Introduction
Granular cell tumors were described as early as 1926 by the Russian pathologist Abrikossoff. They were initially coined granular cell myoblastomas, as they were believed to be of muscular origin. With the advent of immunohistochemical stains and electron microscopy, they are now believed to be Schwannian derivation.[1][2] Notably, a subset of S100-negative “non-neural” granular cell tumors have been identified which may not derive from neural tissue.[3][4][5]
These rare tumors are most commonly reported in the skin, oral cavity, digestive tract, and subcutaneous tissue. However, they can occur anywhere in the body, including breast, bladder, nervous system, respiratory and genitourinary tracts. All age groups and genders can be affected, but it is classically found in women in their 4th to 6th decades of life.[6][7][8][9][10] Granular cell tumors typically present as solitary, painless nodules less than 3-4 cm large and may be found incidentally.[7][11][12]
The vast majority behave indolently. Based on histologic criteria or the presence of metastasis, however, 1% to 2% of these lesions can be malignant, with poor prognosis and few curative options beyond surgical excision.[13][14][11]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The etiology of granular cell tumors to-date is poorly described, and its genetic underpinnings and pathophysiology are not well understood. Although it is known that these tumors may demonstrate recurrent genetic mutations in the setting of specific syndromes, the mutations driving sporadic tumorigenesis have only recently become evident with the use of whole-genome sequencing.
Syndrome Associations
It is known that the clinical finding of multiple granular cell tumors is associated with Noonan syndrome, neurofibromatosis I, and LEOPARD syndrome.[15][16][17][18][19][20] Authors have reported PTPN11 gene mutations in granular cell tumors associated with LEOPARD and Noonan syndromes.[15] It has also been proposed that the etiology of multiple granular cell tumors may be linked to abnormal RAS/MAPK pathway cell signaling, given that this mutation is a shared feature in all 3 syndromes. In another study, granular cell tumor was also associated with germline PTEN mutations in patients with PTEN hamartoma tumor syndrome.[21]
It is important to note that many of these syndrome-associated gene mutations have not yet been tested in large studies and are mostly derived from case studies or small case series. To the contrary, one small follow-on study attempting to confirm the importance of PTEN and PTPN11 mutations in granular cell tumors could not detect either of them and instead found KDR, GNAQ, and ATM mutations in a small sample of granular cell tumors, none of which were recurrent within the sample.[22]
Non-Neural Tumors
Although the question of shared cell lineage with conventional granular cell tumors is debated, there is also a subset of non-neural, S100-negative granular cell tumors which reportedly harbor an ALK gene fusion and which stain positively for Anaplastic Lymphoma Kinase immunohistochemistry.[23]
ATP6AP1 and ATP6AP2 Mutations
Recently, whole-genome sequencing and targeted sequencing analysis have been used to identify mutations in granular cell tumors. In 2018, Pareja et al. used whole-exome sequencing and targeted sequencing analysis to identify recurrent ATP6AP1 and ATP6AP2 inactivating somatic mutations in 72% of their sampled granular cell tumors.[24] Subsequent in-vitro impairment of these pH regulators in Schwann cells not only spurred oncogenesis via increased phosphorylation of PDGFR-B, SFK, and STAT-5, but it also resulted in impaired vesicle acidification, impaired endocytosis, and build-up of intracytoplasmic granules. This re-created the characteristic histologic finding of eosinophilic granules in the cytoplasm. The authors suggest that these 2 mutations are pathognomonic for granular cell tumors, as they are each found in less than 0.3% of other common cancer types. Impairment of ATP6AP1 and ATP6AP2 resulted in impairment of the V-ATPase (H+ ATPase) complex with a subsequent decrease in lysosomal activity. Not only does this correlate with the characteristic cytoplasmic findings, but it also explains the tumor’s positive immunohistochemical staining for TFE3 and wild-type MITF, as lysosomal inhibition induces activation of transcription factors MITF, TFE3, and TFEB. A larger follow-on study in 2019 verified the presence of truncating or splice site mutations in ATP6AP1 and ATP6AP2 as well as ATP6V0C, which also codes for a V-ATPase accessory protein.[25]
Malignant Tumors
Further studies have sought to specifically characterize malignant granular cell tumors via whole-genome sequencing, finding mutations in the new tumor suppressor candidate BRD7 and in GFRA2 of the tyrosine kinase receptor pathway.[26] Other studies have reported potential driver mutations in ATM, ASXL1, NOTCH2, and PARP4 pathways in malignant granular cell tumors, as well as the loss of heterozygosity in chromosomes 9p and 17p in oral granular cell tumors.[27][28][29]
Epidemiology
Granular cell tumors can appear in all age groups but are thought to arise most commonly in the 4th to 6th decades.[8] These tumors are most often seen in women (ranging from 1.8 to 2.4 female: male).[30] While two-thirds of the benign lesions are reported in African-American patients, recent cohort analysis of 113 patients found that over 70% of the patients with malignant cutaneous tumors were Caucasian.[31][32]
These are rare tumors. According to a study by Lack et al. at a single institution over 32 years, the overall incidence of granular cell tumors in surgical specimens was 0.03%.[6]
Although most are solitary, 7% to 29% of patients can have multiple lesions on presentation, 30% of which involve the skin and subcutaneous tissue.[33] In 45 to 65% of cases, they are found in the head and neck region.[30][34][35][36]
Overall, they involve the cutis and subcutaneous tissue in 30% of patients, involve the breasts in 15% of cases, the respiratory tract in 10% of cases, and the gastrointestinal tract in 5% to 11%. 1% to 2% of them are malignant based on histology or clinical presentation.[11][32][37]
Histopathology
Morphology
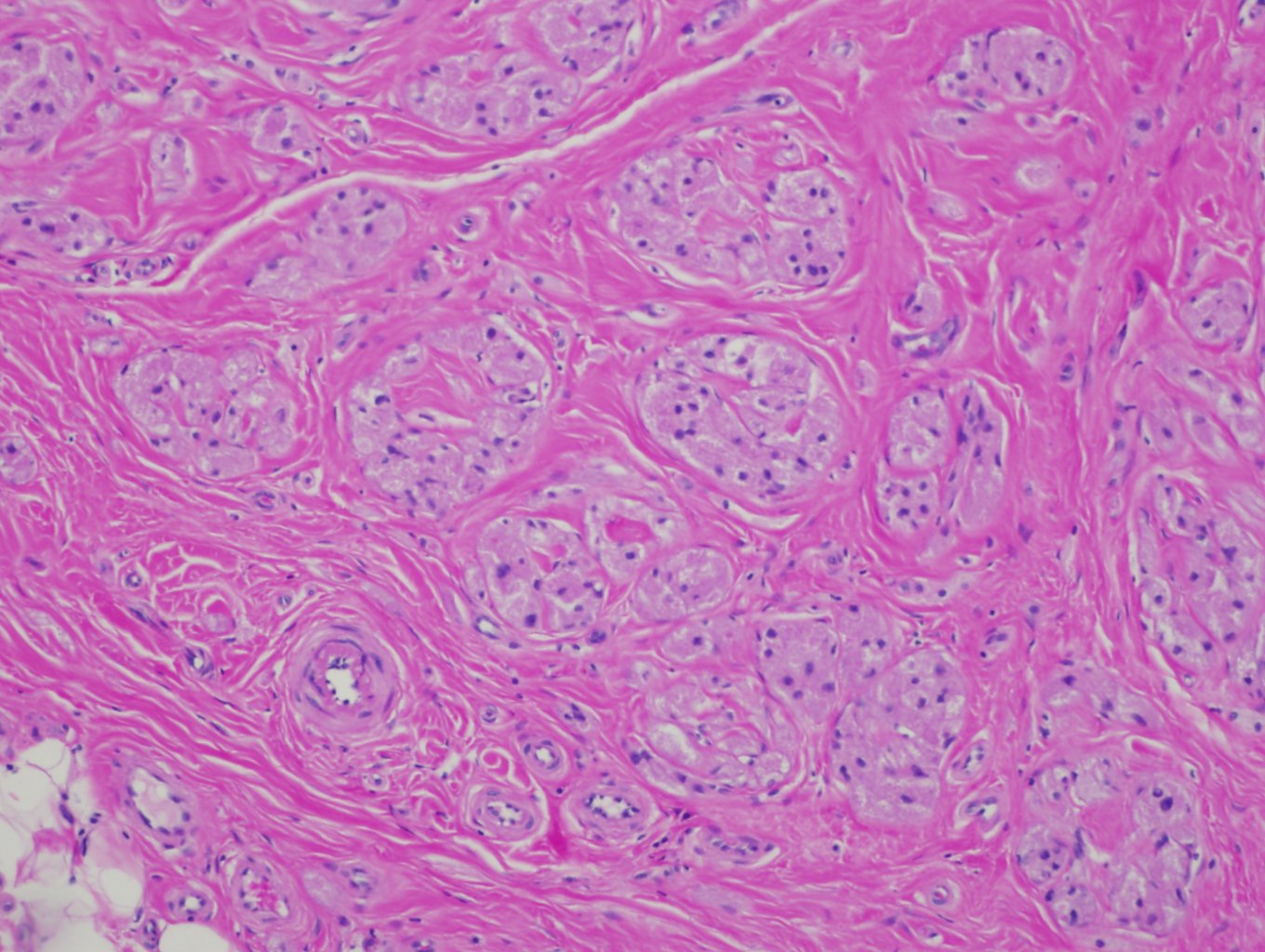
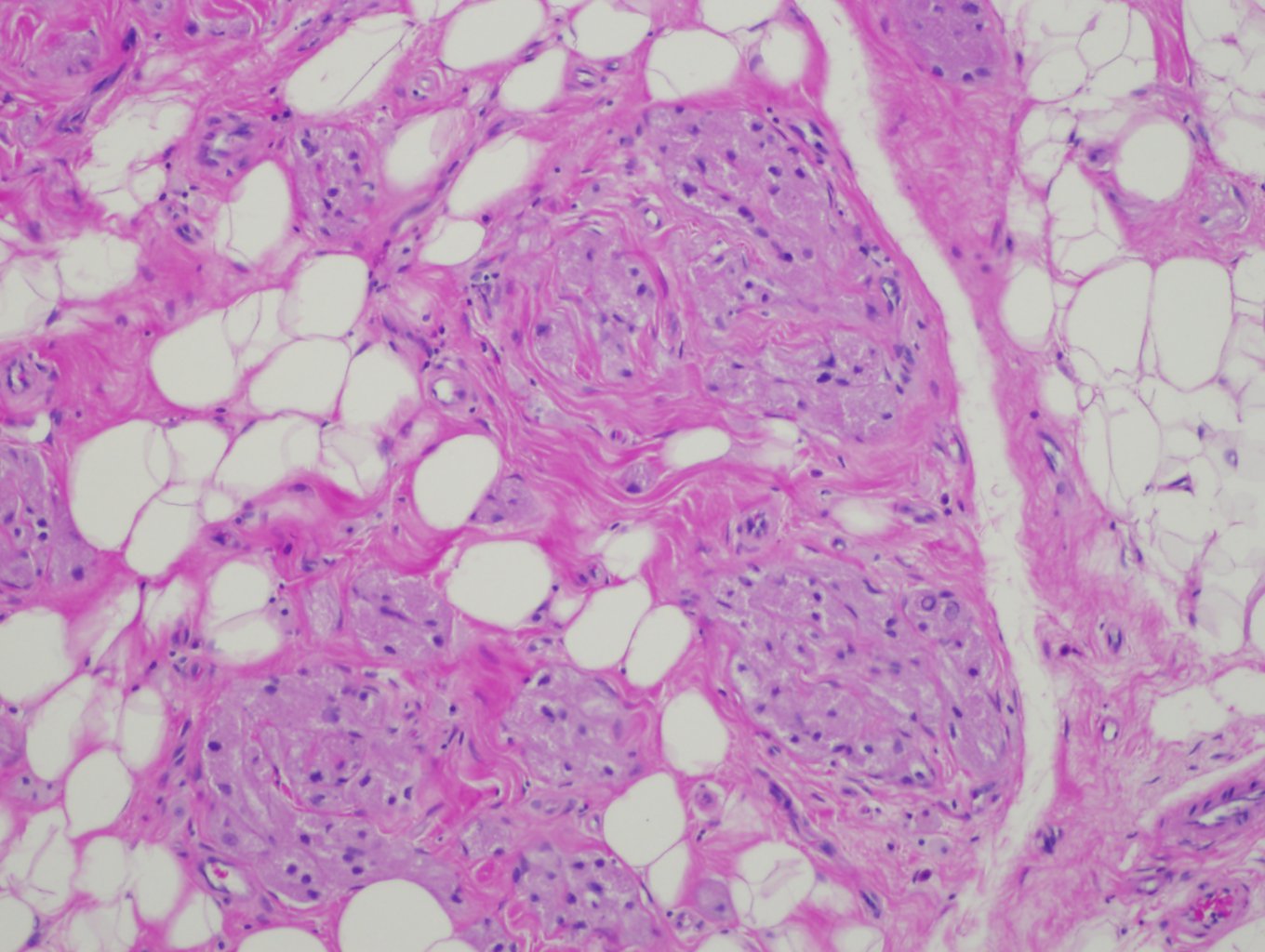
Granular cell tumors are characterized by infiltrative, non-encapsulated nests, cords, or sheets of polygonal and occasionally spindled cells with abundant eosinophilic, finely granular cytoplasm. Many of the tumors have Pustulo-ovoid bodies of Milian, which are large granules with clear halos.[38] The granules are period acid-Schiff (PAS) positive and diastase resistant, and they are thought to represent lysosomes.[8] The nests are often separated by fibrous tissue and located in the dermis, subcutis, or submucosa.[39] Nuclei have dense chromatin, are relatively small, and are centrally placed.
Malignant Features
These lesions can be sub-classified into benign, atypical, and malignant categories based on histologic features. The first system was developed in 1998 by Fanburg-Smith et al. using these six features: necrosis, increased mitotic count (greater than 2 per 10 high power fields), spindled tumor cells, nuclear pleomorphism, prominent nucleoli, and vesicular nuclei, and high nuclear to cytoplasmic ratio.[40] Lesions with none of these features are categorized as benign, and those with 1 or 2 features are categorized as atypical. Tumors with 3 or more are called malignant and have a considerably worse prognosis.
Immunohistochemistry
Both benign and malignant tumor cells typically stain positively for S-100, CD68, neuron-specific enolase, CD57, inhibin, calretinin, TFE3, SOX10, CD56, PGP9.5, and vimentin.[1][41][42][43] There is a rare non-neural variant which stains negatively for S-100 but is positive for CD68, CD10, and occasionally neuron-specific enolase.[5][44] In some studies, proliferation markers ki-67 and PHH3 were shown to be good predictors of atypical histology.[45]
Additional Features
Although benign granular cell tumors are readily identified morphologically, there are some features that can be mistaken for malignancy. Benign lesions located deep to the skin or deep to a mucosal surface can induce reactive pseudoepitheliomatous hyperplasia, which closely mimics squamous cell carcinoma in superficial biopsies and is found in over half of cases.[7][46] Benign tumors can also demonstrate both vascular and perineural invasion, but these histologic features do not confer malignancy or an adverse prognosis.[39]
History and Physical
General
Granular cell tumors typically present as skin-colored or brown-red, solitary, painless, and slow-growing nodules in the head and neck area that are less than 3-4 cm in diameter.[11][47] They are rarely painful or pruritic. They are most reported in the skin, oral cavity, gastrointestinal tract, breast, and respiratory tract. It is postulated that their high frequency in the tongue and skin parallels the large concentration of peripheral nerves in those areas. They have also been reported in every organ system, including the genitourinary tract, thyroid, neurohypophysis, and pancreaticobiliary system.[8]
They may present as multiple nodules, which should prompt an exam for other findings associated with Noonan syndrome, neurofibromatosis type I, and LEOPARD syndrome.[18][48]
Breast
In the breast, granular cell tumors can arise in any location, including all 4 quadrants, the axilla, and nipple. Some authors previously argued that they might arise predominantly in the upper inner quadrant in the distribution of the supraclavicular nerve, although this is debated in recent literature. 70% of these present as palpable masses and others are found incidentally or during screening without a palpable mass on the exam. Most of them are painless, although some patients have reported pain, pruritis, skin retraction, thickening or dimpling, and reactive lymphadenopathy on presentation.[49]
Gastrointestinal Tract
In the gastrointestinal tract, these tumors are often found incidentally during screening, and patients are usually asymptomatic. They occur most frequently in the distal esophagus, followed by the duodenum, anus, and stomach, although cases in the colon, biliary tract, and rectum have also been reported. Some patients present with non-specific symptoms such as belching, dysphagia, abdominal distension, or hematochezia. Endoscopy typically reveals a hard, isolated grey-white submucosal or mucosal nodule with normal overlying mucosa, and the lesions generally do not have unique features to set them apart from other etiologies of gastrointestinal polyps.[50][51][52][53][54][55]
Oral Cavity
In the oral cavity, these are known to occur most frequently on the anterior tongue and are seen as a firm yellow or pink, non-painful, solitary nodule.[56] Although these are also seen on the lip, palate, and buccal mucosa, some studies have found that these locations account for less than 20% of oral cavity granular cell tumors.[57]
Neurohypophysis
In the neurohypophysis, they are most often asymptomatic and found incidentally at autopsy. However, they may rarely present with bitemporal visual impairment due to mass effect, headache, or hyperprolactinemia with hypogonadism. Given their rarity, they are often initially diagnosed with radiology as pituitary adenomas or craniopharyngiomas.[58]
Malignant Tumors
As for the malignant counterpart, the tumors often present as subcutaneous masses, most commonly in the lower extremities.[59] They are usually larger than benign lesions and are known to present with metastatic lesions in the lung, lymph nodes, and bones.[60] Clinical findings associated with malignant lesions include rapid growth, ulceration, invasion of adjacent structures by radiology, and diameter greater than 5 cm.[13][60]
Evaluation
Radiology
Imaging is typically not pursued prior to excisional biopsy for small, benign-appearing nodules in the skin or oral cavity. However, tumors in the gastrointestinal tract, breast, extremity soft tissue, or other uncommon locations are often imaged because they cannot be distinguished clinically from other benign or malignant lesions.
Intramuscular
Although no radiologic findings are unique to granular cell tumors, MRI is the best imaging modality to differentiate benign from malignant lesions. In the muscle, benign granular cell tumors appear iso-intense or brighter than muscle on T1-weighted imaging. On T2-weighted sequences, the center of the lesion is also iso-intense to muscle or suppressed fat, but there is peripheral enhancement. They are round- or oval-shaped, less than 4 cm in diameter, and superficial. By comparison, the malignant counterparts often show high signal intensity on T2-weighted sequences, have the same iso-intensity to muscle on T1-weighted images, are larger than 4 cm in diameter, and may show the invasion of adjacent structures.[35][61]
Breast
In the breast, imaging findings can be difficult to distinguish from carcinoma. They can be seen on mammography as small, round, well-circumscribed lesions that are less than 3 cm in diameter, but they have also been reported to be indistinct, stellate, heterogeneous with hypodense rims, poorly circumscribed, and spiculated without calcifications. Ultrasound findings are also non-specific in the breast. They are the most commonly heterogeneous, solid, and poorly defined masses featuring posterior shadowing and high depth to width ratio. Some series have described them as predominantly hyperechoic, while others have described them as hypoechoic. Rarely, however, they can be well-circumscribed with weak internal echoes and acoustic enhancement.[49][62][63][64][65] On MRI, breast lesions demonstrate similar findings as compared to intramuscular lesions, with the intermediate signal in T1-weighted sequences and peripheral enhancement in T2-weighted images with iso-intensity to muscle.[49][66]
Esophagus
In the esophagus, granular cell tumors are more often hypoechoic, homogeneous, and smooth-edged by ultrasound, although hyperechoic, heterogeneous, and irregular lesions have been described.[37] On MRI, esophageal lesions have been described as having low T1 signal intensity with homogeneous enhancement and high T2 intensity.[67]
Pathology
Given the similar clinical and radiologic findings between granular cell tumors and other benign and malignant entities, diagnosis via biopsy for microscopic analysis is required for all lesions.[49][66]. Both benign and malignant tumors have characteristic histologic findings such that excisional biopsies are readily diagnosed by morphology in combination with immunohistochemical stains. For lesions amenable to it, excisional biopsy is preferred to core biopsy or shave biopsy. This will minimize the likelihood of sampling error, whereby the tumor underlying reactive squamous atypia is missed or where portions of the tumor showing malignant histology are missed.[68]
Treatment / Management
Surgery
Complete excision to negative margins with close clinical follow-up is recommended for granular cell tumors in nearly all locations, whether benign or malignant.[47][49] In the skin, wide local excision is performed for the diagnosis of smaller lesions, but larger lesions may require a biopsy followed by excision. Given the propensity of these tumors to recur with positive margins, Mohs surgery is occasionally employed to ensure complete removal of the tumor, particularly in cosmetically and functionally important locations.[47][69] Sentinel lymph node biopsy is only recommended for lesions suspected to be malignant by clinical impression or histology. Lymph node dissection is generally only suggested for palpable lymph nodes or biopsy-proven metastatic disease, although some authors recommend lymph node dissection upfront in malignant breast tumors.[70][49][65](B3)
Chemotherapy and Radiation Therapy
The current understanding is that there is a limited role for chemotherapy and radiation therapy. There have been rare case reports of these modalities being used with some success on patients with malignant tumors and metastatic disease on presentation.[13][71][72] A handful of case reports have shown that the sarcoma treatment pazopanib can treat some recurrent malignant granular cell tumors, but there is no current standard chemotherapy regimen for malignant or metastatic disease given the lack of randomized clinical trials with this specific lesion. The use of adjuvant radiation therapy has been similarly controversial and ill-defined, with scattered reports recommending its use in recurrent malignant lesions or inoperable metastases.[72][73][74][75] In the United States, only 11.3% of patients with malignant granular cell tumors receive radiation therapy.[31](B3)
Management in the Gastrointestinal Tract
In the gastrointestinal tract, it is important to follow up on the initial endoscopy with endoscopic ultrasound in order to assess tumor size, location, and anatomic depth of invasion. This will determine treatment modality. In contrast to other locations, asymptomatic esophageal granular cell tumors less than 1 cm can be monitored with follow-up endoscopic ultrasounds. Tumors greater than 1 cm, lesions causing symptoms, rapidly growing nodules, or those suspicious for malignancy are resected. Endoscopic mucosal resection is used to resect benign tumors under 2 cm, while submucosal endoscopic resection is used for benign tumors between 2 to 3 cm in the submucosa. Conventional open surgery or video-assisted thoracoscopic surgery are pursued tumors located in the muscularis propria, large or malignant lesions, and in the presence of other contraindications to endoscopic resection.[76][77](B3)
In the colon, endoscopic mucosal resection or endoscopic submucosal dissection are also the best strategies for benign tumors under 2 cm. Some authors recommend endoscopic submucosal excavation for benign tumors up to 5 cm with traditional surgery reserved for those greater than 5 cm and for malignant lesions. Others recommend polypectomy for anything under 4 cm, while some recommend colectomy for any lesion greater than 2 cm.[55][78][79](B3)
Differential Diagnosis
Clinical Differential
The clinical differential diagnosis for granular cell tumors is extensive and varies by the location of the tumor. It may include alveolar soft parts sarcoma, adnexal tumors, apocrine carcinoma, basal cell carcinoma, cholangiocarcinoma, colonic adenoma, cystic lesions, dermatofibroma, dermoid cyst, desmoid tumor, duct ectasia, fat necrosis, fibroadenoma, fibrosarcoma, gastrointestinal stromal tumor, granulomatous mastitis, hidradenoma, hypertrophic scar, invasive mammary carcinoma, irritation fibroma, keloid, leiomyoma, leiomyosarcoma, lipoma, malignant fibrous histiocytoma, neurofibroma, nodular fasciitis, oncocytic renal cell carcinoma, prurigo nodularis, regressing verruca, rhabdomyosarcoma, sclerosing adenosis, schwannoma, steatoma, and traumatic neuroma.
Histologic Differential[80]
The histologic differential largely includes tumors that have similar morphologic findings and those with granular variants. It includes alveolar soft parts sarcoma, ameloblastoma, angiosarcoma, atypical fibroxanthoma, basal cell carcinoma, congenital granular cell epulis, dermatofibroma, dermatofibrosarcoma protuberans, epithelioid histiocytoma, fibroxanthoma, granular cell dermatofibroma, hibernoma, leiomyoma, leiomyosarcoma, lobomycosis, malignant fibrous histiocytoma, malignant peripheral nerve sheath tumor, melanocytic nevus, melanoma, neurofibroma, non-neural granular cell tumor, primitive polypoid granular cell tumor, reactive granular cell change, reticulohistiocytoma, rhabdomyoma, rhabdomyosarcoma, schwannoma, squamous cell carcinoma, trichoblastoma, and xanthoma.
Staging
Currently, no clear staging system exists for malignant granular cell tumors.[31] Nonetheless, for lesions expected to be malignant by clinical presentation or by histology, sentinel lymph node biopsies, and possible lymph node dissection are recommended to evaluate for regional metastasis.[49][65][70]
Prognosis
Benign
The prognosis for benign granular cell tumors is excellent, as complete surgical resection is considered curative.[81] There have only been sporadic case reports of metastatic lesions arising from histologically benign primaries.[82] With wide local excision, the recurrence rate has been reported to be as low as 2% to 8%, although that increases to 21% to 50% with incomplete excision.[83]
Malignant
By contrast, patients with malignant granular cell tumors have a substantially worse prognosis, with 74% and 65% survival rates at 5 years and 10 years, respectively. They see a 32% to 41% rate of recurrence and 11 to 62% rate of metastasis between 3 to 37 months after diagnosis.[11][82][84] Notably, patients with tumors greater than 5 cm see decreased 5-year survival rates of 51% compared to 90% in those with tumors less than 5 cm. Similarly, those with distant metastases at diagnosis have 0% survival at 5 years compared to 81% in those without metastases.
Complications
Complications include the following
- Surgical site infection
- Recurrence
- Metastasis, regional and distant
- Poor aesthetics of surgical scar
- Local invasion and tissue destruction
- Discomfort due to mass effect (dysphagia, nerve impingement, constipation, abdominal fullness, bitemporal hemianopsia, hyperprolactinemia)
Deterrence and Patient Education
Following the resection of granular cell tumors, patients should monitor for metastatic and recurrent lesions. This is especially true with malignant tumors. They should also be reminded to keep their follow-up appointments for appropriate surveillance imaging of metastatic lesions or nodules which were not excised (small esophageal tumors). This will allow for the proper treatment of any newly discovered tumors.
Patients with multiple granular cell tumors should also be educated about the possible association with neurofibromatosis I, Noonan syndrome, and LEOPARD syndrome (lentigines, electrocardiogram (ECG) conduction abnormalities, ocular hypertelorism, pulmonic stenosis, abnormal genitalia, retardation of growth, and sensorineural deafness). They will likely need a thorough exam and additional studies to rule these out.
Pearls and Other Issues
Non-Representative Biopsy
Numerous pitfalls exist in the work-up and diagnosis of granular cell tumors. One well-known pitfall involves a shave biopsy of the superficial skin or mucosa overlying a granular cell tumor. Granular cell tumors are commonly associated with superficial pseudoepitheliomatous hyperplasia, which has a morphologic appearance that is strikingly similar to squamous cell carcinoma. If only the epidermis overlying a deep dermal or subcutaneous nodule is shaved, the patient will be misdiagnosed and receive inappropriate therapy. Similarly, needle biopsies are discouraged for lesions amenable to complete excisional biopsies. Some granular cell tumors may have patchy foci of malignant histology such that a single core may not be representative of the entire tumor.
Incomplete Excision
Another pitfall is not obtaining broad margins on local excisions of lesions where it is feasible. Clinically benign-appearing lesions may still have malignant histology, so wide local excision with negative margins is recommended for all lesions suspected or proven to be granular cell tumors to minimize the possibility of recurrence.
Lymph Node Dissection
Although it is debated in the literature, performing a lymph node dissection for benign or malignant tumors before a sentinel lymph node biopsy may result in unnecessary surgery and morbidity associated with lymph node dissections. Many authors recommend sentinel lymph node biopsies only for malignant tumors before a full lymph node dissection.
Poor Communication
Finally, if there is poor communication between the clinician and the pathologist, the patient may mistakenly be diagnosed with a benign granular cell tumor rather than a malignant one. The history of rapid growth, size over 5 cm, ulceration, and prominent lymphadenopathy, for example, may prompt the diagnosis of a malignant lesion regardless of histologic findings. Failure to communicate that to the pathologist may result in a benign diagnosis and, subsequently, inappropriate follow-up and under-investigation for metastatic lesions.
Enhancing Healthcare Team Outcomes
Granular cell tumors are rare neoplasms whose clinical and radiologic findings are often indistinguishable from other, more common lesions. These tumors, whether malignant or benign, typically present as solitary masses in a wide range of organ systems without any unique features until examined microscopically. Furthermore, while some of the malignant lesions present as such with nodal or distant metastases, many are not found to be malignant until the histology is worked up. Subsequently, while a single physician may be initiating the care of a patient with a granular cell tumor, it is important to consult with an interdisciplinary team of specialists promptly to expedite workup and treatment.
A dermatologist who diagnoses a malignant granular cell tumor of the skin by biopsy or excision may need to order additional imaging and rely on radiology to identify metastatic lesions. An interventional pulmonologist may need to be consulted for biopsy of lung lesions, with the subsequent engagement of thoracic surgery, surgical oncology, medical oncology, and radiation oncology to discuss and pursue management options for metastatic lesions with the patient.
Radiology
Radiologists play an important role in determining the etiology of the lesion, as they can advise the primary physician to keep benign tumors in mind, such as benign granular cell tumors, even when the imaging demonstrates malignant features such as spiculation. If the patient has a prior history of other benign granular cell tumors, it is important to relay that history to the radiologists to ensure they consider multiple foci of such tumors in the differential even if the lesion appears malignant. The radiologist may also be able to discern features suggestive of a malignant versus benign granular cell tumor.
Pathology
Pathologists are also a vital part of the team, as they identify the diagnostic morphologic and immunohistochemical findings in this tumor. They will ultimately communicate with the team whether the lesion is benign or its malignant counterpart. It is important to provide appropriate clinical history and imaging findings to the pathologist when tissue is sent, as this will impact diagnosis as well as classification of the tumor as malignant or benign. Failing to impart the information that lymphadenopathy was noted near the tumor or the fact that synchronous liver lesions were noticed in addition to a skin primary, for example, may lead the pathologist to call a lesion benign or atypical rather than malignant. Alternatively, if it is not communicated to the pathologist that a biopsy was just a superficial shave of a deep dermal lesion, the reactive pseudoepitheliomatous hyperplasia overlying a benign granular cell tumor may be erroneously called squamous cell carcinoma.[46] (Level V)
Nursing and Pharmacy
Leading up to and following surgical excision of the lesion, the nurses play an essential role in the interprofessional group, as they monitor the patient’s pre-operative, intra-operative, and post-operative vital signs and symptoms concerning for infection, and they assist with the education of the patient and family. Pharmacists will ensure the patient is sent home on the appropriate pain medication.
Outcomes
The outcomes of granular cell tumors depend in part on whether the lesions are malignant or benign. Benign tumors have excellent outcomes with wide local excision and rarely recur or metastasize. On the other hand, patients with large, malignant lesions and metastatic disease have dismally poor outcomes. Some studies report up to 41% recurrence after excision and a 62% rate of metastasis in malignant lesions. Another recent study demonstrated that patients with metastatic disease at diagnosis have 0% survival at 5 years.[84] (Level V) It is therefore important to maintain clear communication with diagnostic specialists and to promptly consult with an inter-disciplinary group of sub-specialists. This will decrease the time to treatment and will reduce the likelihood that any given malignant lesion will metastasize or cause further morbidity for the patient.
Media
(Click Image to Enlarge)
(Click Image to Enlarge)
References
Rekhi B,Jambhekar NA, Morphologic spectrum, immunohistochemical analysis, and clinical features of a series of granular cell tumors of soft tissues: a study from a tertiary referral cancer center. Annals of diagnostic pathology. 2010 Jun; [PubMed PMID: 20471560]
FISHER ER,WECHSLER H, Granular cell myoblastoma--a misnomer. Electron microscopic and histochemical evidence concerning its Schwann cell derivation and nature (granular cell schwannoma). Cancer. 1962 Sep-Oct; [PubMed PMID: 13893237]
Lewin MR,Montgomery EA,Barrett TL, New or unusual dermatopathology tumors: a review. Journal of cutaneous pathology. 2011 Sep; [PubMed PMID: 21790713]
Lazar AJ,Fletcher CD, Primitive nonneural granular cell tumors of skin: clinicopathologic analysis of 13 cases. The American journal of surgical pathology. 2005 Jul; [PubMed PMID: 15958858]
Level 3 (low-level) evidenceFernandez-Flores A,Cassarino DS,Riveiro-Falkenbach E,Rodriguez-Peralto JL,Fernandez-Figueras MT,Monteagudo C, Cutaneous dermal non-neural granular cell tumor is a granular cell dermal root sheath fibroma. Journal of cutaneous pathology. 2017 Jun; [PubMed PMID: 28266050]
Lack EE,Worsham GF,Callihan MD,Crawford BE,Klappenbach S,Rowden G,Chun B, Granular cell tumor: a clinicopathologic study of 110 patients. Journal of surgical oncology. 1980; [PubMed PMID: 6246310]
Suchitra G,Tambekar KN,Gopal KP, Abrikossoff's tumor of tongue: Report of an uncommon lesion. Journal of oral and maxillofacial pathology : JOMFP. 2014 Jan; [PubMed PMID: 24959055]
Level 3 (low-level) evidenceMachado I,Cruz J,Lavernia J,Llombart-Bosch A, Solitary, multiple, benign, atypical, or malignant: the [PubMed PMID: 26637199]
Richmond AM,La Rosa FG,Said S, Granular cell tumor presenting in the scrotum of a pediatric patient: a case report and review of the literature. Journal of medical case reports. 2016 Jun 4; [PubMed PMID: 27259474]
Level 3 (low-level) evidenceCollins BM,Jones AC, Multiple granular cell tumors of the oral cavity: report of a case and review of the literature. Journal of oral and maxillofacial surgery : official journal of the American Association of Oral and Maxillofacial Surgeons. 1995 Jun; [PubMed PMID: 7776058]
Level 3 (low-level) evidenceRose B,Tamvakopoulos GS,Yeung E,Pollock R,Skinner J,Briggs T,Cannon S, Granular cell tumours: a rare entity in the musculoskeletal system. Sarcoma. 2009; [PubMed PMID: 20169099]
Gündüz Ö,Erkin G,Bilezikçi B,Adanalı G, Slowly Growing Nodule on the Trunk: Cutaneous Granular Cell Tumor. Dermatopathology (Basel, Switzerland). 2016 Apr-Jun; [PubMed PMID: 27504442]
Aoyama K,Kamio T,Hirano A,Seshimo A,Kameoka S, Granular cell tumors: a report of six cases. World journal of surgical oncology. 2012 Sep 29; [PubMed PMID: 23021251]
Level 3 (low-level) evidenceJobrack AD,Goel S,Cotlar AM, Granular Cell Tumor: Report of 13 Cases in a Veterans Administration Hospital. Military medicine. 2018 Sep 1; [PubMed PMID: 29548015]
Level 3 (low-level) evidenceSchrader KA,Nelson TN,De Luca A,Huntsman DG,McGillivray BC, Multiple granular cell tumors are an associated feature of LEOPARD syndrome caused by mutation in PTPN11. Clinical genetics. 2009 Feb; [PubMed PMID: 19054014]
Level 3 (low-level) evidenceCastagna J,Clerc J,Dupond AS,Laresche C, [Multiple granular cell tumours in a patient with Noonan's syndrome and juvenile myelomonocytic leukaemia]. Annales de dermatologie et de venereologie. 2017 Nov; [PubMed PMID: 28728859]
Park SH,Lee SH, Noonan syndrome with multiple lentigines with PTPN11 (T468M) gene mutation accompanied with solitary granular cell tumor. The Journal of dermatology. 2017 Nov; [PubMed PMID: 28681392]
Ramaswamy PV,Storm CA,Filiano JJ,Dinulos JG, Multiple granular cell tumors in a child with Noonan syndrome. Pediatric dermatology. 2010 Mar-Apr; [PubMed PMID: 20537083]
Level 3 (low-level) evidenceMoos D,Droitcourt C,Rancherevince D,Marec Berard P,Skowron F, Atypical granular cell tumor occurring in an individual with Noonan syndrome treated with growth hormone. Pediatric dermatology. 2012 Sep-Oct; [PubMed PMID: 22329457]
Level 3 (low-level) evidenceSidwell RU,Rouse P,Owen RA,Green JS, Granular cell tumor of the scrotum in a child with Noonan syndrome. Pediatric dermatology. 2008 May-Jun; [PubMed PMID: 18577039]
Level 3 (low-level) evidenceMarchese C,Montera M,Torrini M,Goldoni F,Mareni C,Forni M,Locatelli L, Granular cell tumor in a PHTS patient with a novel germline PTEN mutation. American journal of medical genetics. Part A. 2003 Jul 15; [PubMed PMID: 12833416]
Level 3 (low-level) evidenceFrança JA,de Sousa SF,Moreira RG,Bernardes VF,Guimarães LM,Santos JN,Diniz MG,Gomez RS,Gomes CC, Sporadic granular cell tumours lack recurrent mutations in {i}PTPN11, PTEN{/i} and other cancer-related genes. Journal of clinical pathology. 2018 Jan; [PubMed PMID: 29097601]
Cohen JN,Yeh I,Jordan RC,Wolsky RJ,Horvai AE,McCalmont TH,LeBoit PE, Cutaneous Non-Neural Granular Cell Tumors Harbor Recurrent ALK Gene Fusions. The American journal of surgical pathology. 2018 Sep; [PubMed PMID: 30001233]
Pareja F,Brandes AH,Basili T,Selenica P,Geyer FC,Fan D,Da Cruz Paula A,Kumar R,Brown DN,Gularte-Mérida R,Alemar B,Bi R,Lim RS,de Bruijn I,Fujisawa S,Gardner R,Feng E,Li A,da Silva EM,Lozada JR,Blecua P,Cohen-Gould L,Jungbluth AA,Rakha EA,Ellis IO,Edelweiss MIA,Palazzo J,Norton L,Hollmann T,Edelweiss M,Rubin BP,Weigelt B,Reis-Filho JS, Loss-of-function mutations in ATP6AP1 and ATP6AP2 in granular cell tumors. Nature communications. 2018 Aug 30; [PubMed PMID: 30166553]
Sekimizu M,Yoshida A,Mitani S,Asano N,Hirata M,Kubo T,Yamazaki F,Sakamoto H,Kato M,Makise N,Mori T,Yamazaki N,Sekine S,Oda I,Watanabe SI,Hiraga H,Yonemoto T,Kawamoto T,Naka N,Funauchi Y,Nishida Y,Honoki K,Kawano H,Tsuchiya H,Kunisada T,Matsuda K,Inagaki K,Kawai A,Ichikawa H, Frequent mutations of genes encoding vacuolar H{sup} {/sup} -ATPase components in granular cell tumors. Genes, chromosomes [PubMed PMID: 30597645]
Wei L,Liu S,Conroy J,Wang J,Papanicolau-Sengos A,Glenn ST,Murakami M,Liu L,Hu Q,Conroy J,Miles KM,Nowak DE,Liu B,Qin M,Bshara W,Omilian AR,Head K,Bianchi M,Burgher B,Darlak C,Kane J,Merzianu M,Cheney R,Fabiano A,Salerno K,Talati C,Khushalani NI,Trump DL,Johnson CS,Morrison CD, Whole-genome sequencing of a malignant granular cell tumor with metabolic response to pazopanib. Cold Spring Harbor molecular case studies. 2015 Oct; [PubMed PMID: 27148567]
Level 2 (mid-level) evidenceGomes CC,Fonseca-Silva T,Gomez RS, Evidence for loss of heterozygosity (LOH) at chromosomes 9p and 17p in oral granular cell tumors: a pilot study. Oral surgery, oral medicine, oral pathology and oral radiology. 2013 Feb; [PubMed PMID: 23312918]
Level 3 (low-level) evidenceXu S,Zhao Q,Wei S,Wu Y,Liu J,Shi T,Zhou Q,Chen J, Next Generation Sequencing Uncovers Potential Genetic Driver Mutations of Malignant Pulmonary Granular Cell Tumor. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2015 Oct; [PubMed PMID: 26398830]
Davis R,Deak K,Glass CH, Pulmonary Granular Cell Tumors: A Study of 4 Cases Including a Malignant Phenotype. The American journal of surgical pathology. 2019 Oct; [PubMed PMID: 31180915]
Level 3 (low-level) evidenceBecelli R,Perugini M,Gasparini G,Cassoni A,Fabiani F, Abrikossoff's tumor. The Journal of craniofacial surgery. 2001 Jan; [PubMed PMID: 11314193]
Level 3 (low-level) evidenceMirza FN,Tuggle CT,Zogg CK,Mirza HN,Narayan D, Epidemiology of malignant cutaneous granular cell tumors: A US population-based cohort analysis using the Surveillance, Epidemiology, and End Results (SEER) database. Journal of the American Academy of Dermatology. 2018 Mar; [PubMed PMID: 28989104]
Tamborini F,Cherubino M,Scamoni S,Valdatta LA, Granular cell tumor of the toe: a case report. Dermatology research and practice. 2010; [PubMed PMID: 20862204]
Level 3 (low-level) evidencePushpa G,Karve PP,Subashini K,Narasimhan MN,Ahmad PB, Abrikossoff's Tumor: An Unusual Presentation. Indian journal of dermatology. 2013 Sep; [PubMed PMID: 24082205]
Gross VL,Lynfield Y, Multiple cutaneous granular cell tumors: a case report and review of the literature. Cutis. 2002 May; [PubMed PMID: 12041812]
Level 3 (low-level) evidencePorta N,Mazzitelli R,Cacciotti J,Cirenza M,Labate A,Lo Schiavo MG,Laghi A,Petrozza V,Della Rocca C, A case report of a rare intramuscular granular cell tumor. Diagnostic pathology. 2015 Sep 17; [PubMed PMID: 26377191]
Level 3 (low-level) evidenceHatta J,Yanagihara M,Hasei M,Abe S,Tanabe H,Mochizuki T, Case of multiple cutaneous granular cell tumors. The Journal of dermatology. 2009 Sep; [PubMed PMID: 19712278]
Level 3 (low-level) evidenceBarakat M,Kar AA,Pourshahid S,Ainechi S,Lee HJ,Othman M,Tadros M, Gastrointestinal and biliary granular cell tumor: diagnosis and management. Annals of gastroenterology. 2018 Jul-Aug; [PubMed PMID: 29991888]
Epstein DS,Pashaei S,Hunt E Jr,Fitzpatrick JE,Golitz LE, Pustulo-ovoid bodies of Milian in granular cell tumors. Journal of cutaneous pathology. 2007 May; [PubMed PMID: 17448196]
Battistella M,Cribier B,Feugeas JP,Roux J,Le Pelletier F,Pinquier L,Plantier F, Vascular invasion and other invasive features in granular cell tumours of the skin: a multicentre study of 119 cases. Journal of clinical pathology. 2014 Jan; [PubMed PMID: 23908453]
Level 2 (mid-level) evidenceFanburg-Smith JC,Meis-Kindblom JM,Fante R,Kindblom LG, Malignant granular cell tumor of soft tissue: diagnostic criteria and clinicopathologic correlation. The American journal of surgical pathology. 1998 Jul; [PubMed PMID: 9669341]
Level 2 (mid-level) evidenceAn S,Jang J,Min K,Kim MS,Park H,Park YS,Kim J,Lee JH,Song HJ,Kim KJ,Yu E,Hong SM, Granular cell tumor of the gastrointestinal tract: histologic and immunohistochemical analysis of 98 cases. Human pathology. 2015 Jun; [PubMed PMID: 25882927]
Level 3 (low-level) evidenceSchoolmeester JK,Lastra RR, Granular cell tumors overexpress TFE3 without corollary gene rearrangement. Human pathology. 2015 Aug; [PubMed PMID: 26009539]
Maiorano E,Favia G,Napoli A,Resta L,Ricco R,Viale G,Altini M, Cellular heterogeneity of granular cell tumours: a clue to their nature? Journal of oral pathology [PubMed PMID: 10890560]
Kanno A,Satoh K,Hirota M,Hamada S,Umino J,Itoh H,Masamune A,Egawa S,Motoi F,Unno M,Ishida K,Shimosegawa T, Granular cell tumor of the pancreas: A case report and review of literature. World journal of gastrointestinal oncology. 2010 Feb 15; [PubMed PMID: 21160931]
Level 3 (low-level) evidenceKapur P,Rakheja D,Balani JP,Roy LC,Amirkhan RH,Hoang MP, Phosphorylated histone H3, Ki-67, p21, fatty acid synthase, and cleaved caspase-3 expression in benign and atypical granular cell tumors. Archives of pathology [PubMed PMID: 17227124]
Level 2 (mid-level) evidenceFerreira JC,Oton-Leite AF,Guidi R,Mendonça EF, Granular cell tumor mimicking a squamous cell carcinoma of the tongue: a case report. BMC research notes. 2017 Jan 3; [PubMed PMID: 28057062]
Level 3 (low-level) evidenceChilukuri S,Peterson SR,Goldberg LH, Granular cell tumor of the heel treated with Mohs technique. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2004 Jul; [PubMed PMID: 15209799]
Level 3 (low-level) evidenceBamps S,Oyen T,Legius E,Vandenoord J,Stas M, Multiple granular cell tumors in a child with Noonan syndrome. European journal of pediatric surgery : official journal of Austrian Association of Pediatric Surgery ... [et al] = Zeitschrift fur Kinderchirurgie. 2013 Jun; [PubMed PMID: 22915371]
Level 3 (low-level) evidenceBrown AC,Audisio RA,Regitnig P, Granular cell tumour of the breast. Surgical oncology. 2011 Jun; [PubMed PMID: 20074934]
Xu GQ,Chen HT,Xu CF,Teng XD, Esophageal granular cell tumors: report of 9 cases and a literature review. World journal of gastroenterology. 2012 Dec 21; [PubMed PMID: 23323018]
Level 3 (low-level) evidenceShrestha B,Khalid M,Gayam V,Mukhtar O,Thapa S,Mandal AK,Kaler J,Khalid M,Garlapati P,Iqbal S,Posner G, Metachronous Granular Cell Tumor of the Descending Colon. Gastroenterology research. 2018 Aug; [PubMed PMID: 30116432]
Yang SY,Min BS,Kim WR, A Granular Cell Tumor of the Rectum: A Case Report and Review of the Literature. Annals of coloproctology. 2017 Dec; [PubMed PMID: 29354608]
Level 3 (low-level) evidenceSohn DK,Choi HS,Chang YS,Huh JM,Kim DH,Kim DY,Kim YH,Chang HJ,Jung KH,Jeong SY, Granular cell tumor of colon: report of a case and review of literature. World journal of gastroenterology. 2004 Aug 15; [PubMed PMID: 15285042]
Level 3 (low-level) evidenceYanoma T,Fukuchi M,Sakurai S,Shoji H,Naitoh H,Kuwano H, Granular cell tumor of the esophagus with elevated preoperative serum carbohydrate antigen 19-9: a case report. International surgery. 2015 Feb; [PubMed PMID: 25692443]
Level 3 (low-level) evidenceChen Y,Chen Y,Chen X,Chen L,Liang W, Colonic granular cell tumor: Report of 11 cases and management with review of the literature. Oncology letters. 2018 Aug; [PubMed PMID: 30008819]
Level 3 (low-level) evidencevan de Loo S,Thunnissen E,Postmus P,van der Waal I, Granular cell tumor of the oral cavity; a case series including a case of metachronous occurrence in the tongue and the lung. Medicina oral, patologia oral y cirugia bucal. 2015 Jan 1; [PubMed PMID: 24880452]
Level 2 (mid-level) evidenceAlotaiby FM,Fitzpatrick S,Upadhyaya J,Islam MN,Cohen D,Bhattacharyya I, Demographic, Clinical and Histopathological Features of Oral Neural Neoplasms: A Retrospective Study. Head and neck pathology. 2019 Jun; [PubMed PMID: 29931661]
Level 2 (mid-level) evidenceGagliardi F,Spina A,Barzaghi LR,Bailo M,Losa M,Terreni MR,Mortini P, Suprasellar granular cell tumor of the neurohypophysis: surgical outcome of a very rare tumor. Pituitary. 2016 Jun; [PubMed PMID: 26753850]
Pérez-González YC,Pagura L,Llamas-Velasco M,Cortes-Lambea L,Kutzner H,Requena L, Primary cutaneous malignant granular cell tumor: an immunohistochemical study and review of the literature. The American Journal of dermatopathology. 2015 Apr; [PubMed PMID: 25794371]
Level 3 (low-level) evidencePaul SP,Osipov V, An unusual granular cell tumour of the buttock and a review of granular cell tumours. Case reports in dermatological medicine. 2013; [PubMed PMID: 24066243]
Level 3 (low-level) evidenceBlacksin MF,White LM,Hameed M,Kandel R,Patterson FR,Benevenia J, Granular cell tumor of the extremity: magnetic resonance imaging characteristics with pathologic correlation. Skeletal radiology. 2005 Oct; [PubMed PMID: 16003548]
Level 2 (mid-level) evidenceHammas N,El Fatemi H,Jayi S,Hafid I,Fikri G,El Houari A,Seqqali N,Tizniti S,Melhouf MA,Amarti A, Granular cell tumor of the breast: a case report. Journal of medical case reports. 2014 Dec 26; [PubMed PMID: 25541096]
Level 3 (low-level) evidenceGavriilidis P,Michalopoulou I,Baliaka A,Nikolaidou A, Granular cell breast tumour mimicking infiltrating carcinoma. BMJ case reports. 2013 Feb 18; [PubMed PMID: 23420726]
Level 3 (low-level) evidenceYang WT,Edeiken-Monroe B,Sneige N,Fornage BD, Sonographic and mammographic appearances of granular cell tumors of the breast with pathological correlation. Journal of clinical ultrasound : JCU. 2006 May; [PubMed PMID: 16615051]
Coates SJ,Mitchell K,Olorunnipa OB,DeSimone RA,Otterburn DM,Simmons RM, An unusual breast lesion: granular cell tumor of the breast with extensive chest wall invasion. Journal of surgical oncology. 2014 Sep; [PubMed PMID: 24863566]
Level 3 (low-level) evidenceScaranelo AM,Bukhanov K,Crystal P,Mulligan AM,O'Malley FP, Granular cell tumour of the breast: MRI findings and review of the literature. The British journal of radiology. 2007 Dec; [PubMed PMID: 17940129]
Level 3 (low-level) evidenceLewis RB,Mehrotra AK,Rodriguez P,Levine MS, From the radiologic pathology archives: esophageal neoplasms: radiologic-pathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc. 2013 Jul-Aug; [PubMed PMID: 23842973]
Hwang JS,Beebe KS,Rojas J,Peters SR, Malignant granular cell tumor of the thigh. Orthopedics. 2011 Aug 8; [PubMed PMID: 21815590]
Level 3 (low-level) evidenceGardner ES,Goldberg LH, Granular cell tumor treated with Mohs micrographic surgery: report of a case and review of the literature. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 2001 Aug; [PubMed PMID: 11493306]
Level 3 (low-level) evidenceChen J,Wang L,Xu J,Pan T,Shen J,Hu W,Yuan X, Malignant granular cell tumor with breast metastasis: A case report and review of the literature. Oncology letters. 2012 Jul; [PubMed PMID: 22807961]
Level 3 (low-level) evidenceSingh VA,Gunasagaran J,Pailoor J, Granular cell tumour: malignant or benign? Singapore medical journal. 2015 Sep; [PubMed PMID: 26451054]
Marchand Crety C,Garbar C,Madelis G,Guillemin F,Soibinet Oudot P,Eymard JC,Servagi Vernat S, Adjuvant radiation therapy for malignant Abrikossoff's tumor: a case report about a femoral triangle localisation. Radiation oncology (London, England). 2018 Jun 20; [PubMed PMID: 29925410]
Level 3 (low-level) evidenceLiu TT,Han Y,Zheng S,Li B,Liu YQ,Chen YX,Liu YF,Wang EH, Primary cutaneous malignant granular cell tumor: a case report in China and review of the literature. Diagnostic pathology. 2015 Jul 19; [PubMed PMID: 26187381]
Level 3 (low-level) evidenceKatiyar V,Vohra I,Uprety A,Yin W,Gupta S, Recurrent Unresectable Malignant Granular Cell Tumor With Response to Pazopanib. Cureus. 2020 May 26; [PubMed PMID: 32601562]
Morita S,Hiramatsu M,Sugishita M,Gyawali B,Shibata T,Shimokata T,Urakawa H,Mitsuma A,Moritani S,Kubota T,Ichihara S,Ando Y, Pazopanib monotherapy in a patient with a malignant granular cell tumor originating from the right orbit: A case report. Oncology letters. 2015 Aug; [PubMed PMID: 26622607]
Level 3 (low-level) evidenceChen WS,Zheng XL,Jin L,Pan XJ,Ye MF, Novel diagnosis and treatment of esophageal granular cell tumor: report of 14 cases and review of the literature. The Annals of thoracic surgery. 2014 Jan; [PubMed PMID: 24140217]
Level 3 (low-level) evidenceLu W,Xu MD,Zhou PH,Zhang YQ,Chen WF,Zhong YS,Yao LQ, Endoscopic submucosal dissection of esophageal granular cell tumor. World journal of surgical oncology. 2014 Jul 17; [PubMed PMID: 25030028]
Cha JM,Lee JI,Joo KR,Choe JW,Jung SW,Shin HP,Lim SJ, Granular cell tumor of the descending colon treated by endoscopic mucosal resection: a case report and review of the literature. Journal of Korean medical science. 2009 Apr; [PubMed PMID: 19399282]
Level 3 (low-level) evidenceZnati K,Harmouch T,Benlemlih A,Elfatemi H,Chbani L,Amarti A, Solitary granular cell tumor of cecum: a case report. ISRN gastroenterology. 2011; [PubMed PMID: 21991536]
Level 3 (low-level) evidenceCardis MA,Ni J,Bhawan J, Granular cell differentiation: A review of the published work. The Journal of dermatology. 2017 Mar; [PubMed PMID: 28256763]
Qureshi NA,Tahir M,Carmichael AR, Granular cell tumour of the soft tissues: a case report and literature review. International seminars in surgical oncology : ISSO. 2006 Aug 24; [PubMed PMID: 16930486]
Level 3 (low-level) evidenceThacker MM,Humble SD,Mounasamy V,Temple HT,Scully SP, Case report. Granular cell tumors of extremities: comparison of benign and malignant variants. Clinical orthopaedics and related research. 2007 Feb; [PubMed PMID: 16936589]
Level 3 (low-level) evidenceMeissner M,Wolter M,Schöfer H,Kaufmann R, A solid erythematous tumour. Granular cell tumour (GCT). Clinical and experimental dermatology. 2010 Apr; [PubMed PMID: 20500174]
Level 3 (low-level) evidenceMoten AS,Zhao H,Wu H,Farma JM, Malignant granular cell tumor: Clinical features and long-term survival. Journal of surgical oncology. 2018 Nov; [PubMed PMID: 30196562]