Introduction
H1N1 influenza, a subtype of influenza A virus, is an infectious viral illness that causes both upper and, in some cases, lower respiratory tract infections in its host. H1N1 influenza infections can cause symptoms such as rhinorrhea, cough, decreased appetite, fever, rigors, myalgia, headache, and, possibly, lower respiratory tract disease and gastrointestinal disease.[1][2][3] Although other influenza strains exist, influenza A and B viruses predominantly impact human health.
Three subtypes of swine influenza circulate globally—H3N2, H1N2, and H1N1. The H1N1 influenza gained worldwide attention as "swine flu" during the 2009 pandemic after swine influenza viruses were reassorted with preexisting H1N1 strains.[4] Swine flu emerged from the recombination of various prior swine, avian, and human influenza strains, causing a global pandemic affecting millions of people and impacting industries, including food and tourism.
H1N1 influenza leads to a respiratory disease that can infect pigs' respiratory tract. Humans susceptible to swine influenza are typically exposed through close association with infected pigs, a condition known as zoonotic "swine flu." Swine influenza viruses can potentially infect humans if the antigenic characteristics of the virus change through the reassortment of different influenza strains.[5] This process can enhance replication and transmission, facilitating efficient transfer to human hosts. Such reassortments have led to pandemics, as seen in 1918 and 2009, when the virus acquired efficient person-to-person transmission capabilities.[6]
In 1918, the H1N1 influenza virus, commonly known as the Spanish flu, sparked a devastating pandemic that infected roughly 500 million individuals worldwide and led to the deaths of an estimated 50 to 100 million people, accounting for 3% to 5% of the global population at the time. This made it one of the deadliest pandemics in human history.[7] Similarly, the H1N1 influenza strain in 2009 was classified as a pandemic by the World Health Organization (WHO).[8] The 2009 H1N1 virus spread through airborne droplets from human to human, possibly via fomites contaminated with the virus, and subsequently transferred to the mucosa or upper respiratory tract.[9] Notably, similarities in symptoms of H1N1 in both humans and pigs arose, potentially due to the viral reassortment of preexisting strains. This similarity in symptoms suggested a commonality in viral pathogenesis across multiple hosts, likely facilitated by reassortment, thus enhancing efficient transmission.[4][10] During the pandemic, a common misconception was that individuals could contract swine flu from consuming pig products such as bacon, ham, and other pork items. However, the virus is isolated to the respiratory system and does not involve plasma, making transmission through food unlikely.[11] This misunderstanding resulted in substantial commercial losses in the food and tourism industries.[12]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
The H1N1 influenza virus belongs to the orthomyxovirus family and has a single-stranded negative-sense ribonucleic acid (RNA) genome. Its virions typically measure between 80 and 120 nm in diameter, with an RNA genome size of around 13.5 kb. The influenza genome comprises 8 segmented regions that encode a total of 11 different proteins, as mentioned below.
- Envelope proteins: Hemagglutinin (HA) and neuraminidase (NA).[13]
- Viral RNA polymerases: PB2, PB1, PB1-F2, PA, and PB.
- Matrix proteins: M1 and M2.[14]
- Nonstructural proteins: NS1 and NS2 (NEP), which are crucial for efficient pathogenesis and viral replication.
The H1N1 strain of influenza A is distinguished from other strains, such as H1N2, based on the surface glycoproteins hemagglutinin and neuraminidase, which exhibit metabolic synergy.[15] Hemagglutinin triggers erythrocyte aggregation by binding to sialic acid and facilitates virus attachment to infected cells, enabling endocytosis.[16] Subsequent fusion with the endosome, mediated by matrix proteins, allows viral RNA-dependent polymerase to initiate viral replication.[17] Neuraminidase is crucial during viral budding by cleaving sialic receptors and promoting virus spread to neighboring cells.[18]
Epidemiology
H1N1 influenza was first isolated from pigs in the 1930s by researchers in the United States and was subsequently recognized by pork producers and veterinarians as a cause of influenza infections in pigs worldwide.[19] People closely associated with pigs have been known to develop a disease, and pigs have also been infected with human influenza from these handlers.[20] The virus can potentially cause cross-species transmission through viral reassortment. Selection pressure from human populations immune to established strains of influenza can result in changes in antigenic structure over time to evade host immune responses, also known as antigenic drift.[21]
Significant shifts in the envelope proteins, known as antigenic shifts, can lead to the emergence of new influenza strains capable of evading the host immune response and facilitating human-to-human transmission in previously susceptible populations. For instance, the 2009 H1N1 pandemic, originating in Mexico, resulted from the combination of multiple strains, including Eurasian avian-like H1N1, avian H1N1, and a previously reassorted lineage consisting of avian H1N1, human H3N2, and swine influenza viruses.
Revised estimates from the 2009 H1N1 influenza pandemic indicated 151,700 to 575,500 respiratory and cardiac deaths occurred during the first 12 months of the pandemic, with up to 24% of the global population estimated to have been infected based on seroprevalence studies.[22][23] The 1918 deadly influenza pandemic caused by the H1N1 influenza virus infected approximately 500 million people around the world and caused the death of roughly 50 to 100 million people.[24] The 2009 H1N1 "swine flu" variant is the progeny of the strain that caused the 1918 influenza pandemic.[25] Although variants persist in pigs, the viral descendants of the 1918 virus are also known to infect humans, contributing to the seasonal epidemics of influenza from strains including H2N2 and H3N2.[26]
Influenza strains, including H1N1, are known to transmit between pigs and humans frequently, but human-to-human transmission is uncommon.[27] The 2009 H1N1 strain was easily transmitted between pigs and humans.[28] The potential retention of influenza virus strains in swine after these strains disappear in the human population essentially makes pigs a reservoir where swine influenza viruses can persist.[29] These strains can later emerge to reinfect human populations following reassortment, waned immunity, or a combination of both. As a result, new variants can continue to develop with increased genetic diversity, resulting in new threats to susceptible human populations.[3][30]
Pathophysiology
H1N1 influenza is an acute disease that infects the upper respiratory tract and can cause inflammation of the upper passages, trachea, and, possibly, the lower respiratory tract.[2][31] The known incubation period for the 2009 H1N1 influenza strains has a median duration of 2 days but ranges from 1 to 7 days. The virus replication occurs primarily in the upper and lower respiratory tract passages from the time of inoculation and peaks around 48 hours in most patients.[32] The infectious period begins a day before symptoms develop and lasts approximately 5 to 7 days after the person develops symptoms.[33][34] The period of viral shedding, which correlates with the infectious period, may be up to 15 days in children and last weeks to months in immunocompromised individuals.[35][36] The recommended time of isolation of the infected patient is approximately 7 days.[34]
The acute symptoms of uncomplicated infections persist for 3 days but can range from 1 to 11 days.[2] The disease is mostly self-limited in healthy individuals, but malaise and cough can persist for up to 2 weeks in some patients. Patients with more severe disease may require hospitalization, and this may manifest within 4 to 5 days.[37] The body's immune reaction to the virus and the interferon response are the causes of the viral syndrome, which includes high fever, coryza, and myalgia.[38] Individuals with chronic lung diseases, cardiac conditions, and those who are pregnant face a heightened risk of severe complications, including viral pneumonia, superimposed bacterial pneumonia, hemorrhagic bronchitis, and potentially fatal outcomes. These complications may manifest within 48 hours from the onset of symptoms.[39]
Histopathology
The main area of H1N1 influenza pathogenesis is the upper and lower respiratory tracts. Mild cases usually show a few pathologic changes in the respiratory tract, but severe cases can show clear pathological pneumonia changes. The pathological findings associated with H1N1 influenza include multifocal destruction, potential desquamation of the pseudo-columnar and columnar epithelial cells, and possibly prominent hyperemia and edema in the submucosa.[32] Thrombus formation at the bronchiolar level is also expected. Sometimes, the acute inflammation is severe and is indicated by hemorrhagic tracheobronchitis and desquamative bronchiolitis. This may cause necrosis of the bronchiolar wall. After necrosis occurs, polymorphs and mononuclear cells infiltrate into the affected area.
Although the virus can be observed using electron microscopy, histopathological changes are nonspecific and necessitate additional diagnostic methods for confirmation. These methods include virus isolation through culture, serology, molecular detection via polymerase chain reaction (PCR), or immunohistochemistry (see Image. Electron Microscopic View of H1N1 Influenza Virus Particles).[40] Histological changes in H1N1 influenza pneumonia include interstitial edema with possible inflammatory infiltrate, alveolar proteinaceous exudation associated with membrane formation, thrombosis of capillaries, necrosis of the alveolar septae, intra-alveolar hemorrhage, dislocation of desquamated pneumocytes with pyknotic nuclei into the surrounding alveolar spaces, diffuse alveolar damage with infiltration by the lymphocytes and histiocytes into the interstitium.[41]
In the late stage, patients may exhibit various changes, including diffuse alveolar damage, fibrosis, hyperplasia of type II pneumocytes, epithelial regeneration, and squamous metaplasia. These alterations are indicative of the fibroproliferative stage of acute respiratory distress syndrome (ARDS) and diffuse alveolar destruction. Additionally, bacterial coinfections may be present, with Streptococcus pneumoniae, S pyogenes, Staphylococcus aureus (including community-acquired and methicillin-resistant strains), and Haemophilus influenza being among the most commonly isolated bacteria.[42]
History and Physical
The history and clinical presentations of H1N1 swine influenza can vary widely, ranging from mild upper respiratory tract symptoms to severe respiratory complications, including death. These outcomes depend on factors such as the patient's age, underlying health conditions, influenza vaccination status, and natural immunity to the virus.[2] The signs and symptoms of H1N1 influenza closely resemble those of seasonal influenza, including fevers, chills, cough, runny nose, sore throat, conjunctivitis, muscle pain, difficulty breathing, weight loss, headache, nasal congestion, near-fainting, abdominal discomfort, decreased appetite, and fatigue. However, compared to seasonal influenza, H1N1 tends to present with more frequent episodes of cough, muscle pain, and pleural chest pain.[43] Additionally, the 2009 H1N1 strain was associated with increased vomiting and diarrhea.[44] Given that many symptoms overlap with other conditions, a detailed patient history is essential to differentiate H1N1 influenza, particularly in cases exposed to confirmed H1N1 cases or recent travel to high-prevalence areas.
In severe cases of the 2009 H1N1 pandemic influenza, respiratory failure and shock were the leading causes of death.[37] Other sequelae included encephalopathy, delirium, stroke, gastrointestinal bleeding, secondary bacterial sepsis, myocardial infarction, decompensated cardiac failure, myocarditis, and renal impairment requiring renal replacement therapy.[45] Unlike seasonal influenza strains, the 2009 H1N1 virus was associated with more severe cases and fatalities in children and adults aged 60 and younger.[46]
Additional risk factors for severe disease during the 2009 pandemic included pregnancy, particularly during the second and third trimesters, a body mass index equal to or greater than 35 kg/m2, the presence of chronic medical conditions, and delayed initiation of oseltamivir treatment (≥5 days after symptom onset).[39][47] Chronic medical conditions include chronic obstructive pulmonary disease, bronchial asthma, immunosuppression, chronic liver disease, neurological disorders, and diabetes mellitus.
Evaluation
Influenza A (H1N1) virus infection can manifest across different clinical settings, leading to diverse presentations. It is essential to consider H1N1 as a potential differential diagnosis in patients exhibiting unexplained flu-like symptoms or acute pneumonia, especially in regions with confirmed influenza cases. Initial evaluations should include routine investigations such as hematological, microbiological, biochemical, and radiological tests to establish a diagnosis.
Diagnostic confirmation of influenza A (H1N1) virus infection necessitates obtaining a respiratory sample, such as a nasopharyngeal swab, aspirate, or wash. Various tests can be conducted on these samples, including reverse transcriptase-PCR (RT-PCR), viral isolation by culture, complement fixation testing, haemagglutination assays, and antibody detection via immunofluorescence. Further serological assays during convalescence may be performed to detect seroconversion from immunoglobulin M (IgM) to IgG or a four-fold increase in influenza virus IgG antibody levels.[48][49] In addition to rapid point-of-care tests, these tests are specific for human influenza viruses, including the rapid point-of-care tests, and do not always detect zoonotic variants.[50]
An indication of a novel swine influenza virus may arise if an influenza A virus is detected lacking specific proteins typically found in human influenza viruses, such as hemagglutinins. Diagnosis of zoonotic influenza virus infections may sometimes be achieved retrospectively through serological testing. However, the potential for cross-reactivity with human influenza viruses can complicate this diagnostic process.[51] Another concern is that the neuraminidase (NA) and hemagglutinin (HA) envelope proteins of certain swine influenza viruses, which are the primary targets of host immune-mediated responses, may originate from ancestral human influenza viruses. This could indicate viruses that have previously circulated in human populations. Depending on the jurisdiction, state, regional, or national public health laboratories may be able to conduct genomic analysis and test for novel influenza viruses.[52]
Treatment / Management
Many management strategies applicable to influenza are also relevant to H1N1, except in cases involving variants of zoonotic origin. In such instances, prevention is the most effective approach to managing H1N1 influenza. This involves preventing and controlling swine influenza outbreaks in pigs, minimizing transmission of swine influenza variants from pigs to humans, and ultimately, halting human-to-human transmission.
Prevention of swine influenza in pigs: Prevention of swine influenza in pigs primarily relies on three key methods: facility management, herd management, and vaccination. Facility management involves implementing measures such as disinfection and regulated temperature controls to minimize viral replication in the environment. Herd management strategies include preventing overcrowding and quarantining pigs with influenza-like illnesses away from unexposed pig groups. Additionally, vaccination plays a crucial role.[53][54][55][56] Without complementary prevention and mitigation measures, vaccinations alone may not effectively limit swine influenza spillover events.(B3)
Prevention of swine-to-human viral transmission: Preventing swine-to-human viral transmission is crucial in mitigating the risk of H1N1 transmission to humans. While reducing influenza incidence in swine populations is essential, addressing swine-to-human transmission events is equally important in minimizing outbreak potential. Swine are susceptible to both avian and human strains of H1N1 influenza, earning them the nickname "mixing vessels." In these animals, viral reassortment can occur, leading to the emergence of new strains of swine influenza through antigenic shifts.[57]
Transmission of the influenza virus from swine to humans is usually seen in people closely associated with pigs, such as farmers, pork handlers, and veterinarians.[58] These individuals are strongly encouraged to wear face masks when dealing with infected animals to prevent transmission through respiratory droplets, given their efficacy in preventing influenza transmission.[59] Individuals with an increased risk of acquiring H1N1 influenza through pigs are also advised to employ similar strategies to prevent transmission in humans, emphasizing hand hygiene.(B2)
Prevention of human-to-human transmission: The main routes of zoonotic swine influenza virus spread between humans are most likely via inhalation of respiratory droplets or aerosols, mucosal contact, and fomite exposure, such as when a susceptible individual contacts a contaminated surface and then makes contact with their mucosal membranes in the nose, mouth or eyes.[60] Addressing these potential transmission points is critical during the infectious period of influenza. Documented strategies to prevent the spread of the virus include frequent handwashing with soap and water or alcohol-based sanitizers and disinfecting household, hospital, and public settings by cleaning with a diluted bleach solution.[61] Anyone who resides where the disease is prevalent suspects an infection or presents with flu-like symptoms should stay away from work and public transportation and immediately see a doctor.
During the 2009 H1N1 influenza pandemic, a viable vaccine was approved by the Food and Drug Administration (FDA) in the United States of America following studies by the National Institute of Health (NIH) indicating a single dose within 10 days of illness was sufficient to create protective antibodies against the virus.[62][63] The vaccination is contraindicated in people with a previous severe allergic reaction to the influenza vaccination. Those who are moderate to severely ill, including those with or without a fever, should take the vaccination when they recover or are asymptomatic.(B2)
The treatment of infected patients depends on the severity of symptoms of H1N1 influenza. Mild-to-moderate influenza is usually self-limiting and can be treated at home with rest and oral hydration. Symptomatic treatment for H1N1 influenza may include antipyretics such as paracetamol/acetaminophen, antihistamines for nasal congestion and rhinitis, and simple analgesia like non-steroidal anti-inflammatories or paracetamol/acetaminophen for headaches, myalgias, and arthralgias. Patients experiencing progressive or severe symptoms should be managed in an inpatient setting to monitor for potential intensive care unit (ICU) intervention, especially if signs indicate impending respiratory failure, sepsis, or multiorgan dysfunction. Aggressive supportive measures may include intravenous (IV) hydration, the correction of electrolyte imbalances, and antibiotics for concurrent bacterial infections. Patients developing acute respiratory distress syndrome (ARDS) secondary to influenza should be treated with non-invasive or invasive mechanical ventilation. Severe cases of H1N1-induced ARDS have required extracorporeal membrane oxygenation (ECMO).[64](A1)
Clinicians managing this condition should be aware that early antiviral treatment within 72 hours of symptom onset may decrease severe disease and mortality. Additionally, they should understand which antiviral treatments are effective in this window. Vaccines and other preventative measures are critical in reducing H1N1 influenza transmission during outbreaks. The antiviral medications, oral oseltamivir, IV zanamivir, and IV peramivir, have each been documented to help reduce, or possibly prevent, the effects of H1N1 influenza if the medication is taken within 48 hours of the onset of symptoms.[65][66][67] (A1)
Oseltamivir, a neuraminidase inhibitor, has effectively reduced inpatient readmission rates and mortality associated with influenza, making it a recommended chemoprophylaxis agent during H1N1 influenza outbreaks.[68][69] Common adverse effects of oseltamivir include nausea, vomiting, headache, and skin reactions such as atopic dermatitis and urticaria. Rare but serious adverse reactions may include Steven Johnson syndrome, hepatobiliary enzyme derangement, sporadic transient neuropsychiatric events, and gastrointestinal bleeding.[70][71][72]. Caution should be exercised when prescribing oseltamivir to individuals at higher risk of experiencing these adverse effects.(B2)
Zanamivir is another neuraminidase inhibitor, administered via IV route, that has clinical efficacy in hospitalized influenza patients unable to tolerate oral oseltamivir.[73][74] Individuals treated with zanamivir most commonly experience headaches, but it has been rarely associated with bronchospasm.[75] Zanamivir is contraindicated in individuals with an allergy to its components and in severe milk protein allergy.[76] However, zanamivir and oseltamivir may be used in influenza prophylaxis among older or immunocompromised patients, where vaccination is contraindicated.[66][77](A1)
Peramivir, developed in 2010, is another IV neuraminidase inhibitor alternative to oseltamivir in high-risk patients with influenza, demonstrating fever alleviation comparable to oseltamivir.[78][79] Post-marking experiences revealed mild adverse effects of diarrhea and abnormal behavior were commonly reported, with neutropenia and leukopenia observed rarely.[80] A systemic review of influenza resistance to neuraminidase inhibitors pooled analyses indicated approximately 2.6% of influenza samples were resistant to oseltamivir, 0% were resistant to zanamivir, and 0% to peramivir.[81] Individuals who remain symptomatic after 10 days of treatment should be assessed for secondary infections and potentially screened for antiviral resistance.[61](A1)
Pregnant women who contract the H1N1 are at a greater risk of complications because of hormonal and inflammatory response dysregulation, as well as systemic changes to their cell-mediated immunity to accommodate the growing fetus.[82][83] Neonates born to pregnant patients with H1N1 have higher frequencies of pre-term birth and intrauterine growth restriction.[69] For these reasons, during the 2009 H1N1 pandemic, the United States Centers for Disease Control and Prevention and the WHO recommended all pregnant women be vaccinated to prevent infection. Oseltamivir has been used frequently in pregnancy and has shown efficacy in reducing severe illness with H1N1 influenza in pregnant women within 48 hours of symptom onset.[84] Zanamivir also has demonstrated safety data for H1N1 influenza.[85](B2)
Differential Diagnosis
The conditions included in the differential diagnosis are infections such as HIV, COVID-19, adenovirus, arenavirus, cytomegalovirus, dengue, and echovirus infection, acute respiratory distress syndrome, hantavirus pulmonary syndrome, human parainfluenza virus and other parainfluenza virus infections, Legionnaires disease, Pneumocystis jirovecii pneumonia infection, cryptococcal pneumonia infection, and Mycoplasma pneumoniae and Chlamydia pneumoniae infections.
Among these, the viral infections most closely resembling H1N1 influenza in presentation include COVID-19, seasonal influenza strain, and parainfluenza virus infections.
Prognosis
Pandemic 2009 H1N1 influenza infection was associated with a mortality of approximately 1%.[86] Individuals aged 50 and older had a lower risk of infection compared to younger age groups, while hospitalization rates were higher among those aged 5 or younger and between the ages of 5 and 14. Severe disease was more prevalent in individuals with chronic comorbidities. Key laboratory findings in severe cases included elevated levels of lactate dehydrogenase, creatinine phosphokinase, C-reactive protein, and lymphopenia.[87][88]
Prognostic variables associated with death or ICU admission in H1N1 influenza include diabetes, corticosteroid therapy, histamine-2 receptor use, and morbid obesity, as well as secondary cardiovascular and bacterial complications.[89] Additionally, a study involving 1651 patients during the 2009 pandemic revealed that delaying oseltamivir administration for more than 5 days independently correlated with hospitalization, ICU admission, and heightened odds of mortality.[90]
Complications
The 2009 H1N1 influenza pandemic strain primarily led to respiratory complications, such as pneumonia, exacerbation of chronic pulmonary disease, and, to a lesser extent, ARDS.[91] Cardiovascular complications and secondary bacterial infections could exacerbate outcomes.[88] Neurological complications varied and included seizures, focal neurological deficits, Guillain-Barré syndrome, and myositis.[92] Long-term sequelae from H1N1 influenza-related ARDS have also been observed. One year post-H1N1 influenza, individuals experienced increased exertional dyspnea scores, reduced frequencies of returning to work, and higher rates of anxiety and depression compared to those without H1N1-related ARDS.[93] These potential outcomes emphasize the importance of ensuring appropriate and timely treatment to prevent acute and long-term complications.
Deterrence and Patient Education
The H1N1 influenza virus, as a respiratory pathogen, can be prevented through proper personal hygiene and environmental control measures. Encouraging the distribution and use of masks, along with practicing regular hand hygiene using soap and water, antiseptic hand wash, or alcohol-based hand rubs, is essential. These practices are essential before engaging in activities that involve contact between the hands and face.[61] Education on proper cough and sneeze etiquette, along with social distancing measures, should be provided to minimize exposure to respiratory secretions.[94] Disinfecting contaminated surfaces with agents like alcohol, sodium hypochlorite, or quaternary ammonia compounds is also recommended.[95]
Enhancing Healthcare Team Outcomes
H1N1 influenza is highly infectious and can spread easily and quickly from infected humans or through contact with pigs carrying the virus. The infection can quickly progress to moderate-to-severe symptoms and, in some cases, fatalities. For optimal outcomes, patients with H1N1 influenza should be evaluated and cared for by an interprofessional healthcare team. This team should be aware of high-risk patients, including children, older and immunocompromised individuals, pregnant women, and those suffering from chronic conditions.[96]
The treating clinician, whether primary care physician, pharmacist, or nurse practitioner, should advocate for administering the H1N1 influenza vaccine to both children and adults at risk. Furthermore, it is crucial to encourage vaccination for all pregnant women to mitigate morbidity, mortality, and fetal complications.[96] In emergency situations, the school nurse, in collaboration with school authorities, should assess the need for school closure in the event of H1N1 cases.[97]
Parents should be encouraged to have their children vaccinated against H1N1 influenza and advised to self-isolate if infected to minimize exposure to others. Pharmacists are authorized to administer vaccines in many states within the United States and other countries. In hospital settings, nurses should ensure that infected patients are placed in single isolation rooms with airborne precautions. Notably, taking appropriate measures to prevent contact with bodily fluids and aerosols generated during coughing is essential. Strict adherence to hand hygiene protocols is crucial, and only a limited number of healthcare personnel should have contact with infected patients. Open communication among interprofessional healthcare team members is essential for reducing the morbidity and mortality associated with H1N1 influenza.[98]
Media
(Click Image to Enlarge)
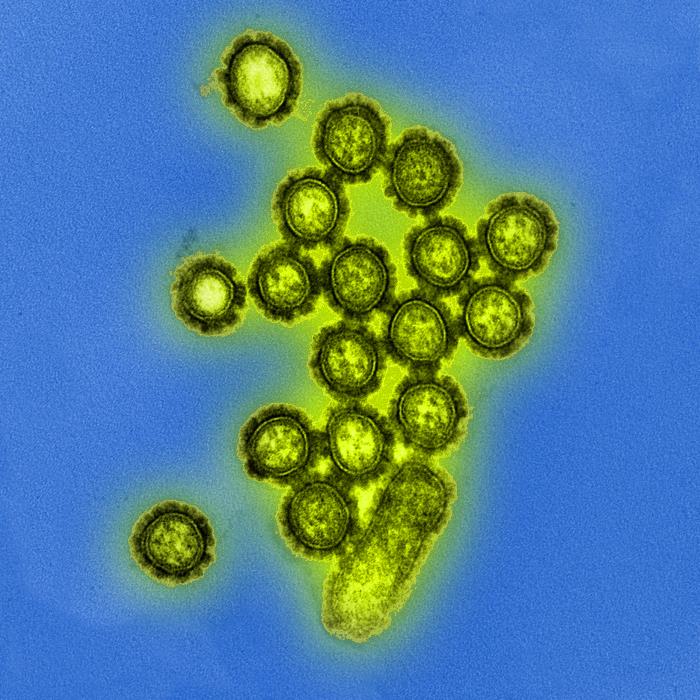
Electron Microscopic View of H1N1 Influenza Virus Particles. Digitally colorized transmission electron microscopic view of H1N1 influenza virus particles.
Public Health Image Library, Public Domain, Centers for Disease Control and Prevention
References
Hackett S, Hill L, Patel J, Ratnaraja N, Ifeyinwa A, Farooqi M, Nusgen U, Debenham P, Gandhi D, Makwana N, Smit E, Welch S. Clinical characteristics of paediatric H1N1 admissions in Birmingham, UK. Lancet (London, England). 2009 Aug 22:374(9690):605. doi: 10.1016/S0140-6736(09)61511-7. Epub [PubMed PMID: 19700001]
Level 3 (low-level) evidenceCao B, Li XW, Mao Y, Wang J, Lu HZ, Chen YS, Liang ZA, Liang L, Zhang SJ, Zhang B, Gu L, Lu LH, Wang DY, Wang C, National Influenza A Pandemic (H1N1) 2009 Clinical Investigation Group of China. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. The New England journal of medicine. 2009 Dec 24:361(26):2507-17. doi: 10.1056/NEJMoa0906612. Epub 2009 Dec 9 [PubMed PMID: 20007555]
Level 3 (low-level) evidenceKshatriya RM, Khara NV, Ganjiwale J, Lote SD, Patel SN, Paliwal RP. Lessons learnt from the Indian H1N1 (swine flu) epidemic: Predictors of outcome based on epidemiological and clinical profile. Journal of family medicine and primary care. 2018 Nov-Dec:7(6):1506-1509. doi: 10.4103/jfmpc.jfmpc_38_18. Epub [PubMed PMID: 30613550]
Level 2 (mid-level) evidenceMena I, Nelson MI, Quezada-Monroy F, Dutta J, Cortes-Fernández R, Lara-Puente JH, Castro-Peralta F, Cunha LF, Trovão NS, Lozano-Dubernard B, Rambaut A, van Bakel H, García-Sastre A. Origins of the 2009 H1N1 influenza pandemic in swine in Mexico. eLife. 2016 Jun 28:5():. pii: e16777. doi: 10.7554/eLife.16777. Epub 2016 Jun 28 [PubMed PMID: 27350259]
Lewis NS, Russell CA, Langat P, Anderson TK, Berger K, Bielejec F, Burke DF, Dudas G, Fonville JM, Fouchier RA, Kellam P, Koel BF, Lemey P, Nguyen T, Nuansrichy B, Peiris JM, Saito T, Simon G, Skepner E, Takemae N, ESNIP3 consortium, Webby RJ, Van Reeth K, Brookes SM, Larsen L, Watson SJ, Brown IH, Vincent AL. The global antigenic diversity of swine influenza A viruses. eLife. 2016 Apr 15:5():e12217. doi: 10.7554/eLife.12217. Epub 2016 Apr 15 [PubMed PMID: 27113719]
Mancera Gracia JC, Van den Hoecke S, Saelens X, Van Reeth K. Effect of serial pig passages on the adaptation of an avian H9N2 influenza virus to swine. PloS one. 2017:12(4):e0175267. doi: 10.1371/journal.pone.0175267. Epub 2017 Apr 6 [PubMed PMID: 28384328]
Johnson NP, Mueller J. Updating the accounts: global mortality of the 1918-1920 "Spanish" influenza pandemic. Bulletin of the history of medicine. 2002 Spring:76(1):105-15 [PubMed PMID: 11875246]
Doshi P. The elusive definition of pandemic influenza. Bulletin of the World Health Organization. 2011 Jul 1:89(7):532-8. doi: 10.2471/BLT.11.086173. Epub [PubMed PMID: 21734768]
Lei H, Li Y, Xiao S, Lin CH, Norris SL, Wei D, Hu Z, Ji S. Routes of transmission of influenza A H1N1, SARS CoV, and norovirus in air cabin: Comparative analyses. Indoor air. 2018 May:28(3):394-403. doi: 10.1111/ina.12445. Epub 2018 Jan 6 [PubMed PMID: 29244221]
Level 2 (mid-level) evidenceKeenliside J. Pandemic influenza A H1N1 in Swine and other animals. Current topics in microbiology and immunology. 2013:370():259-71. doi: 10.1007/82_2012_301. Epub [PubMed PMID: 23254339]
Level 3 (low-level) evidenceLange E, Kalthoff D, Blohm U, Teifke JP, Breithaupt A, Maresch C, Starick E, Fereidouni S, Hoffmann B, Mettenleiter TC, Beer M, Vahlenkamp TW. Pathogenesis and transmission of the novel swine-origin influenza virus A/H1N1 after experimental infection of pigs. The Journal of general virology. 2009 Sep:90(Pt 9):2119-23. doi: 10.1099/vir.0.014480-0. Epub 2009 Jul 10 [PubMed PMID: 19592456]
Level 3 (low-level) evidenceRassy D, Smith RD. The economic impact of H1N1 on Mexico's tourist and pork sectors. Health economics. 2013 Jul:22(7):824-34. doi: 10.1002/hec.2862. Epub [PubMed PMID: 23744805]
Level 3 (low-level) evidenceNogales A, Martinez-Sobrido L, Chiem K, Topham DJ, DeDiego ML. Functional Evolution of the 2009 Pandemic H1N1 Influenza Virus NS1 and PA in Humans. Journal of virology. 2018 Oct 1:92(19):. doi: 10.1128/JVI.01206-18. Epub 2018 Sep 12 [PubMed PMID: 30021892]
Tapia R, García V, Mena J, Bucarey S, Medina RA, Neira V. Infection of novel reassortant H1N2 and H3N2 swine influenza A viruses in the guinea pig model. Veterinary research. 2018 Jul 27:49(1):73. doi: 10.1186/s13567-018-0572-4. Epub 2018 Jul 27 [PubMed PMID: 30053826]
Fouchier RA, Munster V, Wallensten A, Bestebroer TM, Herfst S, Smith D, Rimmelzwaan GF, Olsen B, Osterhaus AD. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. Journal of virology. 2005 Mar:79(5):2814-22 [PubMed PMID: 15709000]
Level 3 (low-level) evidenceHirst GK. THE AGGLUTINATION OF RED CELLS BY ALLANTOIC FLUID OF CHICK EMBRYOS INFECTED WITH INFLUENZA VIRUS. Science (New York, N.Y.). 1941 Jul 4:94(2427):22-3 [PubMed PMID: 17777315]
Zebedee SL, Lamb RA. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. Journal of virology. 1988 Aug:62(8):2762-72 [PubMed PMID: 2455818]
Herrler G, Rott R, Klenk HD, Müller HP, Shukla AK, Schauer R. The receptor-destroying enzyme of influenza C virus is neuraminate-O-acetylesterase. The EMBO journal. 1985 Jun:4(6):1503-6 [PubMed PMID: 2411539]
Level 3 (low-level) evidenceShope RE. SWINE INFLUENZA : III. FILTRATION EXPERIMENTS AND ETIOLOGY. The Journal of experimental medicine. 1931 Jul 31:54(3):373-85 [PubMed PMID: 19869924]
Kaplan MM. Relationships between animal and human influenza. Bulletin of the World Health Organization. 1969:41(3):485-6 [PubMed PMID: 5309460]
Level 3 (low-level) evidence. Antigenic shift and drift. Nature. 1980 Feb 7:283(5747):524-5 [PubMed PMID: 7354835]
Dawood FS, Iuliano AD, Reed C, Meltzer MI, Shay DK, Cheng PY, Bandaranayake D, Breiman RF, Brooks WA, Buchy P, Feikin DR, Fowler KB, Gordon A, Hien NT, Horby P, Huang QS, Katz MA, Krishnan A, Lal R, Montgomery JM, Mølbak K, Pebody R, Presanis AM, Razuri H, Steens A, Tinoco YO, Wallinga J, Yu H, Vong S, Bresee J, Widdowson MA. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. The Lancet. Infectious diseases. 2012 Sep:12(9):687-95. doi: 10.1016/S1473-3099(12)70121-4. Epub 2012 Jun 26 [PubMed PMID: 22738893]
Van Kerkhove MD, Hirve S, Koukounari A, Mounts AW, H1N1pdm serology working group. Estimating age-specific cumulative incidence for the 2009 influenza pandemic: a meta-analysis of A(H1N1)pdm09 serological studies from 19 countries. Influenza and other respiratory viruses. 2013 Sep:7(5):872-86. doi: 10.1111/irv.12074. Epub 2013 Jan 21 [PubMed PMID: 23331969]
Level 2 (mid-level) evidenceNickol ME, Kindrachuk J. A year of terror and a century of reflection: perspectives on the great influenza pandemic of 1918-1919. BMC infectious diseases. 2019 Feb 6:19(1):117. doi: 10.1186/s12879-019-3750-8. Epub 2019 Feb 6 [PubMed PMID: 30727970]
Level 3 (low-level) evidenceCohen J. Swine flu pandemic. What's old is new: 1918 virus matches 2009 H1N1 strain. Science (New York, N.Y.). 2010 Mar 26:327(5973):1563-4. doi: 10.1126/science.327.5973.1563. Epub [PubMed PMID: 20339037]
Level 3 (low-level) evidenceScholtissek C, Rohde W, Von Hoyningen V, Rott R. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology. 1978 Jun 1:87(1):13-20 [PubMed PMID: 664248]
Li X, Guo L, Liu C, Cheng Y, Kong M, Yang L, Zhuang Z, Liu J, Zou M, Dong X, Su X, Gu Q. Human infection with a novel reassortant Eurasian-avian lineage swine H1N1 virus in northern China. Emerging microbes & infections. 2019:8(1):1535-1545. doi: 10.1080/22221751.2019.1679611. Epub [PubMed PMID: 31661383]
Chastagner A, Enouf V, Peroz D, Hervé S, Lucas P, Quéguiner S, Gorin S, Beven V, Behillil S, Leneveu P, Garin E, Blanchard Y, van der Werf S, Simon G. Bidirectional Human-Swine Transmission of Seasonal Influenza A(H1N1)pdm09 Virus in Pig Herd, France, 2018. Emerging infectious diseases. 2019 Oct:25(10):1940-1943. doi: 10.3201/eid2510.190068. Epub [PubMed PMID: 31538914]
Scholtissek C, Bürger H, Kistner O, Shortridge KF. The nucleoprotein as a possible major factor in determining host specificity of influenza H3N2 viruses. Virology. 1985 Dec:147(2):287-94 [PubMed PMID: 2416114]
Level 3 (low-level) evidenceSun H, Xiao Y, Liu J, Wang D, Li F, Wang C, Li C, Zhu J, Song J, Sun H, Jiang Z, Liu L, Zhang X, Wei K, Hou D, Pu J, Sun Y, Tong Q, Bi Y, Chang KC, Liu S, Gao GF, Liu J. Prevalent Eurasian avian-like H1N1 swine influenza virus with 2009 pandemic viral genes facilitating human infection. Proceedings of the National Academy of Sciences of the United States of America. 2020 Jul 21:117(29):17204-17210. doi: 10.1073/pnas.1921186117. Epub 2020 Jun 29 [PubMed PMID: 32601207]
Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R, Jasuja S, Deutscher M, Chen S, Walker JD, Duchin JS, Lett S, Soliva S, Wells EV, Swerdlow D, Uyeki TM, Fiore AE, Olsen SJ, Fry AM, Bridges CB, Finelli L, 2009 Pandemic Influenza A (H1N1) Virus Hospitalizations Investigation Team. Hospitalized patients with 2009 H1N1 influenza in the United States, April-June 2009. The New England journal of medicine. 2009 Nov 12:361(20):1935-44. doi: 10.1056/NEJMoa0906695. Epub 2009 Oct 8 [PubMed PMID: 19815859]
Taubenberger JK, Morens DM. The pathology of influenza virus infections. Annual review of pathology. 2008:3():499-522 [PubMed PMID: 18039138]
Level 3 (low-level) evidenceRoberts KL, Shelton H, Stilwell P, Barclay WS. Transmission of a 2009 H1N1 pandemic influenza virus occurs before fever is detected, in the ferret model. PloS one. 2012:7(8):e43303. doi: 10.1371/journal.pone.0043303. Epub 2012 Aug 29 [PubMed PMID: 22952661]
Level 3 (low-level) evidenceDe Serres G, Rouleau I, Hamelin ME, Quach C, Skowronski D, Flamand L, Boulianne N, Li Y, Carbonneau J, Bourgault A, Couillard M, Charest H, Boivin G. Contagious period for pandemic (H1N1) 2009. Emerging infectious diseases. 2010 May:16(5):783-8. doi: 10.3201/eid1605.091894. Epub [PubMed PMID: 20409367]
Centers for Disease Control and Prevention (CDC). Oseltamivir-resistant novel influenza A (H1N1) virus infection in two immunosuppressed patients - Seattle, Washington, 2009. MMWR. Morbidity and mortality weekly report. 2009 Aug 21:58(32):893-6 [PubMed PMID: 19696719]
Level 3 (low-level) evidenceEsposito S, Daleno C, Baldanti F, Scala A, Campanini G, Taroni F, Fossali E, Pelucchi C, Principi N. Viral shedding in children infected by pandemic A/H1N1/2009 influenza virus. Virology journal. 2011 Jul 13:8():349. doi: 10.1186/1743-422X-8-349. Epub 2011 Jul 13 [PubMed PMID: 21752272]
Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne F, Turgeon AF, Lapinsky S, Ahern SP, Smith O, Siddiqui F, Jouvet P, Khwaja K, McIntyre L, Menon K, Hutchison J, Hornstein D, Joffe A, Lauzier F, Singh J, Karachi T, Wiebe K, Olafson K, Ramsey C, Sharma S, Dodek P, Meade M, Hall R, Fowler RA, Canadian Critical Care Trials Group H1N1 Collaborative. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009 Nov 4:302(17):1872-9. doi: 10.1001/jama.2009.1496. Epub 2009 Oct 12 [PubMed PMID: 19822627]
Killip MJ, Fodor E, Randall RE. Influenza virus activation of the interferon system. Virus research. 2015 Nov 2:209():11-22. doi: 10.1016/j.virusres.2015.02.003. Epub 2015 Feb 9 [PubMed PMID: 25678267]
ANZIC Influenza Investigators, Webb SA, Pettilä V, Seppelt I, Bellomo R, Bailey M, Cooper DJ, Cretikos M, Davies AR, Finfer S, Harrigan PW, Hart GK, Howe B, Iredell JR, McArthur C, Mitchell I, Morrison S, Nichol AD, Paterson DL, Peake S, Richards B, Stephens D, Turner A, Yung M. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. The New England journal of medicine. 2009 Nov 12:361(20):1925-34. doi: 10.1056/NEJMoa0908481. Epub 2009 Oct 8 [PubMed PMID: 19815860]
Level 2 (mid-level) evidenceTaubenberger JK, Layne SP. Diagnosis of influenza virus: coming to grips with the molecular era. Molecular diagnosis : a journal devoted to the understanding of human disease through the clinical application of molecular biology. 2001 Dec:6(4):291-305 [PubMed PMID: 11774194]
Level 3 (low-level) evidenceNin N, Sánchez-Rodríguez C, Ver LS, Cardinal P, Ferruelo A, Soto L, Deicas A, Campos N, Rocha O, Ceraso DH, El-Assar M, Ortín J, Fernández-Segoviano P, Esteban A, Lorente JA. Lung histopathological findings in fatal pandemic influenza A (H1N1). Medicina intensiva. 2012 Jan-Feb:36(1):24-31. doi: 10.1016/j.medin.2011.10.005. Epub 2011 Dec 10 [PubMed PMID: 22154847]
Level 3 (low-level) evidenceMorris DE, Cleary DW, Clarke SC. Secondary Bacterial Infections Associated with Influenza Pandemics. Frontiers in microbiology. 2017:8():1041. doi: 10.3389/fmicb.2017.01041. Epub 2017 Jun 23 [PubMed PMID: 28690590]
Shiley KT, Nadolski G, Mickus T, Fishman NO, Lautenbach E. Differences in the epidemiological characteristics and clinical outcomes of pandemic (H1N1) 2009 influenza, compared with seasonal influenza. Infection control and hospital epidemiology. 2010 Jul:31(7):676-82. doi: 10.1086/653204. Epub [PubMed PMID: 20500086]
Level 2 (mid-level) evidenceCarcione D, Giele C, Dowse GK, Mak DB, Goggin L, Kwan K, Williams S, Smith D, Effler P. Comparison of pandemic (H1N1) 2009 and seasonal influenza, Western Australia, 2009. Emerging infectious diseases. 2010 Sep:16(9):1388-95. doi: 10.3201/eid1609.100076. Epub [PubMed PMID: 20735922]
Lee N, Chan PK, Lui GC, Wong BC, Sin WW, Choi KW, Wong RY, Lee EL, Yeung AC, Ngai KL, Chan MC, Lai RW, Yu AW, Hui DS. Complications and outcomes of pandemic 2009 Influenza A (H1N1) virus infection in hospitalized adults: how do they differ from those in seasonal influenza? The Journal of infectious diseases. 2011 Jun 15:203(12):1739-47. doi: 10.1093/infdis/jir187. Epub [PubMed PMID: 21606532]
Lemaitre M, Carrat F. Comparative age distribution of influenza morbidity and mortality during seasonal influenza epidemics and the 2009 H1N1 pandemic. BMC infectious diseases. 2010 Jun 9:10():162. doi: 10.1186/1471-2334-10-162. Epub 2010 Jun 9 [PubMed PMID: 20534113]
Level 2 (mid-level) evidenceYu H, Feng Z, Uyeki TM, Liao Q, Zhou L, Feng L, Ye M, Xiang N, Huai Y, Yuan Y, Jiang H, Zheng Y, Gargiullo P, Peng Z, Feng Y, Zheng J, Xu C, Zhang Y, Shu Y, Gao Z, Yang W, Wang Y. Risk factors for severe illness with 2009 pandemic influenza A (H1N1) virus infection in China. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011 Feb 15:52(4):457-65. doi: 10.1093/cid/ciq144. Epub 2011 Jan 10 [PubMed PMID: 21220768]
Jernigan DB, Lindstrom SL, Johnson JR, Miller JD, Hoelscher M, Humes R, Shively R, Brammer L, Burke SA, Villanueva JM, Balish A, Uyeki T, Mustaquim D, Bishop A, Handsfield JH, Astles R, Xu X, Klimov AI, Cox NJ, Shaw MW. Detecting 2009 pandemic influenza A (H1N1) virus infection: availability of diagnostic testing led to rapid pandemic response. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011 Jan 1:52 Suppl 1():S36-43. doi: 10.1093/cid/ciq020. Epub [PubMed PMID: 21342897]
Wood JM, Gaines-Das RE, Taylor J, Chakraverty P. Comparison of influenza serological techniques by international collaborative study. Vaccine. 1994 Feb:12(2):167-74 [PubMed PMID: 8147099]
Level 3 (low-level) evidenceVasoo S, Stevens J, Singh K. Rapid antigen tests for diagnosis of pandemic (Swine) influenza A/H1N1. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009 Oct 1:49(7):1090-3. doi: 10.1086/644743. Epub [PubMed PMID: 19725784]
Salvesen HA, Whitelaw CBA. Current and prospective control strategies of influenza A virus in swine. Porcine health management. 2021 Feb 28:7(1):23. doi: 10.1186/s40813-021-00196-0. Epub 2021 Feb 28 [PubMed PMID: 33648602]
Mengual-Chuliá B, Alonso-Cordero A, Cano L, Mosquera MDM, de Molina P, Vendrell R, Reyes-Prieto M, Jané M, Torner N, Martínez AI, Vila J, Díez-Domingo J, Marcos MÁ, López-Labrador FX. Whole-Genome Analysis Surveillance of Influenza A Virus Resistance to Polymerase Complex Inhibitors in Eastern Spain from 2016 to 2019. Antimicrobial agents and chemotherapy. 2021 May 18:65(6):. doi: 10.1128/AAC.02718-20. Epub 2021 May 18 [PubMed PMID: 33782005]
Jeong EK, Bae JE, Kim IS. Inactivation of influenza A virus H1N1 by disinfection process. American journal of infection control. 2010 Jun:38(5):354-60. doi: 10.1016/j.ajic.2010.03.003. Epub 2010 Apr 28 [PubMed PMID: 20430477]
Yamaya M, Nishimura H, Lusamba Kalonji N, Deng X, Momma H, Shimotai Y, Nagatomi R. Effects of high temperature on pandemic and seasonal human influenza viral replication and infection-induced damage in primary human tracheal epithelial cell cultures. Heliyon. 2019 Feb:5(2):e01149. doi: 10.1016/j.heliyon.2019.e01149. Epub 2019 Feb 5 [PubMed PMID: 30839917]
Lee JH, Gramer MR, Joo HS. Efficacy of swine influenza A virus vaccines against an H3N2 virus variant. Canadian journal of veterinary research = Revue canadienne de recherche veterinaire. 2007 Jul:71(3):207-12 [PubMed PMID: 17695596]
Level 3 (low-level) evidenceFablet C, Simon G, Dorenlor V, Eono F, Eveno E, Gorin S, Quéguiner S, Madec F, Rose N. Different herd level factors associated with H1N1 or H1N2 influenza virus infections in fattening pigs. Preventive veterinary medicine. 2013 Nov 1:112(3-4):257-65. doi: 10.1016/j.prevetmed.2013.07.006. Epub 2013 Aug 19 [PubMed PMID: 23968780]
Level 3 (low-level) evidenceMa W, Kahn RE, Richt JA. The pig as a mixing vessel for influenza viruses: Human and veterinary implications. Journal of molecular and genetic medicine : an international journal of biomedical research. 2008 Nov 27:3(1):158-66 [PubMed PMID: 19565018]
Borkenhagen LK, Wang GL, Simmons RA, Bi ZQ, Lu B, Wang XJ, Wang CX, Chen SH, Song SX, Li M, Zhao T, Wu MN, Park LP, Cao WC, Ma MJ, Gray GC. High Risk of Influenza Virus Infection Among Swine Workers: Examining a Dynamic Cohort in China. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020 Jul 27:71(3):622-629. doi: 10.1093/cid/ciz865. Epub [PubMed PMID: 31504322]
Zhang L, Peng Z, Ou J, Zeng G, Fontaine RE, Liu M, Cui F, Hong R, Zhou H, Huai Y, Chuang SK, Leung YH, Feng Y, Luo Y, Shen T, Zhu BP, Widdowson MA, Yu H. Protection by face masks against influenza A(H1N1)pdm09 virus on trans-Pacific passenger aircraft, 2009. Emerging infectious diseases. 2013:19(9):1403-10. doi: 10.3201/eid1909.121765. Epub [PubMed PMID: 23968983]
Level 2 (mid-level) evidenceZhang N, Li Y. Transmission of Influenza A in a Student Office Based on Realistic Person-to-Person Contact and Surface Touch Behaviour. International journal of environmental research and public health. 2018 Aug 9:15(8):. doi: 10.3390/ijerph15081699. Epub 2018 Aug 9 [PubMed PMID: 30096894]
Rewar S, Mirdha D, Rewar P. Treatment and Prevention of Pandemic H1N1 Influenza. Annals of global health. 2015 Sep-Oct:81(5):645-53. doi: 10.1016/j.aogh.2015.08.014. Epub [PubMed PMID: 27036721]
Borse RH, Shrestha SS, Fiore AE, Atkins CY, Singleton JA, Furlow C, Meltzer MI. Effects of vaccine program against pandemic influenza A(H1N1) virus, United States, 2009-2010. Emerging infectious diseases. 2013 Mar:19(3):439-48. doi: 10.3201/eid1903.120394. Epub [PubMed PMID: 23622679]
Level 2 (mid-level) evidenceCenters for Disease Control and Prevention (CDC). Interim results: state-specific influenza A (H1N1) 2009 monovalent vaccination coverage - United States, October 2009-January 2010. MMWR. Morbidity and mortality weekly report. 2010 Apr 2:59(12):363-8 [PubMed PMID: 20360670]
Sukhal S, Sethi J, Ganesh M, Villablanca PA, Malhotra AK, Ramakrishna H. Extracorporeal membrane oxygenation in severe influenza infection with respiratory failure: A systematic review and meta-analysis. Annals of cardiac anaesthesia. 2017 Jan-Mar:20(1):14-21. doi: 10.4103/0971-9784.197820. Epub [PubMed PMID: 28074789]
Level 1 (high-level) evidenceTreanor JJ, Hayden FG, Vrooman PS, Barbarash R, Bettis R, Riff D, Singh S, Kinnersley N, Ward P, Mills RG. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000 Feb 23:283(8):1016-24 [PubMed PMID: 10697061]
Level 1 (high-level) evidenceCheer SM, Wagstaff AJ. Zanamivir: an update of its use in influenza. Drugs. 2002:62(1):71-106 [PubMed PMID: 11790157]
Level 3 (low-level) evidenceKohno S, Kida H, Mizuguchi M, Shimada J, S-021812 Clinical Study Group. Efficacy and safety of intravenous peramivir for treatment of seasonal influenza virus infection. Antimicrobial agents and chemotherapy. 2010 Nov:54(11):4568-74. doi: 10.1128/AAC.00474-10. Epub 2010 Aug 16 [PubMed PMID: 20713668]
Level 1 (high-level) evidenceSharma Y, Horwood C, Hakendorf P, Thompson C. Effectiveness of Oseltamivir in reducing 30-day readmissions and mortality among patients with severe seasonal influenza in Australian hospitalized patients. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2021 Mar:104():232-238. doi: 10.1016/j.ijid.2021.01.011. Epub 2021 Jan 9 [PubMed PMID: 33434667]
Al-Husban N, Obeidat N, Al-Kuran O, Al Oweidat K, Bakri F. H1N1 Infection in Pregnancy; A Retrospective Study of Feto-Maternal Outcome and Impact of the Timing of Antiviral Therapy. Mediterranean journal of hematology and infectious diseases. 2019:11(1):e2019020. doi: 10.4084/MJHID.2019.020. Epub 2019 Mar 1 [PubMed PMID: 30858958]
Level 2 (mid-level) evidenceNordstrom BL, Oh K, Sacks ST, L'Italien GJ. Skin reactions in patients with influenza treated with oseltamivir: a retrospective cohort study. Antiviral therapy. 2004 Apr:9(2):187-95 [PubMed PMID: 15134180]
Level 2 (mid-level) evidenceSmith EV, Pynn MC, Blackford S, Leopold DJ. Stevens-Johnson syndrome secondary to oseltamivir (Tamiflu). The British journal of general practice : the journal of the Royal College of General Practitioners. 2010 Feb:60(571):133-4. doi: 10.3399/bjgp10X483292. Epub [PubMed PMID: 20132714]
Level 3 (low-level) evidenceFang S, Qi L, Zhou N, Li C. Case report on alimentary tract hemorrhage and liver injury after therapy with oseltamivir: A case report. Medicine. 2018 Sep:97(38):e12497. doi: 10.1097/MD.0000000000012497. Epub [PubMed PMID: 30235756]
Level 2 (mid-level) evidenceLee D, Jo H, Jang Y, Bae S, Agura T, Kang D, Kang M, Kim Y, Cho NH, Kim Y, Kang JS. Alloferon and Zanamivir Show Effective Antiviral Activity against Influenza A Virus (H1N1) Infection In Vitro and In Vivo. International journal of molecular sciences. 2022 Dec 30:24(1):. doi: 10.3390/ijms24010678. Epub 2022 Dec 30 [PubMed PMID: 36614125]
Dunn CJ, Goa KL. Zanamivir: a review of its use in influenza. Drugs. 1999 Oct:58(4):761-84 [PubMed PMID: 10551442]
Eiland LS, Eiland EH. Zanamivir for the prevention of influenza in adults and children age 5 years and older. Therapeutics and clinical risk management. 2007 Jun:3(3):461-5 [PubMed PMID: 18488077]
Freund B, Gravenstein S, Elliott M, Miller I. Zanamivir: a review of clinical safety. Drug safety. 1999 Oct:21(4):267-81 [PubMed PMID: 10514019]
Level 3 (low-level) evidenceMonto AS, Robinson DP, Herlocher ML, Hinson JM Jr, Elliott MJ, Crisp A. Zanamivir in the prevention of influenza among healthy adults: a randomized controlled trial. JAMA. 1999 Jul 7:282(1):31-5 [PubMed PMID: 10404908]
Level 1 (high-level) evidenceTakemoto Y, Asai T, Ikezoe I, Yano T, Ichikawa M, Miyagawa S, Matsumoto J. Clinical effects of oseltamivir, zanamivir, laninamivir and peramivir on seasonal influenza infection in outpatients in Japan during the winter of 2012-2013. Chemotherapy. 2013:59(5):373-8. doi: 10.1159/000362436. Epub 2014 May 10 [PubMed PMID: 24821568]
Level 1 (high-level) evidenceNakamura S, Miyazaki T, Izumikawa K, Kakeya H, Saisho Y, Yanagihara K, Miyazaki Y, Mukae H, Kohno S. Efficacy and Safety of Intravenous Peramivir Compared With Oseltamivir in High-Risk Patients Infected With Influenza A and B Viruses: A Multicenter Randomized Controlled Study. Open forum infectious diseases. 2017 Summer:4(3):ofx129. doi: 10.1093/ofid/ofx129. Epub 2017 Jun 19 [PubMed PMID: 28761899]
Level 1 (high-level) evidenceKomeda T, Ishii S, Itoh Y, Ariyasu Y, Sanekata M, Yoshikawa T, Shimada J. Post-marketing safety and effectiveness evaluation of the intravenous anti-influenza neuraminidase inhibitor peramivir. II: a pediatric drug use investigation. Journal of infection and chemotherapy : official journal of the Japan Society of Chemotherapy. 2015 Mar:21(3):194-201. doi: 10.1016/j.jiac.2014.11.009. Epub 2014 Nov 26 [PubMed PMID: 25523716]
Thorlund K, Awad T, Boivin G, Thabane L. Systematic review of influenza resistance to the neuraminidase inhibitors. BMC infectious diseases. 2011 May 19:11():134. doi: 10.1186/1471-2334-11-134. Epub 2011 May 19 [PubMed PMID: 21592407]
Level 1 (high-level) evidenceSomerville LK, Basile K, Dwyer DE, Kok J. The impact of influenza virus infection in pregnancy. Future microbiology. 2018 Feb:13():263-274. doi: 10.2217/fmb-2017-0096. Epub 2018 Jan 11 [PubMed PMID: 29320882]
Littauer EQ, Esser ES, Antao OQ, Vassilieva EV, Compans RW, Skountzou I. H1N1 influenza virus infection results in adverse pregnancy outcomes by disrupting tissue-specific hormonal regulation. PLoS pathogens. 2017 Nov:13(11):e1006757. doi: 10.1371/journal.ppat.1006757. Epub 2017 Nov 27 [PubMed PMID: 29176767]
Creanga AA, Johnson TF, Graitcer SB, Hartman LK, Al-Samarrai T, Schwarz AG, Chu SY, Sackoff JE, Jamieson DJ, Fine AD, Shapiro-Mendoza CK, Jones LE, Uyeki TM, Balter S, Bish CL, Finelli L, Honein MA. Severity of 2009 pandemic influenza A (H1N1) virus infection in pregnant women. Obstetrics and gynecology. 2010 Apr:115(4):717-726. doi: 10.1097/AOG.0b013e3181d57947. Epub [PubMed PMID: 20308830]
Dunstan HJ, Mill AC, Stephens S, Yates LM, Thomas SH. Pregnancy outcome following maternal use of zanamivir or oseltamivir during the 2009 influenza A/H1N1 pandemic: a national prospective surveillance study. BJOG : an international journal of obstetrics and gynaecology. 2014 Jun:121(7):901-6. doi: 10.1111/1471-0528.12640. Epub 2014 Mar 7 [PubMed PMID: 24602087]
Level 2 (mid-level) evidenceVan Kerkhove MD, Vandemaele KA, Shinde V, Jaramillo-Gutierrez G, Koukounari A, Donnelly CA, Carlino LO, Owen R, Paterson B, Pelletier L, Vachon J, Gonzalez C, Hongjie Y, Zijian F, Chuang SK, Au A, Buda S, Krause G, Haas W, Bonmarin I, Taniguichi K, Nakajima K, Shobayashi T, Takayama Y, Sunagawa T, Heraud JM, Orelle A, Palacios E, van der Sande MA, Wielders CC, Hunt D, Cutter J, Lee VJ, Thomas J, Santa-Olalla P, Sierra-Moros MJ, Hanshaoworakul W, Ungchusak K, Pebody R, Jain S, Mounts AW, WHO Working Group for Risk Factors for Severe H1N1pdm Infection. Risk factors for severe outcomes following 2009 influenza A (H1N1) infection: a global pooled analysis. PLoS medicine. 2011 Jul:8(7):e1001053. doi: 10.1371/journal.pmed.1001053. Epub 2011 Jul 5 [PubMed PMID: 21750667]
Nguyen-Van-Tam JS, Openshaw PJ, Hashim A, Gadd EM, Lim WS, Semple MG, Read RC, Taylor BL, Brett SJ, McMenamin J, Enstone JE, Armstrong C, Nicholson KG, Influenza Clinical Information Network (FLU-CIN). Risk factors for hospitalisation and poor outcome with pandemic A/H1N1 influenza: United Kingdom first wave (May-September 2009). Thorax. 2010 Jul:65(7):645-51. doi: 10.1136/thx.2010.135210. Epub [PubMed PMID: 20627925]
Sertogullarindan B, Ozbay B, Gunini H, Sunnetcioglu A, Arisoy A, Bilgin HM, Mermit Cilingir B, Duran M, Yildiz H, Ekin S, Baran A. Clinical and prognostic features of patients with pandemic 2009 influenza A (H1N1) virus in the intensive care unit. African health sciences. 2011 Jun:11(2):163-70 [PubMed PMID: 21857845]
Delgado-Rodríguez M, Castilla J, Godoy P, Martín V, Soldevila N, Alonso J, Astray J, Baricot M, Cantón R, Castro A, Gónzález-Candelas F, Mayoral JM, Quintana JM, Pumarola T, Tamames S, Sáez M, Domínguez A, CIBERESP Cases and Controls in Pandemic Influenza Working Group. Prognosis of hospitalized patients with 2009 H1N1 influenza in Spain: influence of neuraminidase inhibitors. The Journal of antimicrobial chemotherapy. 2012 Jul:67(7):1739-45. doi: 10.1093/jac/dks098. Epub 2012 Mar 30 [PubMed PMID: 22467633]
Level 3 (low-level) evidenceBellei NC, Cabeça TK, Carraro E, Goto JM, Cuba GT, Hidalgo SR, Burattini MN. Pandemic H1N1 illness prognosis: evidence from clinical and epidemiological data from the first pandemic wave in São Paulo, Brazil. Clinics (Sao Paulo, Brazil). 2013 Jun:68(6):840-5. doi: 10.6061/clinics/2013(06)19. Epub [PubMed PMID: 23778481]
Level 2 (mid-level) evidenceViasus D, Oteo Revuelta JA, Martínez-Montauti J, Carratalà J. Influenza A(H1N1)pdm09-related pneumonia and other complications. Enfermedades infecciosas y microbiologia clinica. 2012 Oct:30 Suppl 4():43-8. doi: 10.1016/S0213-005X(12)70104-0. Epub [PubMed PMID: 23116792]
Davis LE. Neurologic and muscular complications of the 2009 influenza A (H1N1) pandemic. Current neurology and neuroscience reports. 2010 Nov:10(6):476-83. doi: 10.1007/s11910-010-0135-1. Epub [PubMed PMID: 20697982]
Luyt CE, Combes A, Becquemin MH, Beigelman-Aubry C, Hatem S, Brun AL, Zraik N, Carrat F, Grenier PA, Richard JM, Mercat A, Brochard L, Brun-Buisson C, Chastre J, REVA Study Group. Long-term outcomes of pandemic 2009 influenza A(H1N1)-associated severe ARDS. Chest. 2012 Sep:142(3):583-592. doi: 10.1378/chest.11-2196. Epub [PubMed PMID: 22948576]
Level 2 (mid-level) evidenceDel Rio C, Hernandez-Avila M. Lessons from previous influenza pandemics and from the Mexican response to the current influenza pandemic. Archives of medical research. 2009 Nov:40(8):677-80. doi: 10.1016/j.arcmed.2009.12.005. Epub [PubMed PMID: 20304256]
Level 3 (low-level) evidenceBridges CB, Kuehnert MJ, Hall CB. Transmission of influenza: implications for control in health care settings. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2003 Oct 15:37(8):1094-101 [PubMed PMID: 14523774]
Level 3 (low-level) evidenceFell DB, Sprague AE, Liu N, Yasseen AS 3rd, Wen SW, Smith G, Walker MC, Better Outcomes Registry & Network (BORN) Ontario. H1N1 influenza vaccination during pregnancy and fetal and neonatal outcomes. American journal of public health. 2012 Jun:102(6):e33-40. doi: 10.2105/AJPH.2011.300606. Epub 2012 Apr 19 [PubMed PMID: 22515877]
Level 2 (mid-level) evidenceJackson C, Vynnycky E, Hawker J, Olowokure B, Mangtani P. School closures and influenza: systematic review of epidemiological studies. BMJ open. 2013:3(2):. doi: 10.1136/bmjopen-2012-002149. Epub 2013 Feb 26 [PubMed PMID: 23447463]
Level 1 (high-level) evidenceCauchemez S, Ferguson NM, Wachtel C, Tegnell A, Saour G, Duncan B, Nicoll A. Closure of schools during an influenza pandemic. The Lancet. Infectious diseases. 2009 Aug:9(8):473-81. doi: 10.1016/S1473-3099(09)70176-8. Epub [PubMed PMID: 19628172]