Introduction
The first system to develop due to the growing embryo's ever-increasing metabolic demands is the cardiovascular system. Initially, simple diffusion of necessary nutrients is sufficient but eventually becomes inadequate to supply oxygen and nutrients. Cardiac development is a complicated interplay of molecular communication, ensuring the proper formation of structures and spatial configuration changes in the appropriate timing. Interference with this process, whether genetic or environmental, leads to the formation of congenital heart diseases.
Development
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Development
The cardiovascular system's embryological development begins with cardiac progenitor cells' migration in the epiblast, just lateral to the primitive streak. These cardiac progenitor cells eventually develop into cardiac myoblasts. Within this same splanchnic layer of the mesoderm, so-called "blood islands" eventually undergo a period of vasculogenesis to form vascular structures. Coalescence of the blood islands eventually forms a region known as the cardiogenic field. The cardiogenic field is initially horseshoe-shaped and surrounded by cardiac myoblasts with the cardiogenic field's apex, eventually developing into primitive ventricles along with their respective outflow tracts. Ultimately, the cardiogenic field changes its configuration by cephalocaudal rotation. By doing so, it forms a primitive heart tube continuous with vascular structures.
The cranial aspect of the heart tube directs blood into the dorsal aorta, while the caudal aspect serves as a conduit for systemic venous return. The cephalocaudal rotation is not the only change in configuration at this developmental stage. The closure of the neural tube and the anterior displacement of the buccopharyngeal membrane facilitate the embryological heart's movement into the thorax. The primitive heart tube is composed of three layers, which are analogous to the adult human heart. The endocardium forms the endothelial lining of the embryonic heart. The myocardium forms the muscular bulk of the embryonic heart while the visceral pericardium forms the embryonic heart tube's external surface.
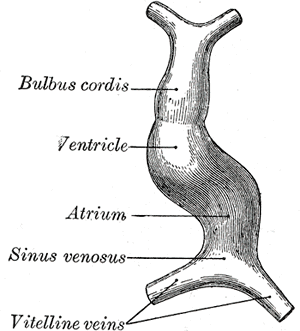
By approximately day 22-23, the heart tube elongates and alters its configuration again, forming a cardiac loop. The cardiac loop forms when the cranial aspect of the heart tube bends ventrocaudally and to the right while the caudal aspect of the heart tube bends toward the dorsocranial aspect and towards the left. The formation of the cardiac loop takes approximately five days and usually complete by day 28. The different parts of the heart tube develop in the following pattern:
- The proximal aspect of the heart tube forms the bulbus cordis, which develops into the trabeculated parts of the right ventricle.
- The middle segment of the heart tube is the conus cordis and is the precursor for the ventricular outflow tracts.
- The distal portion of the heart tube is referred to as the truncus arteriosus. The truncus arteriosus gives rise to the proximal portion of the aorta and pulmonary artery.
Prior to the end of the cardiac loop formation, the heart tube is essentially smooth-walled. However, near the end of the loop formation, trabeculated regions begin to form, and these trabeculated regions serve as primitive ventricles. The impaired development (or total lack of development) of the trabecular meshwork has associations with embryonic lethality.[1]
The heart's septa typically form between the 27th and 37th days of development via fusion of tissue masses. These tissue masses are known as the endocardial cushions, and they contribute to the formation of the atrial/ventricular septa, the AV canals and valves, and the aortic/pulmonary channels. By the end of the 4th developmental week, the common atrium's roof develops a crest-like structure known as the septum primum, which is sickle-shaped. The two inferior limbs of the septum primum migrate toward the endocardial cushion. As the septum primum and the endocardial cushions do not fully fuse initially, there remains an opening called the ostium primum.
The endocardial cushions eventually fuse with the septum primum. Physiologic apoptosis produces perforations in the septum primum, which ultimately coalesce to form a structure called the ostium secundum. The ostium secundum allows for blood from the right primitive atrium to the left primitive atrium. Eventually, expansion of the right atrium occurs, during which a new fold develops in the right atrium. This fold of tissue is called the septum secundum, and it never completely partitions the atrium. The anterior aspect of the septum secundum extends inferiorly towards the fused endocardial cushions.
The septum secundum forms a crescent-shaped configuration that incompletely overlaps the ostium secundum. The remaining opening is the foramen ovale, which is one of two fetal structures responsible for directing blood flow from the developing lungs (the other structure is the ductus arteriosus). After overlapping of the septum secundum over the ostium secundum, the septum primum gradually disappears, its remnant forming the valve of the foramen ovale. After birth, the increased oxygen tension taken from the newborn's first breath allows for increased blood into the lungs. The increased blood flow going into the lungs increases the left interatrial pressure, allowing for blood to close the valve of the foramen ovale against the septum secundum.
In the adult heart, four pulmonary veins feed into the left atrium. In the embryo, there is initially a single pulmonary vein next to the posterior left of the septum primum. With the lung buds' co-development, the pulmonary vein and its branches become a part of the left atrium. This location is the smooth-walled portion of the left atrium (contrasting with the trabeculated left atrial appendage). The adult's right atrium also divides into a trabeculated right atrial appendage and a smooth-walled sinus venarum (originating from the right horn of the sinus venosus).
By the end of the 4th week of gestation, the atrioventricular canal develops two atrioventricular cushions along the superior and inferior borders and two lateral cushions. The superior and inferior endocardial cushions eventually project into the lumen and eventually fuse, although the lateral cushions do not participate in this process. The result is the atrioventricular canal division into two distinct orifices (the left atrioventricular canal and the right atrioventricular canal). Mesenchymal tissue surrounds the peripheral edges of each atrioventricular canal. This mesenchymal tissue eventually thins to form atrioventricular valves. The valves themselves connect to thick papillary muscles via the chordae tendinae. The trabecular meshwork is an essential morphological development.
During the 5th week of development, swellings appear in the truncus. The right superior truncus swelling migrates towards the left, while the left inferior truncus swelling moves towards the right. The truncus swellings eventually rotate around each other to fuse, forming the aorticopulmonary septum. The septum formation divides the truncus into two channels: the aortic channel and the pulmonary channel. As the truncus swellings begin to form, the walls of the conus cordis also start to develop structures called the conus swellings. The conus swellings grow toward one another to eventually fuse to form the primitive outflow tracts. The septum divides the conus cordis into two portions: an anterolateral and a posteromedial portion with the anterolateral portion becoming the outflow tract of the right ventricle and the posteromedial portion becoming the outflow tract of the left ventricle. The semilunar valves' development begins near the completion of the truncus partitioning, starting with primordial semilunar valves located on the truncal swellings. These primordial semilunar valves start as tubercles on the main truncus swellings and eventually thin to become the semilunar valves.
Septum formation of the ventricles has a somewhat different approach from the developmental standpoint, as opposed to the atria. The ventricle walls start to expand by approximately the 4th week, with gradual apposition and merger of the medial ventricular walls, forming a muscular interventricular septum. The membranous part of the interventricular septum forms from the complete closure of the interventricular foramen, which closes from tissue growth from the endocardial cushions. The pacemaker of the primitive heart tube is located in the caudal portion. Later in development, the sinus venosus (a location in the mammalian embryological heart between the two venae) assumes the pacemaker's role of the embryonic heart. Its incorporation into the right atrium serves as the origin of the sinoatrial node.
Studies determined that the sinoatrial node, atrioventricular node, and proximal bundle branches originate from separate lineages early in embryonic development in mouse subjects.[2] Although numerous genetic factors bear on the heart's structural development, several factors play a role in the development of the conduction system itself. In particular, the Shox2 factors have been speculated to lead to abnormal cardiac phenotypes, particularly congenital bradycardic arrhythmia.[3]
Cellular
Cardiac cells will assume their function depending on the position they will have. The cells that make up the cardiac layers (endocardium and the pericardium) are in the same area of the cells that will constitute the myocardium.
Molecular Level
Cardiac development is a highly regulated process, and several molecular factors are involved in the process. Perhaps the most documented of these is the NOTCH signaling pathway. Mutations in the NOTCH signaling pathway result in congenital cardiac abnormalities in both humans and mice. The primary purpose of NOTCH signaling is to regulate tissue patterning via local cell interactions.[4] The NOTCH signaling pathway's two major ligands include Delta and Jagged, each having their respective ligand subtypes (Dll 1, Dll 3, Dll 4, and Jag1, Jag2).[5]
The establishment of laterality is an essential aspect of cardiac development, facilitated by cilia configuration within the primitive node. Ciliary structures found in the primitive node have a unique design that allows for overall leftward migration with the failure of such a process, leading to dextrocardia conditions. The motile cilia rotate in a clockwise fashion to generate this movement. The determination of the left-right cardiac axis is via several genes typically induced by NODAL expression. In turn, the NODAL expression induces expression of left-right determination factor (LEFTY) gene expression and paired-like homeodomain transcription factor 2 (PITX2).[6] The PITX2 gene is considered the primary gene responsible for the determination of left-right orientation. The PITX2 gene has several roles in cardiac development. In addition to establishing left-right orientation, PITX2 gene mutations have associations with isolated cardiac congenital heart disease, including atrial septal defect, ventricular septal defect, and tetralogy of Fallot.[7] Even aside from mutations, some studies have emphasized the global effects of regulatory gene knockout on embryonic lethalities, such as the demonstrated importance of VangI2 on cardiac outflow tract development and Noggin on contractility.[8]
Mechanism
The cardiovascular system is the initial system to develop due to the growing embryo’s growing metabolic demands. Compared to the adult cardiovascular system, one notes several distinctions, particularly that of a well developed pulmonary system in the adult. In an amniotic fluid environment, the fetus would not need a developed pulmonary system (as the placenta serves as a means of oxygen exchange). Several cardiovascular structures (such as the foramen ovale and the ductus arteriosus) minimize blood flow to the fetal lungs by directing blood into the systemic circulation and away from the lungs. The minimal blood flow to the developing pulmonary system prevents the premature development of the lungs.
The ductus arteriosus is a small vessel connecting the pulmonary artery and the aorta. It allows for blood flow from the right ventricle into the systemic circulatory system, bypassing the nonfunctional fetal lungs. Prostaglandins maintain its patency, and it closes after birth. The vestigial remnant of this structure is the ligamentum arteriosum. Premature closure of the ductus arteriosus is usually due to pharmacological agents that block prostaglandin synthesis (i.e., NSAIDs).
Birth presents an interesting change in the cardiovascular dynamics of the fetus. The fetus is no longer in an amniotic environment and now requires functioning lungs to meet respiratory needs — the first breath of oxygen that a newborn takes increases the inspired oxygen tension, facilitating blood flow to the lungs. The increased blood flow to the lungs leads to increased blood flow to the left atrium, closing the foramen ovale.
Pathophysiology
zA significant majority of congenital heart defects occur due to genetic mutations that interfere with cardiac development. Other conditions are also associated with abnormalities in the various developmental stages, often due to the influence of teratogens such as alcohol, rubella, etc. Chromosomal lesions (as those found in DiGeorge syndrome or Holt-Oram syndrome) are also noted to contribute to congenital heart disease development. The number one genetic cause of congenital heart disease is trisomy 21, which often manifests itself as endocardial cushion defects.The significant features of congenital heart disease can follow one or more elements of the following classifications: malformations causing shunting (right-to-left or left-to-right) or malformations causing an obstruction. Shunts are abnormal communications between systemic circulation (the left heart) and the pulmonary circulation (the right side).
The term shunt refers to abnormal communication between vascular structures, and these shunts permit non-physiologic blood flow along pressure gradients. Blood flowing from the right side of the heart to the left side is termed a “right-to-left” shunt, while blood flowing from the left side of the heart to the right side is a “left-to-right” shunt. Right-to-left shunt implies the circulation of deoxygenated blood (i.e., blood that has yet to reach the pulmonary system) to the systemic circulation. Patients with a right to left shunts present with cyanosis and at increased risk for paradoxical embolism and the associated complications like infarction and abscess formation. In contrast, left-to-right shunts do not present with cyanosis since the systemic circulation still receives oxygenated blood. However, the increase in pulmonary blood load from the left-to-right shunt leads to profound pathological responses, partly due to the pulmonary system being a “low-pressure” system incapable of withstanding the increased pressure.The pulmonary arteries typically respond to the increased blood flow and pressure via hypertrophy and vasoconstriction. Eventually, pulmonary vascular resistance approaches systemic levels, creating a shunt reversal (now right-to-left) to distribute deoxygenated blood into the systemic system. The name given this condition of shunt reversal is Eisenmenger syndrome. Once pulmonary hypertension develops, the congenital heart condition progresses beyond irreparability.
The most commonly associated left-to-right shunts include atrial septal defect, ventricular septal defect, patent ductus arteriosus, and atrioventricular septal defects. Common right-to-left shunts include Fallot’s tetralogy, transposition of the great arteries, persistent truncus arteriosus, total anomalous pulmonary venous connection, and tricuspid atresia.Calreticulin is a Ca (2 +) - binding chaperone and is found in the endoplasmic reticulum. Studies reveal that it is a fundamental substance in the complex process of cardiac development. An alteration of the calreticulin function will cause cardiac disorders such as hypertrophy and dilated cardiomyopathy.
Clinical Significance
Understanding the embryological cardiac development is necessary to understand the pathophysiology of congenital heart disease. The medical community’s knowledge of cardiovascular embryology and congenital heart disease has expanded tremendously. An understanding of the genetic influences in addition to teratogenic effects at various developmental stages has led to a more profound view of congenital heart disease management. Embryological development of the heart is highly regulated but still influenced by genetic as well as environmental influences.
Addressing parents whose child may have a congenital heart condition requires a great deal of sensitivity and empathy. “Congenital heart disease” or “heart defect” are words that frighten most parents and rightfully so. While it is true that some congenital heart defects are incompatible with life, a significant portion of congenital heart disease patients can grow into adulthood and live fulfilling lives with proper monitoring and management.
Media
(Click Image to Enlarge)
References
Wu M. Mechanisms of Trabecular Formation and Specification During Cardiogenesis. Pediatric cardiology. 2018 Aug:39(6):1082-1089. doi: 10.1007/s00246-018-1868-x. Epub 2018 Mar 28 [PubMed PMID: 29594501]
Mohan RA, Boukens BJ, Christoffels VM. Developmental Origin of the Cardiac Conduction System: Insight from Lineage Tracing. Pediatric cardiology. 2018 Aug:39(6):1107-1114. doi: 10.1007/s00246-018-1906-8. Epub 2018 May 17 [PubMed PMID: 29774393]
Hu W, Xin Y, Zhao Y, Hu J. Shox2: The Role in Differentiation and Development of Cardiac Conduction System. The Tohoku journal of experimental medicine. 2018 Mar:244(3):177-186. doi: 10.1620/tjem.244.177. Epub [PubMed PMID: 29503396]
MacGrogan D, Münch J, de la Pompa JL. Notch and interacting signalling pathways in cardiac development, disease, and regeneration. Nature reviews. Cardiology. 2018 Nov:15(11):685-704. doi: 10.1038/s41569-018-0100-2. Epub [PubMed PMID: 30287945]
Nakanishi T, Markwald RR, Baldwin HS, Keller BB, Srivastava D, Yamagishi H, Luxán G, D’Amato G, de la Pompa JL. Intercellular Signaling in Cardiac Development and Disease: The NOTCH pathway. Etiology and Morphogenesis of Congenital Heart Disease: From Gene Function and Cellular Interaction to Morphology. 2016:(): [PubMed PMID: 29787142]
Kloesel B, DiNardo JA, Body SC. Cardiac Embryology and Molecular Mechanisms of Congenital Heart Disease: A Primer for Anesthesiologists. Anesthesia and analgesia. 2016 Sep:123(3):551-69. doi: 10.1213/ANE.0000000000001451. Epub [PubMed PMID: 27541719]
Franco D, Sedmera D, Lozano-Velasco E. Multiple Roles of Pitx2 in Cardiac Development and Disease. Journal of cardiovascular development and disease. 2017 Oct 11:4(4):. doi: 10.3390/jcdd4040016. Epub 2017 Oct 11 [PubMed PMID: 29367545]
Dees E, Baldwin HS. Making a heart: advances in understanding the mechanisms of cardiac development. Current opinion in pediatrics. 2016 Oct:28(5):584-9. doi: 10.1097/MOP.0000000000000401. Epub [PubMed PMID: 27428484]
Level 3 (low-level) evidence