Introduction
Capillary hemangiomas of infancy are the most common benign orbital neoplasms in children.[1] Historically, they have been referred to by many names such as infantile hemangiomas, juvenile hemangiomas, hemangioblastomas, or strawberry nevi. Currently, the universal vocabulary of capillary hemangioma is followed.
Initially, these vascular anomalies were classified as angiomas by John Mulliken and Julie Glowacki, as early as 1982: they were thought to be proliferating lesions with an independent life cycle. However, these classifications have undergone several revisions. Capillary hemangiomas have been reclassified as benign vascular neoplasms as per the revised International Society for the study of vascular anomalies (ISSVA). According to this classification, hemangiomas are benign neoplasms which are true tumors that arise de novo and undergo clonal proliferation and growth out of proportion to the patient. These lesions have a predictable life cycle and most often do not require any treatment in the absence of complications. This is in contrast to the other category of vascular malformations which often present at birth, undergo slow growth, and persist into adult life.[2]
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Hereditary factors have not been shown to play any role in developing these neoplasms, but several reports have found them to be uncommon in African races. Factors such as prematurity and low birth weight are associated with a higher risk of developing these neoplasms after birth.[3]
Epidemiology
Capillary hemangiomas constitute 8-10% of benign tumors in the pediatric age group, 80% of which occur in the head and neck region.[1] They are more common in females with a female to male ratio of 3:2 to 5:1.[4]
Pathophysiology
The natural course of capillary hemangiomas was described as early as 1900. These tumors are generally not present at birth but first appear within the first few weeks after birth. They appear as small flat plaques of telangiectatic vessels. They undergo rapid proliferation (proliferative phase) between 3-12 months of age. During this phase, the endothelial cells undergo rapid proliferation causing new vessel growth, and the lesions become nodular with a scarlet-colored hue: thus the name ‘strawberry nevus.’ This phase is followed by spontaneous involution of the lesions (involutional phase) usually from about three years of age. Endothelial proliferation comes to a halt, and the lesions are replaced by fibrous tissue.[4]
Histopathology
The tumor consists of anastomosing vascular channels lined by plump endothelial cells and pericytes during the proliferative phase. As the lesion progresses to the involutional stage, the plump endothelial cells become flattened, and there is increased deposition of fibrous tissue between the vascular channels.[4]
History and Physical
Clinical Features
Presenting features depend on the location of the lesion. Hemangiomas are classified into the following types: [5]
- Superficial type
- Deep type
- Mixed type
Superficial Lesions
They appear as bright red, nodular masses typically involving the eyelids. Lesions below the dermis tend to have a deep blue to purple hue. These lesions can present as skin discoloration, a cosmetic blemish, or an eyelid mass. Capillary hemangiomas may cause mechanical ptosis obscuring the visual axis and producing astigmatism and amblyopia.
Deep Lesions
Deep orbital lesions are invisible to the naked eye. They present with gradually progressive proptosis, strabismus, or decreased visual acuity due to optic nerve compression.
Mixed Lesions
It consists of both superficial and deep components.
A characteristic clinical feature of capillary hemangiomas is an increase in size or change in color to dark blue when the child cries or strains which are often noted by the parents. This is due to the increased accumulation of deoxygenated blood. This situation, however, is not pathognomonic of capillary hemangiomas and can present in other vascular anomalies.
Systemic Associations
Cutaneous hemangiomas are sometimes associated with visceral lesions. The presence of four or more superficial lesions should raise suspicion of visceral hemangiomas. The most common site of visceral involvement is the liver.
Kasabach – Merritt Syndrome[6]
Large hemangiomas can result in entrapment and consumption of platelets and other clotting factors resulting in life-threatening hemorrhagic thrombocytopenia.
PHACES Syndrome[7]
First described in 1996, children presenting with large hemangiomas of the face, neck, and scalp can have associated defects involving the brain, blood vessels, eyes, heart, and chest. It is seen more commonly in females. The syndrome name is an acronym of the different anomalies it compromises. (PHACES – Posterior fossa anomalies, Hemangiomas, Arterial anomalies, Cardiac anomalies, and Eye anomalies)
Evaluation
Imaging Features[4]
Ultrasound: Capillary hemangiomas appear as irregular lesions in orbit, demonstrating high internal reflectivity with irregular acoustic structures. A scan shows low to medium reflectivity with high spikes produced by the septae. Increased flow within a lesion can be demonstrated by Doppler echography.
Computed tomography (CT) scan: Capillary hemangiomas appear as well-defined to irregular pre-septal or post-septal, intraconal, or extraconal-heterogenous soft tissue masses, which enhance with contrast. There is no evidence of calcification or bony erosion. They may appear well-defined or ill-defined.
Magnetic resonance imaging (MRI): they appear as well-defined or ill-defined lesions that are hypointense on T1 weighted images and hyperintense on T2 weighted images. The characteristic feature is the presence of flow voids within the lesion. They demonstrate diffuse enhancement with Gadolinium contrast which is best appreciated in fat-suppressed images.
Treatment / Management
Capillary hemangiomas are most often asymptomatic thus require no intervention. Around 10% of hemangiomas can present with astigmatism, visually obscuring ptosis, amblyopia, ulceration or bleeding, significant proptosis causing optic nerve compression or exposure keratopathy: these cases which require management.[8](A1)
Beta-Blockers
Topical or systemic beta; blockers are currently the mainstay of management for capillary hemangiomas.
Following their accidental discovery by Leaute – Labreze and colleagues in 2008, who noticed a regression in cutaneous hemangiomas in children treated with beta; blockers for cardiac and renal problems, propranolol has replaced oral steroids as the treatment modality of choice.[9](B3)
beta; blockers suppress vascular endothelial growth factors (VEGF) and fibroblast growth factors (FGF) which are responsible for proliferation. They also cause down-regulation of cyclic – AMP needed for cell signaling and induce apoptosis of proliferating cells.[10]
Dosage
Oral Propranolol – It is started as a low dosage of 0.16 mg/kg and gradually increased in the absence of complications to 2 mg/kg/day in three divided doses. [10]
Topical Timolol – It is useful for superficial lesions and applied as 0.5% gel preparation twice daily until the lesions regress.
Reduction in size and change in color can be noticed as early as 1-week after commencing treatment. Treatment should continue throughout the proliferative phase (up to 12 months of age) and gradually tapered and stopped to avoid a rebound increase in size.
Adverse effects: Potential side effects include hypotension, bradycardia, hypoglycemia, bronchospasm, sleep disturbances, diarrhea, and hyperkalemia. These can be overcome by close monitoring at the time of initial administration and proper parental counseling.
Corticosteroids
Before the advent of beta; blockers, corticosteroids were administered orally or intralesionally. This was the treatment of choice for capillary hemangiomas. [11] Steroids are effective only during the early proliferative phase and are associated with significant side effects. Complications such as weight gain, cushingoid features, adrenal suppression, hypertension, localized hypopigmentation of skin, fat atrophy, and central retinal artery occlusion have been reported, thus making this modality a less preferred choice. Steroid injections into lesions that are being removed surgically are still used.
Agents such as interferon – α, vincristine, cyclophosphamide have been tried in steroid-resistant cases. However, these agents are associated with complications such as bone marrow suppression and hepatotoxicity.
Lasers
Superficial hemangiomas can be treated with pulsed–dye lasers, which diminish the size and lighten the color of the lesions.
Surgery
Surgical debulking of the lesion is reserved only for large vision-threatening lesions. As these lesions are irregular and unencapsulated, a complete removal is not possible. However, with careful surgical dissection, the majority of large orbital and eyelid hemangiomas can be successfully treated. Concurrent steroids are injected into any residual hemangioma tissue during surgery.
Differential Diagnosis
Based on the location and size of the lesion they may be mistaken for other benign conditions such as lymphangioma, arterio-venous malformations, mucocele or meningocele.
Lesions presenting with rapid onset proptosis can mimick rhabdomyosarcoma.
Prognosis
The overall prognosis for capillary hemangioma of infancy is excellent as most often, these lesions involute spontaneously. Large lesions respond very well to oral propranolol, which results in complete to the near-complete resolution of the lesions.[10]
Complications
Although most capillary hemangiomas involute over time, some will undergo rapid growth and can cause amblyopia, proptosis with exposure keratitis, or optic nerve compression. Ulceration and bleeding may occur but are infrequently seen.
Complications of oral steroids include hypotension, bradycardia, hypoglycemia, bronchospasm, sleep disturbances, diarrhea, and hyperkalemia. These can be overcome by close monitoring at the time of initial administration and proper parental counseling.
Complications of surgical resection include injury to the surrounding tissues like the levator aponeurosis and levator muscle. Orbital capillary hemangioma resection will often result in a residual tumor in the orbit because these lesions are irregular with no capsulation.
Deterrence and Patient Education
It is important to remind parents of the natural history of capillary hemangiomas. With modern treatments using oral propranolol, most hemangiomas that need treatment will respond, although the procedure may need to be continued for a prolonged period.
Enhancing Healthcare Team Outcomes
Capillary hemangiomas are common benign lesions, and parents can present to either pediatricians, ophthalmologists, oculoplastic surgeons, or dermatologists with complaints of reddish discoloration of the skin or as an eyelid mass. Health care physicians should be aware of this common clinical entity and its features which enable one to distinguish it from other more soft severe tissue lesions presenting during childhood. Early treatment with oral propranolol in coordination with a pediatrician shows excellent results. Careful attention should be paid to the assessment of refraction and vision to detect astigmatism, refractive error, or amblyopia. In the presence of proptosis due to an intraorbital hemangioma, sequential imaging may be necessary. The cornea should be carefully examined for any evidence of exposure keratopathy.
Media
(Click Image to Enlarge)
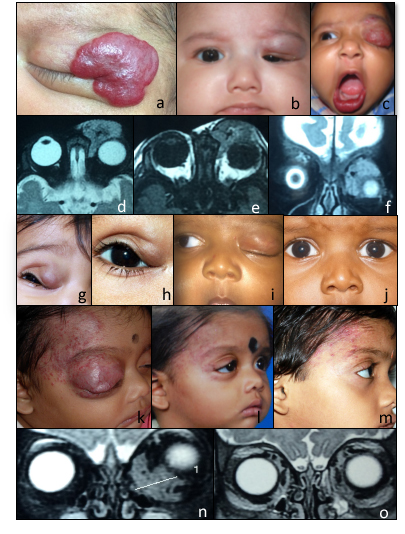
a. Clinical picture showing large superficial hemangioma. b,c. Partial and complete obscuration of visual axis due to hemangioma of the upper lid. d,e,f. MRI scan axial and coronal view showing homogenous solid pre and post septal soft tissue lesion which is isointense on T1W1 and hyperintense on T2W2 with characterictic flow voids within. g,h,i,j. Clinical pictures showing partial and complete resolution of upper lid hemangioma with oral propranolol therapy. k,l,m. Clinical picture showing CHI at presentation and significant reduction in size and colour of lesion at 3 months and 12 months post treatment with oral propranolol. n,o. Pre and post treatment MRI scan showing complete resolution of lesion located along the medial and inferior extraconal space with oral propranolol. Contributed by Professor Bhupendra C. K. Patel MD, FRCS and Dr. Kirti Koka MD
References
Spiteri Cornish K, Reddy AR. The use of propranolol in the management of periocular capillary haemangioma--a systematic review. Eye (London, England). 2011 Oct:25(10):1277-83. doi: 10.1038/eye.2011.164. Epub 2011 Jul 8 [PubMed PMID: 21738233]
Level 1 (high-level) evidenceMulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plastic and reconstructive surgery. 1982 Mar:69(3):412-22 [PubMed PMID: 7063565]
Pandey V, Tiwari P, Gangopadhyay AN, Gupta DK, Sharma SP, Kumar V. Propranolol for infantile haemangiomas: experience from a tertiary center. Journal of cutaneous and aesthetic surgery. 2014 Jan:7(1):37-41. doi: 10.4103/0974-2077.129975. Epub [PubMed PMID: 24761098]
Haik BG, Karcioglu ZA, Gordon RA, Pechous BP. Capillary hemangioma (infantile periocular hemangioma). Survey of ophthalmology. 1994 Mar-Apr:38(5):399-426 [PubMed PMID: 8009426]
Level 3 (low-level) evidenceStass-Isern M. Periorbital and orbital infantile hemangiomas. International ophthalmology clinics. 2014 Summer:54(3):73-82. doi: 10.1097/IIO.0000000000000039. Epub [PubMed PMID: 24879105]
Osman NM. Kasabach - Merritt syndrome: A case report. Sudanese journal of paediatrics. 2013:13(1):49-52 [PubMed PMID: 27493358]
Level 3 (low-level) evidenceFrieden IJ, Reese V, Cohen D. PHACE syndrome. The association of posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities. Archives of dermatology. 1996 Mar:132(3):307-11 [PubMed PMID: 8607636]
Level 3 (low-level) evidenceBuckmiller LM, Munson PD, Dyamenahalli U, Dai Y, Richter GT. Propranolol for infantile hemangiomas: early experience at a tertiary vascular anomalies center. The Laryngoscope. 2010 Apr:120(4):676-81. doi: 10.1002/lary.20807. Epub [PubMed PMID: 20112413]
Level 1 (high-level) evidenceLéauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. The New England journal of medicine. 2008 Jun 12:358(24):2649-51. doi: 10.1056/NEJMc0708819. Epub [PubMed PMID: 18550886]
Level 3 (low-level) evidenceKoka K, Mukherjee B, Agarkar S. Effect of oral propranolol on periocular Capillary Hemangiomas of Infancy. Pediatrics and neonatology. 2018 Aug:59(4):390-396. doi: 10.1016/j.pedneo.2017.11.021. Epub 2017 Dec 6 [PubMed PMID: 29301720]
Aletaha M, Salour H, Bagheri A, Raffati N, Amouhashemi N. Oral propranolol for treatment of pediatric capillary hemangiomas. Journal of ophthalmic & vision research. 2012 Apr:7(2):130-3 [PubMed PMID: 23275821]
Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics. 2011 Aug:128(2):e259-66. doi: 10.1542/peds.2010-0029. Epub 2011 Jul 25 [PubMed PMID: 21788220]
Level 1 (high-level) evidence