High-Grade Squamous Intraepithelial Lesion of the Cervix
High-Grade Squamous Intraepithelial Lesion of the Cervix
Introduction
High-grade squamous intraepithelial lesion (HSIL) is a squamous cell abnormality associated with human papillomavirus (HPV). It encompasses the previously used terms cervical intraepithelial neoplasia grades 2 and 3 (CIN 2 and CIN 3), moderate and severe dysplasia, and carcinoma in situ. In 1988 the Bethesda System for Reporting Cervical Cytology (TBS) introduced the current terminology for HSIL, which has since been adopted for histology specimens by the Lower Anogenital Squamous Terminology Standardization Consensus Conference (LAST) and the World Health Organization (WHO) in 2012 and 2014, respectively.[1] Though not all HSIL will progress to cancer, it is considered a precancerous lesion and therefore is usually treated aggressively. Though HSIL can involve various cutaneous and mucosal sites within the anogenital tract, this summary will focus on cervical HSIL.
Etiology
Register For Free And Read The Full Article
Search engine and full access to all medical articles
10 free questions in your specialty
Free CME/CE Activities
Free daily question in your email
Save favorite articles to your dashboard
Emails offering discounts
Learn more about a Subscription to StatPearls Point-of-Care
Etiology
Scientific studies have established HPV as the primary etiologic agent in the pathogenesis of cervical dysplasia and carcinoma. HPV is a non-enveloped double-stranded DNA virus within the Papillomaviridae family. There are over 150 genotypes of HPV, with 40 known to infect the anogenital tract. These 40 genotypes are divided into high-risk and low-risk groups based on evidence of their oncogenic potential. HPV 16 and HPV 18 are high-risk genotypes found in over 70% of HSILs and cervical squamous cell carcinomas. Contrary to low-grade squamous intraepithelial lesions (LSIL), which represent transient HPV infections that are cleared within 2 to 5 years and have a low risk of malignancy, HSILs are associated with persistent infection and a greater risk of progression to invasive cervical cancer, especially if the persistent infection is a high-risk genotype such as HPV 16 and/or HPV 18.[2]
Epidemiology
Since HSIL is caused by HPV infection, it is found more commonly in women with specific genetic and behavioral factors which increase their risk of acquiring HPV. HPV prevalence is highest in young, sexually active females, then progressively drops until menopause, with some studies showing a slight increase after menopause. This decline in middle age is thought to result from an effective immune response after exposure to HPV and the likelihood of less exposure to HPV. An immunocompromised state, such as after transplant therapy and in HIV-infected individuals, increases a patient’s risk of persistent HPV infection and the development of a squamous intraepithelial lesion (SIL).
Studies indicate a younger age at sexual debut and a higher number of sexual partners increase the risk of HPV infection as well as more recent sexual activity. Male partner promiscuity is also a factor. Condom use and circumcision can reduce the risk of HPV infection.[3] Studies have demonstrated no difference in the prevalence of HPV infection based on sexual orientation.[4] Multiparous women, with specifically >7 births, are also at increased risk.
There is a strong association between smoking and cervical neoplasia, independent of HPV status, presumably due to the presence of carcinogens within the cervical mucus. Certain HLA class II alleles and haplotypes, notably HLA DRB1*07 and HLA-DQB1*03, have a positive association with SILs and invasive cancer, with evidence suggesting that the haplotypes may influence HPV antigen presentation and immune response.[5][6] Other HLA class II haplotypes have been found to be protective. Oral contraceptive use may somewhat increase a patient’s risk of cervical neoplasia, but there is a minimal negative impact on the absolute risk.[7][8][9][10]
Pathophysiology
The genome of HPVs includes the early genes (E1, E2, E4, E5, E6, E7), involved in the regulation of the vegetative and proliferative phases of the viral life cycle, the late genes (L1, L2), which encode the capsid proteins, and a non-coding long control region (LCR) involved in viral replication and transcription regulation. HPV infects the basal epithelial cells of the transformation zone after gaining entry through microabrasions. A class of cell surface receptors on the keratinocytes called heparan sulfate proteoglycans (HSPG) are thought to be the initial receptors for the virus, attaching to L1 of the capsid and inducing conformational changes and subsequent cleavage of L2. The viral genome is slowly internalized over a 12-hour period through clathrin or caveolae-mediated endocytosis. Entry of the viral genome into the nucleus occurs through nuclear membrane breaks. Viral replication then begins. As the basal cells mature and reach the terminally differentiated layer of epithelium, expression of the L1 and L2 capsid proteins occurs to enable the assembly of viral particles, which are sloughed off along with the dead squamous cells, allowing continued transmission and infection of the virus. This is the typical life cycle of most HPV genotypes.[2][11]
High-risk HPV types, especially HPV 16, have often been shown to integrate their genome into the human genome. Integration has been proposed as an early event in the progression of LSIL to HSIL. It involves the viral oncoproteins E6 and E7 maintaining double-strand breakpoints in host DNA by attenuating the DNA-damage response (DDR) involved in repairing such breaks so that the viral genome can integrate at these breakpoints. The high-risk HPV E6 oncoprotein is known to interfere with p53, a cellular tumor-suppressor protein required for sensing base excision repair machinery and repairing oxidative damage, by inducing increased proteasome-dependent degradation of p53. High-risk HPV E7 oncoprotein inactivates members of the retinoblastoma (Rb) tumor suppressor protein family.
Retinoblastoma is coupled with an E2F transcription factor. Inactivation of Rb uncouples E2F, thus allowing it to upregulate genes required for S-phase entry and progression. The aforementioned E6 and E7 interactions are just glimpses of how these oncoproteins interact with various proteins in the nucleus. Essentially, overexpression of E6/E7 causes cell cycle dysregulation. Studies suggest integration also causes host gene expression changes, amplifying oncogenes and disrupting tumor suppressor genes. As the HPV genome is double-stranded and integrated into the host genome, it effectively evades the immune response leading to persistent infection. Epigenetic modification of various genes and changes in the expression of microRNAs have also been shown to play a role in carcinogenesis. Much remains to be elucidated regarding the molecular pathogenesis of cervical neoplasia.[2][11]
Histopathology
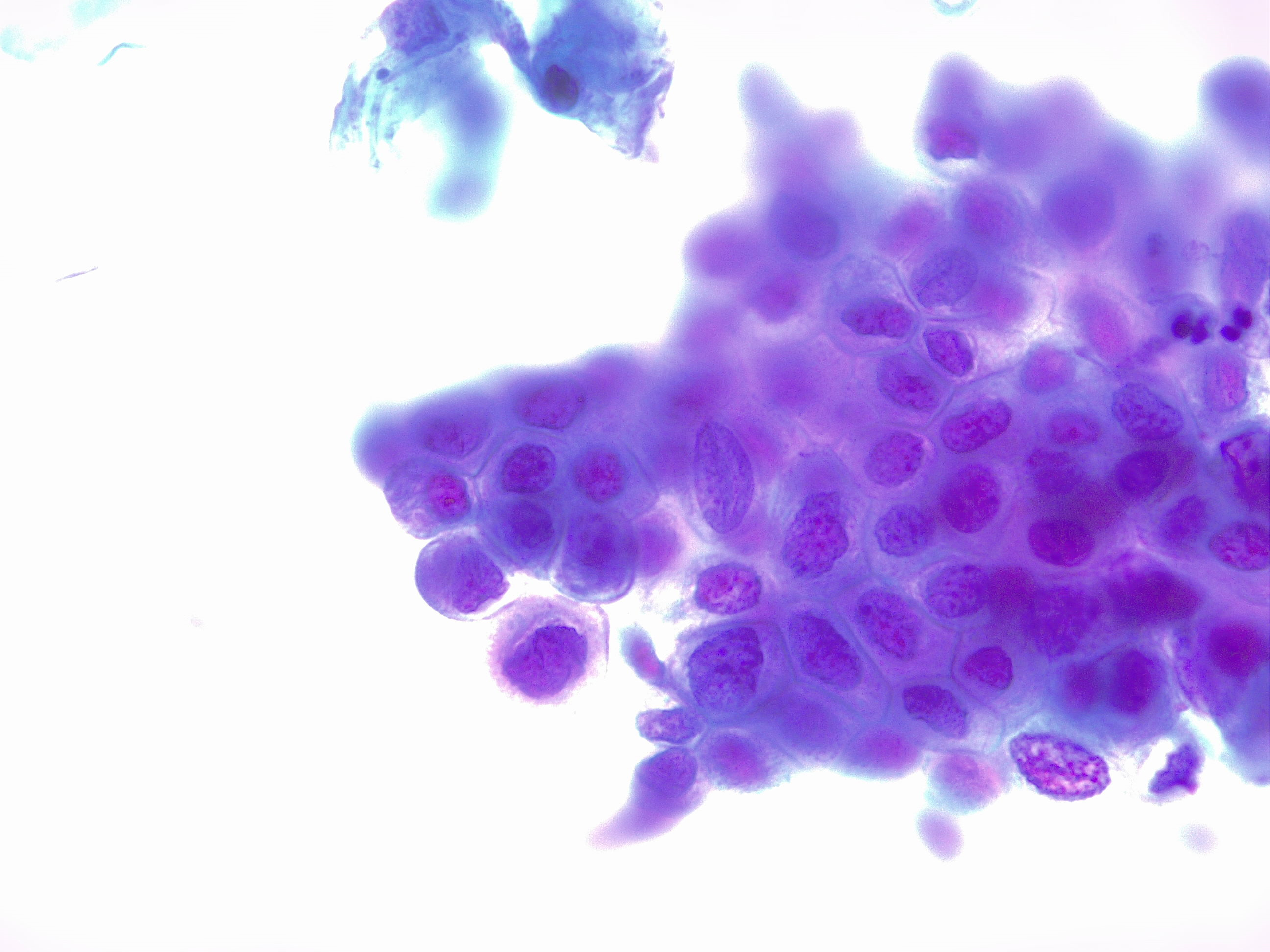
Diagnosis of HSIL on cytology requires specific criteria to be met. The cells are smaller, with less cytoplasmic maturity than that of LSIL. Occasionally, the cytoplasm may be densely keratinized. HSIL cells occur singly as well as in sheets or syncytial aggregates. Though the size of the nucleus itself is variable, the cells must have a high nuclear-to-cytoplasmic ratio (see Image 1. HSIL of the Cervix, Nuclear-to-Cytoplasmic Ratio). The nuclei are often hyperchromatic but can range from normal to hyperchromatic. The chromatin can range from evenly distributed and fine to coarsely granular. Nuclear contours must be distinctly irregular with prominent indentations and/or grooves. Nucleoli are usually not a feature of HSIL, though may be seen when HSIL involves the endocervical glands.
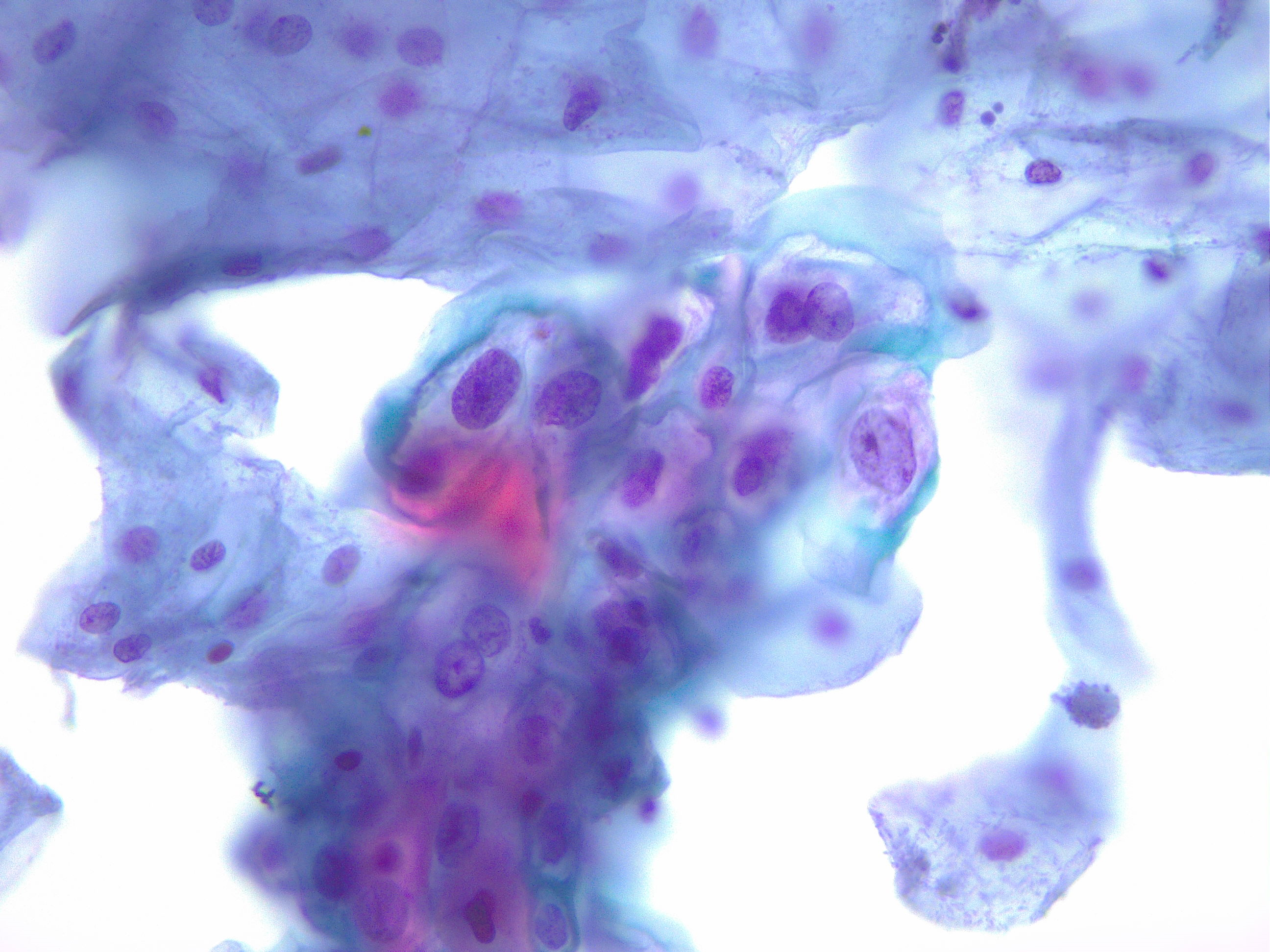
Histologic criteria for HSIL exceeds the extent and degree of nuclear atypia allowed for a diagnosis of LSIL and includes less maturation, a higher nuclear-to-cytoplasmic ratio, decreased organization from the lower immature cell layers to the superficial mature layers (loss of polarity), a greater degree of nuclear pleomorphism, highly irregular nuclear contours, increased mitotic index, and abnormal mitotic figures, especially within more superficial layers of the epithelium (see Image 2. HSIL of the Cervix, Nuclear Membrane). CIN 3 must have full-thickness atypia. When faced with challenging biopsy specimens where the pathologist is debating between benign mimics of HSIL, such as immature metaplasia or atypical atrophy, utilizing the biomarker p16 may help distinguish them, as p16 shows intense and continuous staining in HSILs and suggests infection with a high-risk HPV type.[12][13][14]
History and Physical
Women with biopsy-proven HSIL will likely have a history of multiple risk factors for HPV infection, a positive HPV test, and/or a history of abnormal Pap tests. Abnormal colposcopic findings characteristic of high-grade changes include dense acetowhite epithelium, rapid appearance of acetowhitening, cuffed crypt openings, coarse mosaicism, coarse punctuation, a sharp border, an inner border sign, and a ridge sign. The inner border sign is a sharply demarcated acetowhite area within a less opaque acetowhite area. The ridge sign is the presence of thick ledges of opaque acetowhite epithelium growing irregularly within the squamocolumnar junction.[15][16][17]
Evaluation
A Papanicolaou (Pap) test is the preferred initial method of screening for cervical neoplasia. This test is performed by opening the vaginal canal with a speculum, fully visualizing the cervix, using a cervical broom or spatula to sample cells from the transformation zone, and transferring the cells either into liquid preservative (liquid-based cytology) or directly onto a microscope slide (conventional cytology). Pathology will process the specimens according to the type of test received.
The current American College of Obstetricians and Gynecologists (ACOG) recommendations for cervical carcinoma screening in women is dependent upon age, HIV infection/immunodeficiency, and pregnancy status. Screening should be initiated at 21 years of age. Women ages 21 to 29 should be screened by cytology every 3 years. Women ages 30 to 65 should be screened with cytology and HPV co-testing every 5 years or by cytology alone every 3 years. Depending on the HPV test used, the test will provide pooled results for high-risk HPV subtypes and/or individual genotype results for HPV 16 and 18. The risk of HSIL in a patient with a positive HPV test and an abnormal Pap test is approximately 20% and increases to 33% if HPV positive at more than 1 visit.[18]
The American Society for Colposcopy and Cervical Pathology (ASCCP) publishes comprehensive guidelines for managing patients based on their Pap-test and HPV-test results and risk. For women ages 21 to 24, colposcopy is recommended following an HSIL cytology diagnosis. Women older than 24 years should also have a colposcopy performed, though management with an excisional procedure is acceptable. About 60% of women with HSIL cytology will have at least CIN 2 on biopsy, with approximately 2% showing invasive cancer, though the latter is more likely in older women. Women older than 30 years have an 8% 5-year risk of cervical cancer after a diagnosis of HSIL. Biopsies taken during colposcopy are examined for histology.[19]
After spontaneous resolution or pertinent management of CIN 2, CIN 3, or adenocarcinoma in situ, routine screening should not be discontinued for at least 20 years (even if this extends beyond age 65).[20]
Treatment / Management
Women aged 21 to 24 with HSIL cytology are recommended to undergo colposcopy. If CIN 2 or greater is not diagnosed on biopsy, it is recommended the patient follow up with cytology and colposcopy every 6 months for a 24-month period, as long as exams are adequate and reveal no squamous intraepithelial lesions (or at most LSIL). If HSIL cytology or a high-grade colposcopic lesion is found during this time, a biopsy should be performed. In patients where HSIL cytology persists for 24 months but no high-grade lesion is identified on biopsy, a diagnostic excisional procedure is recommended. If colposcopy is inadequate, CIN 3 is specified on biopsy, or CIN 2 or CIN 3 persists for 24 months, a diagnostic excisional procedure is recommended. If CIN 2 is specified on biopsy, observation for 12 months using both cytology and colposcopy every 6 months is recommended. This guideline exists because CIN 2 has a higher regression rate and less risk of progression to cancer than CIN 3, especially in younger women. Once the patient has 2 consecutive negative results on cytology and no evidence of a colposcopic abnormality, co-testing is recommended a year later. If negative, a second co-test is recommended after three years. If either co-test is abnormal, colposcopy is recommended.
For women 25 years old or older without special circumstances and with a HSIL Pap test result, expedited treatment is preferred if the immediate risk of CIN 3+ is ≥60% and acceptable if the immediate risk of CIN 3+ is between 25% and 60%. Expedited treatment is defined as treatment without a preceding colposcopic biopsy. Expedited treatment is preferred in women with HSIL and positive testing for HPV 16. Consideration of future pregnancy risks and shared decision-making should be considered when discussing expedited treatment.[21][22] (B3)
In women with HSIL, if the colposcopic exam is inadequate, a diagnostic excisional procedure is recommended. If the colposcopy is adequate and HSIL (CIN 2 or CIN 3) is confirmed on biopsy, ablation of the transformation zone or excision is considered acceptable. However, only a diagnostic excisional procedure is acceptable if the colposcopy is inadequate or the endocervical curettage shows a high-grade lesion.
Once the patient is treated, regardless of age, the recommended follow-up is HPV co-testing at 12 and 24 months post-treatment. If both are negative, she can be retested in 3 years. If this test is negative, she can return to routine screening for at least the next 20 years. An abnormal test should result in a colposcopy with endocervical sampling.[18][19](B3)
Pregnant women with HSIL cytology should not undergo excisional treatment; only colposcopy is acceptable. If a histologic diagnosis of a high-grade lesion is made, she may have additional cytologic and colposcopic exams up to every 12 weeks. If cytology results suggest invasive cancer or the colposcopic appearance of the lesion worsens, a biopsy is recommended. It is also considered acceptable to defer re-evaluation until the patient is at least 6 weeks postpartum.[21](B3)
Differential Diagnosis
Conditions that can be mistaken for HSIL on biopsy include early invasive cervical carcinoma, atrophy, squamous metaplasia, transitional metaplasia, and reactive atypia.
Prognosis
HSILs are associated with persistent HPV infection and a greater risk of progression to invasive cancer, especially if the persistent infection is a high-risk genotype such as HPV 16 and/or HPV 18.
Deterrence and Patient Education
Patient education regarding the risk factors for exposure to HPV as well as safe sexual practices in general, may reduce the risk of HPV infection.
Vaccines have been developed against high-risk HPV types 16 and 18 and low-risk HPV types 6 and 11. These vaccines are effective at preventing initial and persistent infection as well as the associated squamous intraepithelial lesions. As a majority of high-grade lesions are associated with HPV 16, it is predicted that vaccination can reduce the incidence of HGSIL/CIN 2/CIN 3 by up to 87%, although it remains to be validated in the general population.[23]
Discussion of the risks and benefits of treatment for HSIL and its effects on future pregnancy outcomes is essential.[21]
Enhancing Healthcare Team Outcomes
One of the most important functions of the interprofessional medical team is to educate patients regarding the risk factors for HPV infection as well as safe sexual practices that may reduce the risk of HPV exposure. A coordinated team approach to patient education will ultimately reduce the burden of cervical cancer on individuals and communities. Treatment decisions for HSILs are multifaceted and require a collaborative effort. A team-based approach enables clinicians to discuss individual patient factors, such as age, desire for future fertility, and comorbidities, in order to personalize the management strategy. Regular communication and follow-up among the health care team members play a crucial role in monitoring patients with HSILs. Shared decision-making and a coordinated approach to surveillance protocols ensure that patients receive appropriate follow-up care, including repeat cytology, HPV testing, and colposcopy at recommended intervals. This collaborative effort allows for early detection of disease progression or recurrence, facilitating timely intervention and preventing the development of invasive carcinoma.
Media
(Click Image to Enlarge)
HSIL of the Cervix, Nuclear-to-Cytoplasmic Ratio. High-grade squamous intraepithelial lesion of the cervix (HSIL). High nuclear-to-cytoplasmic ratio, nuclear abnormalities of high-grade dysplasia. Compare with the normal squamous cell nuclear size of the surrounding cells. Pap stain, 40x.
Contributed by Fabiola Farci, MD
(Click Image to Enlarge)
References
Darragh TM. The LAST Project and the diagnostic bottom line. Cytopathology : official journal of the British Society for Clinical Cytology. 2015 Dec:26(6):343-5. doi: 10.1111/cyt.12299. Epub [PubMed PMID: 26767600]
Senapati R, Senapati NN, Dwibedi B. Molecular mechanisms of HPV mediated neoplastic progression. Infectious agents and cancer. 2016:11():59 [PubMed PMID: 27933097]
Morris BJ, Hankins CA, Banerjee J, Lumbers ER, Mindel A, Klausner JD, Krieger JN. Does Male Circumcision Reduce Women's Risk of Sexually Transmitted Infections, Cervical Cancer, and Associated Conditions? Frontiers in public health. 2019:7():4. doi: 10.3389/fpubh.2019.00004. Epub 2019 Jan 31 [PubMed PMID: 30766863]
Piróg M, Grabski B, Jach R, Zmaczyński A, Dutsch-Wicherek M, Wróbel A, Stangel-Wójcikiewicz K. Human Papillomavirus Infection: Knowledge, Risk Perceptions and Behaviors among SMW and AFAB. Diagnostics (Basel, Switzerland). 2022 Mar 29:12(4):. doi: 10.3390/diagnostics12040843. Epub 2022 Mar 29 [PubMed PMID: 35453891]
Brown MA, Leo PJ. Genetic susceptibility to cervical neoplasia. Papillomavirus research (Amsterdam, Netherlands). 2019 Jun:7():132-134. doi: 10.1016/j.pvr.2019.04.002. Epub 2019 Apr 5 [PubMed PMID: 30954690]
Bahls L, Yamakawa R, Zanão K, Alfieri D, Flauzino T, Delongui F, de Abreu A, Souza R, Gimenes F, Reiche E, Borelli S, Consolaro M. Human Leukocyte Antigen Class I and Class II Polymorphisms and Serum Cytokine Profiles in Cervical Cancer. International journal of molecular sciences. 2017 Aug 31:18(9):. doi: 10.3390/ijms18091478. Epub 2017 Aug 31 [PubMed PMID: 28858203]
Ades S, Koushik A, Duarte-Franco E, Mansour N, Arseneau J, Provencher D, Gilbert L, Gotlieb W, Ferenczy A, Coutlée F, Roger M, Franco EL, Biomarkers of Cervical Cancer Risk (BCCR) Study Team. Selected class I and class II HLA alleles and haplotypes and risk of high-grade cervical intraepithelial neoplasia. International journal of cancer. 2008 Jun 15:122(12):2820-6. doi: 10.1002/ijc.23459. Epub [PubMed PMID: 18351579]
Level 2 (mid-level) evidenceKjellberg L, Hallmans G, Ahren AM, Johansson R, Bergman F, Wadell G, Angström T, Dillner J. Smoking, diet, pregnancy and oral contraceptive use as risk factors for cervical intra-epithelial neoplasia in relation to human papillomavirus infection. British journal of cancer. 2000 Apr:82(7):1332-8 [PubMed PMID: 10755410]
Level 2 (mid-level) evidenceCoker AL, Sanders LC, Bond SM, Gerasimova T, Pirisi L. Hormonal and barrier methods of contraception, oncogenic human papillomaviruses, and cervical squamous intraepithelial lesion development. Journal of women's health & gender-based medicine. 2001 Jun:10(5):441-9 [PubMed PMID: 11445043]
Level 2 (mid-level) evidenceGadducci A, Cosio S, Fruzzetti F. Estro-progestin Contraceptives and Risk of Cervical Cancer: A Debated Issue. Anticancer research. 2020 Nov:40(11):5995-6002. doi: 10.21873/anticanres.14620. Epub [PubMed PMID: 33109537]
Vanajothi R, Srikanth N, Vijayakumar R, Palanisamy M, Bhavaniramya S, Premkumar K. HPV-mediated Cervical Cancer: A Systematic Review on Immunological Basis, Molecular Biology, and Immune Evasion Mechanisms. Current drug targets. 2022:23(8):782-801. doi: 10.2174/1389450123666211221160632. Epub [PubMed PMID: 34939539]
Level 1 (high-level) evidenceSolomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T Jr, Young N, Forum Group Members, Bethesda 2001 Workshop. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002 Apr 24:287(16):2114-9 [PubMed PMID: 11966386]
Level 1 (high-level) evidenceHorn LC, Reichert A, Oster A, Arndal SF, Trunk MJ, Ridder R, Rassmussen OF, Bjelkenkrantz K, Christiansen P, Eck M, Lorey T, Skovlund VR, Ruediger T, Schneider V, Schmidt D. Immunostaining for p16INK4a used as a conjunctive tool improves interobserver agreement of the histologic diagnosis of cervical intraepithelial neoplasia. The American journal of surgical pathology. 2008 Apr:32(4):502-12. doi: 10.1097/PAS.0b013e31815ac420. Epub [PubMed PMID: 18223479]
Zhu Y, Ren C, Yang L, Zhang X, Liu L, Wang Z. Performance of p16/Ki67 immunostaining, HPV E6/E7 mRNA testing, and HPV DNA assay to detect high-grade cervical dysplasia in women with ASCUS. BMC cancer. 2019 Mar 27:19(1):271. doi: 10.1186/s12885-019-5492-9. Epub 2019 Mar 27 [PubMed PMID: 30917784]
Sláma J. [The new colposcopic signs--ridge sign and inner border]. Ceska gynekologie. 2012 Feb:77(1):22-4 [PubMed PMID: 22536636]
Vercellino GF, Erdemoglu E, Chiantera V, Vasiljeva K, Drechsler I, Cichon G, Schneider A, Böhmer G. Validity of the colposcopic criteria inner border sign, ridge sign, and rag sign for detection of high-grade cervical intraepithelial neoplasia. Obstetrics and gynecology. 2013 Mar:121(3):624-631. doi: 10.1097/AOG.0b013e3182835831. Epub [PubMed PMID: 23635627]
Level 2 (mid-level) evidenceHariprasad R, Mittal S, Basu P. Role of colposcopy in the management of women with abnormal cytology. CytoJournal. 2022:19():40. doi: 10.25259/CMAS_03_15_2021. Epub 2022 Jun 14 [PubMed PMID: 35928528]
Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, Solomon D, Wentzensen N, Lawson HW, 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstetrics and gynecology. 2013 Apr:121(4):829-846. doi: 10.1097/AOG.0b013e3182883a34. Epub [PubMed PMID: 23635684]
Level 3 (low-level) evidenceBentley J, EXECUTIVE COUNCIL OF THE SOCIETY OF CANADIAN COLPOSCOPISTS, SPECIAL CONTRIBUTORS. Colposcopic management of abnormal cervical cytology and histology. Journal of obstetrics and gynaecology Canada : JOGC = Journal d'obstetrique et gynecologie du Canada : JOGC. 2012 Dec:34(12):1188-1202. doi: 10.1016/S1701-2163(16)35468-8. Epub [PubMed PMID: 23231803]
Smith RA, Andrews KS, Brooks D, Fedewa SA, Manassaram-Baptiste D, Saslow D, Wender RC. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA: a cancer journal for clinicians. 2019 May:69(3):184-210. doi: 10.3322/caac.21557. Epub 2019 Mar 15 [PubMed PMID: 30875085]
Perkins RB, Guido RS, Castle PE, Chelmow D, Einstein MH, Garcia F, Huh WK, Kim JJ, Moscicki AB, Nayar R, Saraiya M, Sawaya GF, Wentzensen N, Schiffman M, 2019 ASCCP Risk-Based Management Consensus Guidelines Committee. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors. Journal of lower genital tract disease. 2020 Apr:24(2):102-131. doi: 10.1097/LGT.0000000000000525. Epub [PubMed PMID: 32243307]
Level 3 (low-level) evidence. 2019 ASCCP Risk-Based Management Consensus Guidelines for Abnormal Cervical Cancer Screening Tests and Cancer Precursors: Erratum. Journal of lower genital tract disease. 2020 Oct:24(4):427. doi: 10.1097/LGT.0000000000000563. Epub [PubMed PMID: 32732649]
Level 3 (low-level) evidenceKops NL, Hohenberger GF, Bessel M, Correia Horvath JD, Domingues C, Kalume Maranhão AG, Alves de Souza FM, Benzaken A, Pereira GF, Wendland EM. Knowledge about HPV and vaccination among young adult men and women: Results of a national survey. Papillomavirus research (Amsterdam, Netherlands). 2019 Jun:7():123-128. doi: 10.1016/j.pvr.2019.03.003. Epub 2019 Mar 16 [PubMed PMID: 30885798]
Level 3 (low-level) evidence