Introduction
The spontaneous respiratory drive is among the most vital evolutionary qualities in mammalian existence, and the neurocircuitry and physiology behind the human respiratory drive are complex. While the motivation to inspire oxygen is certainly evident, there is also the consideration that the over-expansion of the lungs via inspiratory drive can be damaging, and therefore requires monitoring and inhibition. Among these interactions, the most widely recognized description of such reflexive respiratory control was first conceptualized by Ewald Hering, and Joseph Breuer in 1868, who illustrated that sustained lung inflation triggers reflexive expiration to prevent over-inflation related lung injury. Since its discovery, the Hering-Breuer reflex (HBR) has been thoroughly investigated within the realm of physiology and pathology and is consistently identical across other mammalian species.[1] As such, it is an essential mechanism for respiratory control and protection in animals, enabling volume-dependent regulation.[2][3] Interestingly, initially, this reflex was not believed to influence breathing at eupneic tidal volumes in adult humans.[4] However, data has since demonstrated that this reflex can be identified in adults, most easily with the cessation of chest wall movement.[5][6] Also, although this reflex is present and (in select circumstances detectable) in adult humans, under ordinary circumstances, the volume necessary to activate the HBR exceeds physiologic tidal volumes and is therefore considered undetectable.[4][7] One interesting caveat is the consideration by which temperature influences the relationship between lung volume and duration of inspiration, which is still observable among adults. With increasing temperature, the threshold (maximum volume) for inspiratory discontinuation is achieved sooner, thereby affecting the tidal volume and respiratory tone.[8]
Although adults demonstrated reduced ability to illustrate the Hering Breuer reflex, it is much more prominent among preterm infants and newborns. Furthermore, the HBR may play a vital function, as there are suggestions that it plays a role in controlling lung volume at an early age.[9] Additionally, abnormal development of these vagal pathways may contribute towards several breathing disorders in infancy (see Section: Clinical Significance).
Herein, we discuss the physiologic mechanism behind the Herin-Breuer reflex, the neural circuitry involved, its role in human development, and the clinical and pathologic sequelae associated with malfunctioning with this system.
Development
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Development
Since its discovery, the HBR is considered major regulatory feedback for stimulating and maintaining rhythmic respiratory activity. As such, the HBR is an important respiratory control mechanism, particularly in newborns.[10] Among infants, the reflex is particularly prominent and can be evoked by briefly occluding the airway at the end of inspiration.[11][12] The HBR has been shown to decrease in prominence with age, and can only be evoked at lung volumes well above the tidal range. In fact, the HBR does not influence the respiratory pattern until attaining a high lung volume (and is thereby unattainable with resting breathing.[13][14][15] This diminished influence of the HBR on respiratory patterns appears to be related to postnatal HBR habituation.[16]
Despite this physiologic change and reduced need for the HBR to establish a respiratory rhythm, the HBR does still maintain important functionality among juvenile and adult animals. Primarily, sensory feedback regarding the tidal volume and lung expansion appear to be responsible for altering breathing patterns secondary to behavioral and emotional stressors (such as prolonged vocalization or abrupt physical exercise).
Function
When considering mammalian survival and evolution, the motivation to inspire is paramount; however, one must consider that too much inspiration can also be harmful. Therefore, this balance requires close monitoring. The Hering-Breuer reflex fulfills this function. This function is particularly useful in adults. Also, infants and neonates possess a separate necessity for the Hering Breuer reflex in the form of maintaining a rhythmic breathing pattern.[17] (Discussed in more detail in the clinical significance section)
Mechanism
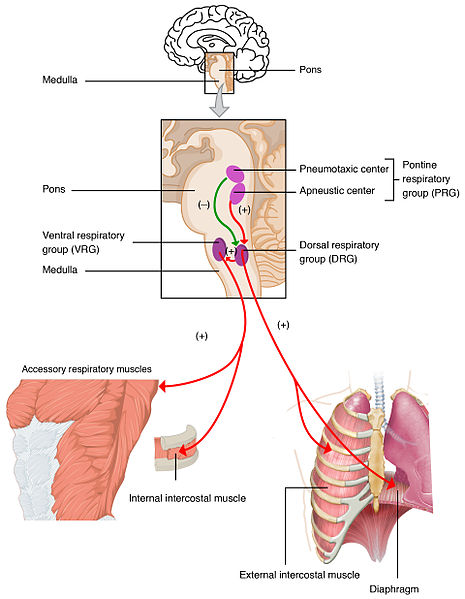
The neurologic mechanism behind the Hering Breuer reflex is complex and is a prime example of an inhibitory feedback loop. In human adults, the reflex begins with prolonged inspiration well exceeding eupneic tidal volume. This thoracic expansion subsequently and gradually activates, slowly adapting pulmonary stretch receptors. These receptors relay a signal through the vagus nerve (on a breath-by-breath basis) to "pump" cells" located within the ventrolateral nucleus of the solitary tract.[1][4][6][18][19][20][21] These pump cells receive these vagal inputs and project the information to the medullary post-inspiratory neurons.[22] These neurons subsequently project inhibitory signals back to the inspiratory neurons along the lateral portion of the respiratory column, thereby terminating inspiration and beginning a prolonged expiration.[23][24][25][26]
Related Testing
Despite the clinical significance behind the Hering Breuer reflex (especially with regards to obstructive sleep apnea and traumatic related vagotomy), there is limited research to date regarding possible diagnostic testing to assess its viability in vivo.
Clinical Significance
Several conditions and syndromes hold relevance to the Hering Breuer reflex. For one, understanding the neural circuitry and interplay among airway mechanoreceptor reflexes have been extensively studied in the past due to their clinical relevance in maintaining airway protection and upper airway motor tone. One potential clinical circumstance includes sleep and the possible dysregulation in patients who suffer from obstructive sleep apnea.[27]
Second, as discussed previously, the HBR loop maintains breathing pattern stabilization in neonates and even in preterm infants.[17] As infants mature, gradual habituation occurs, and the threshold for HBR stimulation increases. In adults, the HBR threshold is well above tidal volume, thus reducing the global influence of the HBR. In certain instances, attenuating the habituation processing (as well as desensitization of pontine input) of the HBR is evident, and may be protective.[28] For example, in the case of Rett syndrome, animal models of mice with MECP2 deficiency lead to exaggerated post-inspiratory activation within the vagus nerve, which can contribute to lung over-inflation.[29] It is thereby plausible that given the lack of HBR habituation in such instances may contribute to breathing pattern instability due to competing for sensory and central neural signaling.[16]
Another clinical circumstance with regards to the HBR is removing feedback altogether (via reduced vagal nerve stimulation or vagotomy). Research has shown such an occurrence to reduce the respiratory rhythm while increasing the inspiratory amplitude and prolonging the duration of expiration.[4] Such an event is not exclusive to the case of bilateral denervation, as in the case of a unilateral vagotomy. Here, both lungs respond by altering respiratory patterns to reflect a slowing and deepening of respiratory movement.[30]
Media
(Click Image to Enlarge)
References
Miyazaki M,Arata A,Tanaka I,Ezure K, Activity of rat pump neurons is modulated with central respiratory rhythm. Neuroscience letters. 1998 Jun 12; [PubMed PMID: 9672389]
Level 3 (low-level) evidenceWIDDICOMBE JG. Respiratory reflexes in man and other mammalian species. Clinical science. 1961 Oct:21():163-70 [PubMed PMID: 14006725]
Level 3 (low-level) evidenceGrunstein MM, Younes M, Milic-Emili J. Control of tidal volume and respiratory frequency in anesthetized cats. Journal of applied physiology. 1973 Oct:35(4):463-76 [PubMed PMID: 4743002]
Level 3 (low-level) evidenceClark FJ, von Euler C. On the regulation of depth and rate of breathing. The Journal of physiology. 1972 Apr:222(2):267-95 [PubMed PMID: 5033464]
Level 3 (low-level) evidenceBuSha BF, Judd BG, Manning HL, Simon PM, Searle BC, Daubenspeck JA, Leiter JC. Identification of respiratory vagal feedback in awake normal subjects using pseudorandom unloading. Journal of applied physiology (Bethesda, Md. : 1985). 2001 Jun:90(6):2330-40 [PubMed PMID: 11356800]
BuSha BF, Stella MH, Manning HL, Leiter JC. Termination of inspiration by phase-dependent respiratory vagal feedback in awake normal humans. Journal of applied physiology (Bethesda, Md. : 1985). 2002 Sep:93(3):903-10 [PubMed PMID: 12183484]
Hamilton RD,Winning AJ,Horner RL,Guz A, The effect of lung inflation on breathing in man during wakefulness and sleep. Respiration physiology. 1988 Aug; [PubMed PMID: 3420318]
Bradley GW, von Euler C, Marttila I, Roos B. Steady state effects of CO-2 and temperature on the relationship between lung volume and inspiratory duration (Hering-Breuer threshold curve). Acta physiologica Scandinavica. 1974 Nov:92(3):351-63 [PubMed PMID: 4454993]
Level 3 (low-level) evidenceTrippenbach T. Pulmonary reflexes and control of breathing during development. Biology of the neonate. 1994:65(3-4):205-10 [PubMed PMID: 8038284]
Level 3 (low-level) evidenceCROSS KW, KLAUS M, TOOLEY WH, WEISSER K. The response of the new-born baby to inflation of the lungs. The Journal of physiology. 1960 Jun:151(3):551-65 [PubMed PMID: 13813016]
Rabbette PS,Costeloe KL,Stocks J, Persistence of the Hering-Breuer reflex beyond the neonatal period. Journal of applied physiology (Bethesda, Md. : 1985). 1991 Aug; [PubMed PMID: 1938718]
Rabbette PS, Fletcher ME, Dezateux CA, Soriano-Brucher H, Stocks J. Hering-Breuer reflex and respiratory system compliance in the first year of life: a longitudinal study. Journal of applied physiology (Bethesda, Md. : 1985). 1994 Feb:76(2):650-6 [PubMed PMID: 8175574]
Bechbache RR, Chow HH, Duffin J, Orsini EC. The effects of hypercapnia, hypoxia, exercise and anxiety on the pattern of breathing in man. The Journal of physiology. 1979 Aug:293():285-300 [PubMed PMID: 501598]
Cunningham DJ, Gardner WN. A quantitative description of the pattern of breathing during steady-state CO2 inhalation in man, with special emphasis on expiration. The Journal of physiology. 1977 Nov:272(3):613-32 [PubMed PMID: 592205]
Duffin J, Mohan RM, Vasiliou P, Stephenson R, Mahamed S. A model of the chemoreflex control of breathing in humans: model parameters measurement. Respiration physiology. 2000 Mar:120(1):13-26 [PubMed PMID: 10786641]
Dutschmann M, Bautista TG, Mörschel M, Dick TE. Learning to breathe: habituation of Hering-Breuer inflation reflex emerges with postnatal brainstem maturation. Respiratory physiology & neurobiology. 2014 May 1:195():44-9. doi: 10.1016/j.resp.2014.02.009. Epub 2014 Feb 22 [PubMed PMID: 24566392]
Level 3 (low-level) evidenceHand IL, Noble L, Wilks M, Towler E, Kim M, Yoon JJ. Hering-Breuer reflex and sleep state in the preterm infant. Pediatric pulmonology. 2004 Jan:37(1):61-4 [PubMed PMID: 14679491]
Berger AJ, Dick TE. Connectivity of slowly adapting pulmonary stretch receptors with dorsal medullary respiratory neurons. Journal of neurophysiology. 1987 Dec:58(6):1259-74 [PubMed PMID: 3437333]
Level 3 (low-level) evidenceBonham AC, McCrimmon DR. Neurones in a discrete region of the nucleus tractus solitarius are required for the Breuer-Hering reflex in rat. The Journal of physiology. 1990 Aug:427():261-80 [PubMed PMID: 2213599]
Level 3 (low-level) evidenceBonham AC, Coles SK, McCrimmon DR. Pulmonary stretch receptor afferents activate excitatory amino acid receptors in the nucleus tractus solitarii in rats. The Journal of physiology. 1993 May:464():725-45 [PubMed PMID: 8229827]
Level 3 (low-level) evidenceMiyazaki M, Tanaka I, Ezure K. Excitatory and inhibitory synaptic inputs shape the discharge pattern of pump neurons of the nucleus tractus solitarii in the rat. Experimental brain research. 1999 Nov:129(2):191-200 [PubMed PMID: 10591893]
Level 3 (low-level) evidenceBerger AJ. Dorsal respiratory group neurons in the medulla of cat: spinal projections, responses to lung inflation and superior laryngeal nerve stimulation. Brain research. 1977 Oct 28:135(2):231-54 [PubMed PMID: 922474]
Level 3 (low-level) evidenceEzure K, Tanaka I. GABA, in some cases together with glycine, is used as the inhibitory transmitter by pump cells in the Hering-Breuer reflex pathway of the rat. Neuroscience. 2004:127(2):409-17 [PubMed PMID: 15262331]
Level 3 (low-level) evidencevon Euler C. The contribution of sensory inputs to the pattern generation of breathing. Canadian journal of physiology and pharmacology. 1981 Jul:59(7):700-6 [PubMed PMID: 6274493]
Level 3 (low-level) evidenceBianchi AL, Denavit-Saubié M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiological reviews. 1995 Jan:75(1):1-45 [PubMed PMID: 7831394]
Level 3 (low-level) evidenceKubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. Journal of applied physiology (Bethesda, Md. : 1985). 2006 Aug:101(2):618-27 [PubMed PMID: 16645192]
Level 3 (low-level) evidenceWang J, Wang Y, Feng J, Chen BY, Cao J. Complex sleep apnea syndrome. Patient preference and adherence. 2013:7():633-41. doi: 10.2147/PPA.S46626. Epub 2013 Jul 3 [PubMed PMID: 23861580]
Siniaia MS, Young DL, Poon CS. Habituation and desensitization of the Hering-Breuer reflex in rat. The Journal of physiology. 2000 Mar 1:523 Pt 2(Pt 2):479-91 [PubMed PMID: 10699090]
Level 3 (low-level) evidenceDhingra RR, Zhu Y, Jacono FJ, Katz DM, Galán RF, Dick TE. Decreased Hering-Breuer input-output entrainment in a mouse model of Rett syndrome. Frontiers in neural circuits. 2013:7():42. doi: 10.3389/fncir.2013.00042. Epub 2013 Apr 3 [PubMed PMID: 23565077]
Level 3 (low-level) evidenceMoore RL. A STUDY OF THE HERING-BREUER REFLEX. The Journal of experimental medicine. 1927 Oct 31:46(5):819-37 [PubMed PMID: 19869374]