to 10% of neonates with gastroschisis have associated atresia, most commonly jejunal or ileal.[42] Patients born with atresia have significantly worse outcomes.[33] The timing of surgical management of the atresia depends on the state of the bowel as a bowel anastomosis in the setting of an extensive inflammatory peel will not hold sutures well. A retrospective database review examined outcomes of early versus late operations for intestinal atresia associated with gastroschisis (defined as before or after 21 days of life) found there was no significant difference in outcomes and potential for early feeding between the two groups.[43] If the atresia is known, it may be reduced and re-explored at a 4 to 6-week interval to allow for decreased adhesions and inflammation. Atresia of the bowel can also be diagnosed following several weeks of no bowel function with a confirmatory contrast study. At the initial assessment of the gastroschisis, obvious bowel atresia can be turned into an end ostomy to allow the earlier resumption of feeding while waiting for the intestines to normalize.
Closing Gastroschisis
A “closing gastroschisis” is when the gastroschisis defect size decreases before delivery.[44] As the hole gets smaller, the blood supply to the bowel progressively diminishes, resulting in atresia and a variable loss of bowel. When a large amount of the intestine is lost in utero, it usually results in short bowel syndrome.
Consultations
It is essential to consult with an interprofessional team of specialists, including an obstetrician, neonatologist, and pediatric surgeon. Often a prenatal consultation is performed with the family before delivery. The obstetrician assists in safe modes and timing of delivery and the management of the mother and patient. The neonatologist assists in the critical care of the infant after birth. The pediatric surgeon assists in forming an operative strategy for managing the exposed bowel and slowly reducing it into the abdominal cavity to close the abdominal wall. Closure of the abdominal wall defect may occur with the aid of a silo (that the bowel is contained within it temporarily externally) as it is slowly reduced into the abdominal cavity.
Deterrence and Patient Education
Families are given an option for a prenatal consultation to meet an interprofessional team when the diagnosis of gastroschisis becomes known. This allows the family to understand the diagnosis and prepare for the needs of the infant. The family is consulted on what gastroschisis is (full-thickness abdominal wall defect to the right of the umbilical cord). The most crucial issue is that as the organs are developing, the bowel, which has not returned to the infant's abdominal cavity, is in contact with the amniotic fluid. Early signs are bowel floating outside of the infant on ultrasound. Later signs include new organs being visualized through the defect, including the liver, stomach, and bladder. What is the treatment: the infant is delivered in a hospital that has access to surgeons and specialists to care for the infant immediately after birth. The best time for delivery is unknown, and most commonly, infants are delivered near term. When the infant is born, a nasogastric tube is inserted to decompress and drain the stomach, a breathing tube may be required to assist in breathing, peripheral intravenous access, and fluids will be administered. The bowel that is exposed will be protected with moist dressings and/or a lower body bag to avoid evaporative fluid losses as well as to monitor the bowel perfusion (blood flow). The surgical team will then assist in deciding if the bowel needs to be placed into a silo (a temporary plastic bag) that allows gravity to get the intestine back into the abdomen over a few days or if the abdominal wall defect can be closed at birth. There are many ways to close the abdominal wall opening, which include primary closure or sutureless closure, and the surgeon will discuss which option is best for your child. Because the bowels have been exposed to the amniotic fluid, they may not be able to tolerate food for several weeks. Therefore, nutrition will be given through the infant's veins until they can start feeding.
In some cases reducing the organs and bowel back through the abdominal wall opening may injure the organs. The intestines may also have a chance of having blood supply issues, such as when the space is too tight in the tummy or outside the body, and these issues can happen both in-utero and after birth. Up to one-third of infants experience an infection called necrotizing enterocolitis, and 10% to 15% of infants have intestinal atresia where the intestines are not in continuity and require additional operation(s) a few weeks after birth. Once the child goes home, there are usually no restrictions on a diet. There are no activity restrictions. The surgeon reviews wound care. If the infant develops feeding intolerance (vomiting) or the incision becomes red, then the surgical team needs to be called.
Pearls and Other Issues
Gastroschisis is a full-thickness right-sided paraumbilical abdominal wall defect without a covering membrane. The pathogenesis is unknown but occurs early in embryologic development around the 4th week of gestation. Maternal serum alpha-fetoprotein (AFP) is elevated in pregnancies with gastroschisis. Prenatal ultrasound visualizes free-floating bowel loops and a paraumbilical abdominal wall defect. Compared with other abdominal wall defects diagnosed prenatally, such as omphalocele, only 10 percent of cases with gastroschisis are associated with malformations outside of the gastrointestinal tract. Additional gastrointestinal problems (e.g., intestinal atresia, stenosis, perforation, necrosis, malrotation, volvulus) are present in up to 25% of cases.
Pregnancy complications include increased risk of intrauterine growth restriction, fetal demise, spontaneous preterm birth, and bowel thickening and dilation. A fetal microarray molecular testing should be offered when gastroschisis is associated with additional nongastrointestinal structural abnormalities. Infants may benefit from delivery in a facility with resources such as high-risk obstetrics, neonatology, and neonatal intensive care unit, and a pediatric surgeon. A trial of labor rather than scheduled cesarean birth for most patients (grade 2C), except if the liver is significantly herniated. There is no consensus on the optimum timing of delivery of these pregnancies, and it is between 37 to 38 weeks gestation. In the delivery room, an orogastric tube is placed, as are peripheral IVs. The airway is stabilized. The exposed bowel is wrapped with sterile saline dressings covered with plastic wrap to preserve heat and minimize insensible fluid loss. The lower half of the infant is placed into a plastic bag to minimize evaporative losses and aid in bowel visualization. Overall survival is greater than 90%.
Enhancing Healthcare Team Outcomes
Gastroschisis poses several challenges in the prenatal and early neonatal management of the infant with exposed bowel from an in-utero full-thickness abdominal wall defect with exposed bowel. These infants rarely have additional congenital issues besides intrauterine growth restriction (IUGR). The cause of gastroschisis is unknown and presumed to be multifactorial. While the physical exam at birth reveals an infant with exposed bowel, which may be matted depending on the duration of exposure to amniotic fluid, liver, stomach, and bladder may also have herniated through the abdominal wall defect depending on the size.
It is essential to consult with an interprofessional team of specialists, including an obstetrician, neonatologist, and pediatric surgeon. The nurses are also vital members of the interprofessional group as they will monitor the patient's vital signs and assist with the education of the patient and family. The obstetrician assists in safe modes and timing of delivery and the management of the mother and patient. The neonatologist assists in the critical care of the infant after birth. The pediatric surgeon assists in forming an operative strategy for managing the exposed bowel and slowly reducing it into the abdominal cavity to close the abdominal wall. Closure of the abdominal wall defect may occur with the aid of a silo (that the bowel is contained within it temporarily externally) as it is slowly reduced into the abdominal cavity.
The outcomes of gastroschisis depend on the infant's prematurity, additional comorbidities, and the state of the bowel from cumulative effects pre and antenatally (atresia, vanishing gastroschisis, vascular compromise of the mesentery supplying bowel before and after birth). However, to improve outcomes, prompt consultation with an interprofessional group of specialists is recommended.
Media
(Click Image to Enlarge)
References
Robinson TD, Whyte C. Closed Gastroschisis. The New England journal of medicine. 2021 Apr 15:384(15):e56. doi: 10.1056/NEJMicm2029281. Epub 2021 Apr 10 [PubMed PMID: 33853208]
Feldkamp ML, Alder SC, Carey JC. A case control population-based study investigating smoking as a risk factor for gastroschisis in Utah, 1997-2005. Birth defects research. Part A, Clinical and molecular teratology. 2008 Nov:82(11):768-75. doi: 10.1002/bdra.20519. Epub [PubMed PMID: 18985693]
Level 2 (mid-level) evidenceGoodman M, Mandel JS, DeSesso JM, Scialli AR. Atrazine and pregnancy outcomes: a systematic review of epidemiologic evidence. Birth defects research. Part B, Developmental and reproductive toxicology. 2014 Jun:101(3):215-36. doi: 10.1002/bdrb.21101. Epub 2014 May 2 [PubMed PMID: 24797711]
Level 3 (low-level) evidenceAgopian AJ, Langlois PH, Cai Y, Canfield MA, Lupo PJ. Maternal residential atrazine exposure and gastroschisis by maternal age. Maternal and child health journal. 2013 Dec:17(10):1768-75. doi: 10.1007/s10995-012-1196-3. Epub [PubMed PMID: 23184502]
Baird PA, MacDonald EC. An epidemiologic study of congenital malformations of the anterior abdominal wall in more than half a million consecutive live births. American journal of human genetics. 1981 May:33(3):470-8 [PubMed PMID: 6454342]
Lap CC, Brizot ML, Pistorius LR, Kramer WL, Teeuwen IB, Eijkemans MJ, Brouwers HA, Pajkrt E, van Kaam AH, van Scheltema PN, Eggink AJ, van Heijst AF, Haak MC, van Weissenbruch MM, Sleeboom C, Willekes C, van der Hoeven MA, van Heurn EL, Bilardo CM, Dijk PH, van Baren R, Francisco RP, Tannuri AC, Visser GH, Manten GT. Outcome of isolated gastroschisis; an international study, systematic review and meta-analysis. Early human development. 2016 Dec:103():209-218. doi: 10.1016/j.earlhumdev.2016.10.002. Epub 2016 Oct 27 [PubMed PMID: 27825040]
Level 1 (high-level) evidenceIslam S. Clinical care outcomes in abdominal wall defects. Current opinion in pediatrics. 2008 Jun:20(3):305-10. doi: 10.1097/MOP.0b013e3282ffdc1e. Epub [PubMed PMID: 18475100]
Level 3 (low-level) evidenceD'Antonio F, Virgone C, Rizzo G, Khalil A, Baud D, Cohen-Overbeek TE, Kuleva M, Salomon LJ, Flacco ME, Manzoli L, Giuliani S. Prenatal Risk Factors and Outcomes in Gastroschisis: A Meta-Analysis. Pediatrics. 2015 Jul:136(1):e159-69. doi: 10.1542/peds.2015-0017. Epub [PubMed PMID: 26122809]
Level 1 (high-level) evidencePage R, Ferraro ZM, Moretti F, Fung KF. Gastroschisis: antenatal sonographic predictors of adverse neonatal outcome. Journal of pregnancy. 2014:2014():239406. doi: 10.1155/2014/239406. Epub 2014 Dec 22 [PubMed PMID: 25587450]
Level 1 (high-level) evidenceGirsen AI, Do S, Davis AS, Hintz SR, Desai AK, Mansour T, Merritt TA, Oshiro BT, El-Sayed YY, Blumenfeld YJ. Peripartum and neonatal outcomes of small-for-gestational-age infants with gastroschisis. Prenatal diagnosis. 2015 May:35(5):477-82. doi: 10.1002/pd.4562. Epub [PubMed PMID: 25613462]
Level 2 (mid-level) evidenceBlumenfeld YJ, Do S, Girsen AI, Davis AS, Hintz SR, Desai AK, Mansour T, Merritt TA, Oshiro BT, El-Sayed YY, Shamshirsaz AA, Lee HC. Utility of third trimester sonographic measurements for predicting SGA in cases of fetal gastroschisis. Journal of perinatology : official journal of the California Perinatal Association. 2017 May:37(5):498-501. doi: 10.1038/jp.2016.275. Epub 2017 Jan 26 [PubMed PMID: 28125100]
Level 3 (low-level) evidenceMastroiacovo P, Lisi A, Castilla EE, Martínez-Frías ML, Bermejo E, Marengo L, Kucik J, Siffel C, Halliday J, Gatt M, Annerèn G, Bianchi F, Canessa MA, Danderfer R, de Walle H, Harris J, Li Z, Lowry RB, McDonell R, Merlob P, Metneki J, Mutchinick O, Robert-Gnansia E, Scarano G, Sipek A, Pötzsch S, Szabova E, Yevtushok L. Gastroschisis and associated defects: an international study. American journal of medical genetics. Part A. 2007 Apr 1:143A(7):660-71 [PubMed PMID: 17357116]
Level 1 (high-level) evidenceAmin R, Domack A, Bartoletti J, Peterson E, Rink B, Bruggink J, Christensen M, Johnson A, Polzin W, Wagner AJ. National Practice Patterns for Prenatal Monitoring in Gastroschisis: Gastroschisis Outcomes of Delivery (GOOD) Provider Survey. Fetal diagnosis and therapy. 2019:45(2):125-130. doi: 10.1159/000487541. Epub 2018 May 23 [PubMed PMID: 29791899]
Level 3 (low-level) evidenceJaparaj RP, Hockey R, Chan FY. Gastroschisis: can prenatal sonography predict neonatal outcome? Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2003 Apr:21(4):329-33 [PubMed PMID: 12704738]
Nicholas SS, Stamilio DM, Dicke JM, Gray DL, Macones GA, Odibo AO. Predicting adverse neonatal outcomes in fetuses with abdominal wall defects using prenatal risk factors. American journal of obstetrics and gynecology. 2009 Oct:201(4):383.e1-6. doi: 10.1016/j.ajog.2009.06.032. Epub 2009 Aug 29 [PubMed PMID: 19716531]
Santiago-Munoz PC, McIntire DD, Barber RG, Megison SM, Twickler DM, Dashe JS. Outcomes of pregnancies with fetal gastroschisis. Obstetrics and gynecology. 2007 Sep:110(3):663-8 [PubMed PMID: 17766615]
Overcash RT, DeUgarte DA, Stephenson ML, Gutkin RM, Norton ME, Parmar S, Porto M, Poulain FR, Schrimmer DB, University of California Fetal Consortium*. Factors associated with gastroschisis outcomes. Obstetrics and gynecology. 2014 Sep:124(3):551-557. doi: 10.1097/AOG.0000000000000425. Epub [PubMed PMID: 25162255]
Level 2 (mid-level) evidenceLogghe HL, Mason GC, Thornton JG, Stringer MD. A randomized controlled trial of elective preterm delivery of fetuses with gastroschisis. Journal of pediatric surgery. 2005 Nov:40(11):1726-31 [PubMed PMID: 16291160]
Level 1 (high-level) evidenceZalles-Vidal C, Peñarrieta-Daher A, Bracho-Blanchet E, Ibarra-Rios D, Dávila-Perez R, Villegas-Silva R, Nieto-Zermeño J. A Gastroschisis bundle: effects of a quality improvement protocol on morbidity and mortality. Journal of pediatric surgery. 2018 Nov:53(11):2117-2122. doi: 10.1016/j.jpedsurg.2018.06.014. Epub 2018 Jun 19 [PubMed PMID: 30318281]
Level 2 (mid-level) evidenceRosen O, Angert RM. Gastroschisis Simulation Model: Pre-surgical Management Technical Report. Cureus. 2017 Mar 22:9(3):e1109. doi: 10.7759/cureus.1109. Epub 2017 Mar 22 [PubMed PMID: 28439484]
Jansen LA, Safavi A, Lin Y, MacNab YC, Skarsgard ED, Canadian Pediatric Surgery Network (CAPSNet). Preclosure fluid resuscitation influences outcome in gastroschisis. American journal of perinatology. 2012 Apr:29(4):307-12. doi: 10.1055/s-0031-1295639. Epub 2011 Nov 17 [PubMed PMID: 22094919]
Poola AS, Aguayo P, Fraser JD, Hendrickson RJ, Weaver KL, Gonzalez KW, St Peter SD. Primary Closure versus Bedside Silo and Delayed Closure for Gastroschisis: A Truncated Prospective Randomized Trial. European journal of pediatric surgery : official journal of Austrian Association of Pediatric Surgery ... [et al] = Zeitschrift fur Kinderchirurgie. 2019 Apr:29(2):203-208. doi: 10.1055/s-0038-1627459. Epub 2018 Feb 19 [PubMed PMID: 29458229]
Level 1 (high-level) evidenceBruzoni M, Jaramillo JD, Dunlap JL, Abrajano C, Stack SW, Hintz SR, Hernandez-Boussard T, Dutta S. Sutureless vs Sutured Gastroschisis Closure: A Prospective Randomized Controlled Trial. Journal of the American College of Surgeons. 2017 Jun:224(6):1091-1096.e1. doi: 10.1016/j.jamcollsurg.2017.02.014. Epub 2017 Mar 6 [PubMed PMID: 28279777]
Level 1 (high-level) evidenceFraser JD, Deans KJ, Fallat ME, Helmrath MA, Kabre R, Leys CM, Burns RC, Corkum K, Dillon PA, Downard CD, Gadepalli SK, Grabowski JE, Hernandez E, Hirschl RB, Johnson KN, Kohler JE, Landman MP, Landisch RM, Lawrence AE, Mak GZ, Minneci PC, Rymeski B, Sato TT, Scannell M, Slater BJ, Wilkinson KH, Wright TN, St Peter SD, Midwest Pediatric Surgery Consortium. Sutureless vs sutured abdominal wall closure for gastroschisis: Operative characteristics and early outcomes from the Midwest Pediatric Surgery Consortium. Journal of pediatric surgery. 2020 Nov:55(11):2284-2288. doi: 10.1016/j.jpedsurg.2020.02.017. Epub 2020 Feb 20 [PubMed PMID: 32151403]
Olesevich M, Alexander F, Khan M, Cotman K. Gastroschisis revisited: role of intraoperative measurement of abdominal pressure. Journal of pediatric surgery. 2005 May:40(5):789-92 [PubMed PMID: 15937815]
Level 2 (mid-level) evidenceSantos Schmidt AF, Goncalves A, Bustorff-Silva JM, Oliveira-Filho AG, Miranda ML, Oliveira ER, Marba S, Sbragia L. Monitoring intravesical pressure during gastroschisis closure. Does it help to decide between delayed primary or staged closure? The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstetricians. 2012 Aug:25(8):1438-41. doi: 10.3109/14767058.2011.640366. Epub 2012 Apr 25 [PubMed PMID: 22098652]
Level 2 (mid-level) evidenceIonescu S, Mocanu M, Andrei B, Bunea B, Carstoveanu C, Gurita A, Tabacaru R, Licsandru E, Stanescu D, Selleh M. Differential diagnosis of abdominal wall defects - omphalocele versus gastroschisis. Chirurgia (Bucharest, Romania : 1990). 2014 Jan-Feb:109(1):7-14 [PubMed PMID: 24524464]
Williams AP, Marayati R, Beierle EA. Pentalogy of Cantrell. Seminars in pediatric surgery. 2019 Apr:28(2):106-110. doi: 10.1053/j.sempedsurg.2019.04.006. Epub 2019 Apr 9 [PubMed PMID: 31072457]
Revels JW, Wang SS, Nasrullah A, Revzin M, Iyer RS, Deutsch G, Katz DS, Moshiri M. An Algorithmic Approach to Complex Fetal Abdominal Wall Defects. AJR. American journal of roentgenology. 2020 Jan:214(1):218-231. doi: 10.2214/AJR.19.21627. Epub 2019 Nov 12 [PubMed PMID: 31714849]
Lepigeon K, Van Mieghem T, Vasseur Maurer S, Giannoni E, Baud D. Gastroschisis--what should be told to parents? Prenatal diagnosis. 2014 Apr:34(4):316-26. doi: 10.1002/pd.4305. Epub 2014 Jan 20 [PubMed PMID: 24375446]
Fullerton BS, Velazco CS, Sparks EA, Morrow KA, Edwards EM, Soll RF, Modi BP, Horbar JD, Jaksic T. Contemporary Outcomes of Infants with Gastroschisis in North America: A Multicenter Cohort Study. The Journal of pediatrics. 2017 Sep:188():192-197.e6. doi: 10.1016/j.jpeds.2017.06.013. Epub 2017 Jul 13 [PubMed PMID: 28712519]
Arnold MA, Chang DC, Nabaweesi R, Colombani PM, Bathurst MA, Mon KS, Hosmane S, Abdullah F. Risk stratification of 4344 patients with gastroschisis into simple and complex categories. Journal of pediatric surgery. 2007 Sep:42(9):1520-5 [PubMed PMID: 17848242]
Bergholz R, Boettcher M, Reinshagen K, Wenke K. Complex gastroschisis is a different entity to simple gastroschisis affecting morbidity and mortality-a systematic review and meta-analysis. Journal of pediatric surgery. 2014 Oct:49(10):1527-32. doi: 10.1016/j.jpedsurg.2014.08.001. Epub 2014 Sep 4 [PubMed PMID: 25280661]
Level 1 (high-level) evidenceBradnock TJ, Marven S, Owen A, Johnson P, Kurinczuk JJ, Spark P, Draper ES, Knight M, BAPS-CASS. Gastroschisis: one year outcomes from national cohort study. BMJ (Clinical research ed.). 2011 Nov 15:343():d6749. doi: 10.1136/bmj.d6749. Epub 2011 Nov 15 [PubMed PMID: 22089731]
Level 2 (mid-level) evidenceFawley JA, Abdelhafeez AH, Schultz JA, Ertl A, Cassidy LD, Peter SS, Wagner AJ. The risk of midgut volvulus in patients with abdominal wall defects: A multi-institutional study. Journal of pediatric surgery. 2017 Jan:52(1):26-29. doi: 10.1016/j.jpedsurg.2016.10.014. Epub 2016 Oct 25 [PubMed PMID: 27847120]
Koivusalo A, Lindahl H, Rintala RJ. Morbidity and quality of life in adult patients with a congenital abdominal wall defect: a questionnaire survey. Journal of pediatric surgery. 2002 Nov:37(11):1594-601 [PubMed PMID: 12407546]
Level 2 (mid-level) evidenceYardley IE, Bostock E, Jones MO, Turnock RR, Corbett HJ, Losty PD. Congenital abdominal wall defects and testicular maldescent--a 10-year single-center experience. Journal of pediatric surgery. 2012 Jun:47(6):1118-22. doi: 10.1016/j.jpedsurg.2012.03.011. Epub [PubMed PMID: 22703780]
Level 2 (mid-level) evidenceHarris EL, Hart SJ, Minutillo C, Ravikumara M, Warner TM, Williams Y, Nathan EA, Dickinson JE. The long-term neurodevelopmental and psychological outcomes of gastroschisis: A cohort study. Journal of pediatric surgery. 2016 Apr:51(4):549-53. doi: 10.1016/j.jpedsurg.2015.08.062. Epub 2015 Sep 16 [PubMed PMID: 26490011]
Youssef F, Laberge JM, Puligandla P, Emil S, Canadian Pediatric Surgery Network (CAPSNet). Determinants of outcomes in patients with simple gastroschisis. Journal of pediatric surgery. 2017 May:52(5):710-714. doi: 10.1016/j.jpedsurg.2017.01.019. Epub 2017 Jan 28 [PubMed PMID: 28188037]
Youssef F, Cheong LH, Emil S, Canadian Pediatric Surgery Network (CAPSNet). Gastroschisis outcomes in North America: a comparison of Canada and the United States. Journal of pediatric surgery. 2016 Jun:51(6):891-5. doi: 10.1016/j.jpedsurg.2016.02.046. Epub 2016 Mar 3 [PubMed PMID: 27004440]
Emil S, Canvasser N, Chen T, Friedrich E, Su W. Contemporary 2-year outcomes of complex gastroschisis. Journal of pediatric surgery. 2012 Aug:47(8):1521-8. doi: 10.1016/j.jpedsurg.2011.12.023. Epub [PubMed PMID: 22901911]
Level 2 (mid-level) evidenceAbdullah F, Arnold MA, Nabaweesi R, Fischer AC, Colombani PM, Anderson KD, Lau H, Chang DC. Gastroschisis in the United States 1988-2003: analysis and risk categorization of 4344 patients. Journal of perinatology : official journal of the California Perinatal Association. 2007 Jan:27(1):50-5 [PubMed PMID: 17036030]
Alshehri A, Emil S, Laberge JM, Skarsgard E, Canadian Pediatric Surgery Network. Outcomes of early versus late intestinal operations in patients with gastroschisis and intestinal atresia: results from a prospective national database. Journal of pediatric surgery. 2013 Oct:48(10):2022-6. doi: 10.1016/j.jpedsurg.2013.04.003. Epub [PubMed PMID: 24094951]
Houben C, Davenport M, Ade-Ajayi N, Flack N, Patel S. Closing gastroschisis: diagnosis, management, and outcomes. Journal of pediatric surgery. 2009 Feb:44(2):343-7. doi: 10.1016/j.jpedsurg.2008.10.084. Epub [PubMed PMID: 19231531]
Level 2 (mid-level) evidence